-
Medical journals
- Career
Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures
Authors: Lorentzon A. Kanis H. Johansson N. C. Harvey V. Gudnason G. Sigurdsson K. Siggeirsdottir M. J. 1,2 1,2 3,4 5,6 5 5 1,7; Mccloskey Liu L. Vandenput E. V. E. 1 1,8 2,9
Authors‘ workplace: Mary McKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia 1; Centre for Metabolic Bone Diseases, University of Sheffield Medical School, Beech Hill Road, Sheffield S10 2RX, UK 2; MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK 3; NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, UK 4; Icelandic Heart Association Research Institute, Kopavogur, Iceland 5; University of Iceland, Reykjavik, Iceland 6; Geriatric Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden 7; Department of Internal Medicine and Clinical Nutrition, Institute of Medicine and Clinical Nutrition, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden 8; Mellanby Centre for bone research, Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK 9
Published in: Clinical Osteology 2020; 25(4): 177-188
Category:
Overview
The risk of a recurrent fragility fracture is particularly high immediately following the fracture. This study provides adjustments to FRAX-based fracture probabilities accounting for the site of a recent fracture.
Keywords:
Fracture probability – FRAX adjustment – Imminent risk – Prior fracture – risk assessment – Sentinel fracture
Introduction
In 2008, the then World Health Organization (WHO) Collaborating Centre at Sheffield, UK, released FRAX®, a fracture risk assessment tool for estimating individualised 10-year probability of hip and major osteoporotic fracture (MOF; hip, clinical spine, distal forearm or proximal humerus) [1]. The FRAX tool integrates seven dichotomous clinical risk factors (CRFs; prior fragility fracture, parental hip fracture, smoking, systemic glucocorticoid use, excess alcohol intake, rheumatoid arthritis and other causes of secondary osteoporosis) which, in addition to age, sex and body mass index (BMI), contribute to a 10-year fracture probability estimate independently of bone mineral density (BMD) [1, 2]. BMD at the femoral neck is an optional input variable. FRAX tools are country specific to take account of the heterogeneity of fracture risk and mortality worldwide [3]. Since its release, 71 models have been made available for 66 countries covering more than 80% of theworld population [4]. The tool provides metrics which are increasingly used in health technology assessment [5–7] and regulatory guidance [8].
Prior fragility fracture, a well-established risk factor for a future fracture [9–13], is already accommodated within FRAX [1]. The population relative risk of having a hip fracture or other osteoporotic fracture is approximately 2-fold higher for most types of prior fracture. However, the increase in risk is not constant with time or age. For example, a large meta-analysis showed that a prior fracture history was a significant risk factor for hip fracture at all ages but was highest at younger ages and decreased progressively with age [12]. There is also a growing body of evidence that the risk of a subsequent osteoporotic fracture is particularly acute immediately after an index fracture and wanes progressively with time [14–20]. A recent populationbased study examined the age dependency of this immediate increase in fracture risk [21] and showed that the phenomenon of immediate risk was also age dependent, the transient effect being more evident at older ages. The immediate risk is high and then wanes over time for approximately 2 years. Thereafter, a nadir is reached but the risk remains higher than that of the general population. The early phase of particularly high risk has been termed imminent risk [21].
This transiency, which is not currently accommodated in the FRAX algorithm, suggests that treatment given to such patients immediately after fracture might avoid a higher number of new fractures compared with treatment given at a later date. This reinforces a rationale for very early intervention immediately after fractures to avoid recurrent fractures. Furthermore, it mandates the use of the most effective therapies early in the course of treatment, rather than delaying their use to a time of lower fracture risk. Thus, the quantification of imminent risk enables the targeting of anabolic treatments to individuals identified to be at very high risk [22]. In order to provide a simple means of operationalising this concept of imminent risk, the aim of the present study was to determine the impact of recency of index fractures on fracture probability as defined by FRAX.
Methods
Study cohort
The study cohort consists of 30,795 men and women, comprising all residents in the greater Reykjavik Area on December 1, 1967, born between 1907 and 1935 (both years included), which represented 55% of the total Icelandic population in this age range at that time [23, 24]. The current study is based on 18,872 participants (71.8% of the cohort) who attended during the recruitment period in 1967–1991, comprising 9116 men and 9756 women. Individuals were followed-up for a median time of 28 years until death, emigration or December 31st, 2012, yielding a total of 510,265 person-years of observation. The study was approved by the National Bioethics Committee and the Data Protection Authority in Iceland. All participants gave informed written consent.
Assessment of fractures
The Reykjavik Study fracture register documented all incident fractures and their date of occurrence in all participants fromtheir entry into the study until December 31, 2012. All medical records for the participants, including referral letters if needed,were manually examined and verified. All fractures were registered according to the International Classification of Diseases (ICD version 10 or ICD version 9). Avulsions less than 5 × 6 mm, fractures secondary to malignancy and stress fractures were excluded, as were fractures at skeletal sites not considered to be associated with osteoporosis (e.g. face, skull, hands, feet) [25]. The register has been shown to have a capture rate of about 97% for hip, forearm and clinical vertebral fractures [26]. The circumstances of the trauma leading to the fracturewere assessed, but all fractures were counted regardless of trauma. In order to minimise double counting, subsequent consecutive fractures that occurred at the same site were excluded where the interval between fractures was less than 30 days.
Four categories of sentinel fractures were defined, comprising clinical vertebral fracture (ICD 10 codes S12.0–S12.2, S12.7, S22.0–22.1, S32.0), humeral fractures (S42.2–42.3), distal forearm fracture (S52.5–52.6) and hip fracture (S72.0-S72.2).
Time horizon
Ten-year probabilities were chosen to determine the impact of recency on fracture risk. Although imminent risk appears to apply over a relatively short time frame, the magnitude of the effect is such that it impacts on 10-year probabilities. This hasbeen tested in a pilot study for prior vertebral fracture using the UK as the reference population [22].
Fracture probabilities
FRAX computes the 10-year probability of hip or major osteoporotic fracture. In the case of a prior fracture, FRAX makes no distinction between the site of fracture or its recency. In order to determine an adjustment to FRAX for these variables, fracture probabilities were calculated using the Reykjavik study. In this way, the 10-year probability of fracture, for example, after a humeral fracture 1 year ago, can be compared with the 10-year probability of a prior fragility fracture irrespective of its site or recency (as provided in the current FRAX algorithm). The ratio of the two probabilities provides an adjustment to FRAX hereafter referred to as the probability ratio or adjustment ratio.
The hazard function for a second fracture (MOF or hip fracture) after a first forearm, vertebral, humerus or hip fracture was calculated. A modification of the Poisson regression model [27, 28] was used to study the relationship between sex, age and the time since previous fracture on the one hand and on the other hand, the risk of a second fracture. Note that the model determined the hazard function for fracture and not fracture probability. Follow-up was measured in personyears and the observation period of each participant was divided in intervals of 1 month. The hazard function was assumed to be exp(β0 + β1 × sex + β2 × current time from fracture + β3 × current age). The beta coefficients reflect the importance of the variables, and βx = 0 denotes that the corresponding variable does not contribute to fracture risk. All associations were adjusted for age and time since baseline.
The fracture risk with time after previous fracture was investigated with spline functions with time since previous fracture as a continuous variable. When analysing time to second fracture, only the first fracture after the sentinel fracture was counted. When studying the association between risk of a second fracture and the time since first fracture, spline functions were fitted using knots at 0.5, 2.5 and 15 years after the first fracture. The splines were second-order functions between the breakpoints and linear functions at the tails resulting in a smooth curve. The hazard functions for death were also investigated with spline functions with time since previous fracture as a continuous variable, with the same knots as above. From the hazard functions for fracture and death, the 10-year probability of hip fracture and major osteoporotic fracture following a recent sentinel fracture occurring within 0–2 years were calculated [29, 30].
It is important to note that the probability models used were based on purpose-built models similar, but not identical, to FRAX. The Reykjavik study did not document the presence or absence of other FRAX variables, and the admixture of clinical risk factors is important in estimating the probability within any particular segment of the population. Take for example an individual with a prior humerus fracture: the probability of sustaining a subsequent hip fracture will depend not only on the recency of the humeral fracture, age and sex but also the presence of other clinical risk factors (e.g. parental history). Thus, probability ratios should, on the one hand, compare individuals with a recent prior fracture at a particular site, including some with other clinical risk factors, with, on the other hand, individuals with a prior fracture at any site, irrespective of its recency, including some with other clinical risk factors. The latter required the construct of a simulated Icelandic population (described below).
Simulation
No information on the FRAX risk variables was available for the Reykjavik Study apart from age, sex and date and site of incident fractures. The distribution of other risk indicators in the Icelandic population was undertaken using simulation samples of men and women in each 5-year age interval from the age of 39 years upwards. A small proportion of the simulated cohort was younger than 40 year (2.8%of men and 2.6% of women age 39 years). Data from these were not used in the calculations of probability ratios. Within each age interval, sample sizes were in proportion to the population of Iceland in 2015 [31]; for both men and women, a total population of 73,500 individuals was simulated. Simulations were used to provide age-specific data that reproduced the prevalence of the clinical risk factors (e.g. at the age of 65 years rather than in women aged 65 years or more). The estimates assumed that the distribution of the risk scores was the same in Icelandic men and women as that of the population-based cohorts used to synthesise the FRAX algorithms [32, 33]. The age - and sexspecific body mass index (BMI) was taken from the Icelandic REFINE (Risk Evaluation For INfarct Estimates) cohort [34] which was a random sample of 6940 men and women born in 1935–1985, living in the Reykjavik area. Bone mineral density was not simulated.
Data from the cohorts used to synthesise FRAX were used to identify appropriate regression equations needed to generate data for other risk factors in the Icelandic population as described previously [35–37]. In particular, linear (for BMI) or logistic regression (for dichotomous risk factors) was used to examine the conditional probability of the association of the risk factor to be simulated with age, sex, BMI, previous fracture, glucocorticoid use, rheumatoid arthritis, parental history of a fracture, current smoking, secondary osteoporosis and alcohol intake.
The equations identified in the logistic regressions for the dichotomous risk factors were then applied in the simulation of an Icelandic population to predict the probability of having a positive value for the risk factor for each individual. Next, a random number between 0 and 1 was generated using a computer programme, which was then compared with the predicted probability for that variable for that individual. If the random number was less than or equal to the predicted probability, the individual was assigned a positive value for the risk factor. If the random number was greater than the predicted probability, the person was assigned a negative value for the risk factor.
In order to check the adequacy of assuming that the distribution of the risk scores was the same in Icelandic men and women as that of the population-based cohorts used to synthesise the FRAX algorithms, we compared 10-year fracture probabilities of the simulated cohort with that of the population-based Age, Gene/Environment Susceptibility – Reykjavik Study (AGES Study) [38]. The AGES study recruited 3287 men and women age 65 years or more in whom FRAX was available and these data were compared to FRAX probabilities of the simulated cohort (age 65 years or more) matched for age.
Data presentation
Probability estimates are presented for each sentinel fracture sustained within the previous 2 years. For brevity, a fracture within the previous 2 years is termed a recent fracture unless otherwise noted.
Results
Characteristics of the simulated Icelandic population by sex are given in Table 1. As expected, fracture probabilities were higher in women than in men (Table 2). The mean 10-year probability of a major osteoporotic fracture in men was 8.1% and of a hip fracture was 2.5%. For women, the respective probabilities were 12.2 and 4.1%. In both men and women fracture probabilities were skewed to the left. Using the agematched population (65 years or more), fracture probabilities were very similar comparing the simulated cohort with the AGES cohort. The 10-year probability of a major osteoporotic fracture in women was 22.9% and 23.0%, respectively, and for men 10.7% and 12.6%, respectively. Hip fracture probabilities in women were 10.8% and 10.9% for the respective cohorts and in men were 5.0% and 6.4%.
1. Characteristics of the simulated Icelandic population 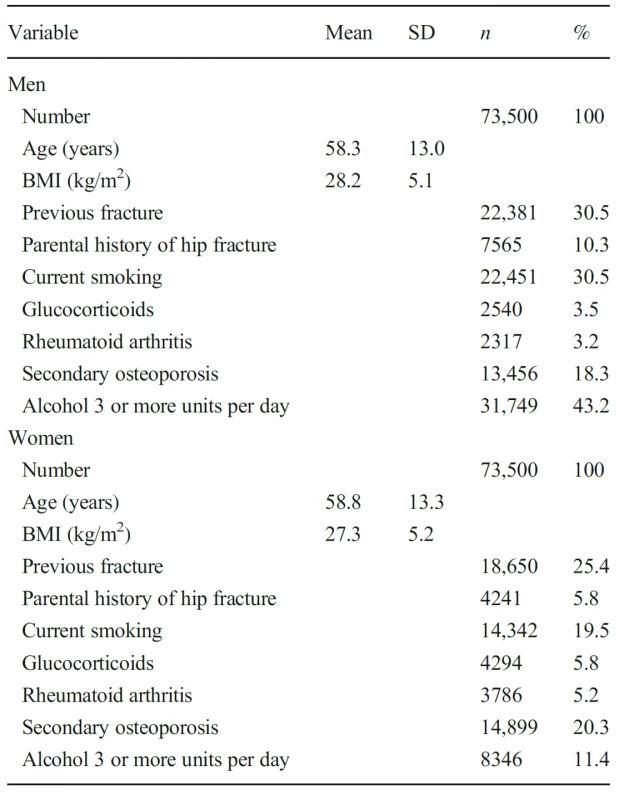
2. The 10-year probability (%) of a major osteoporotic fracture and of a hip fracture calculated without BMD in the simulated Icelandic population 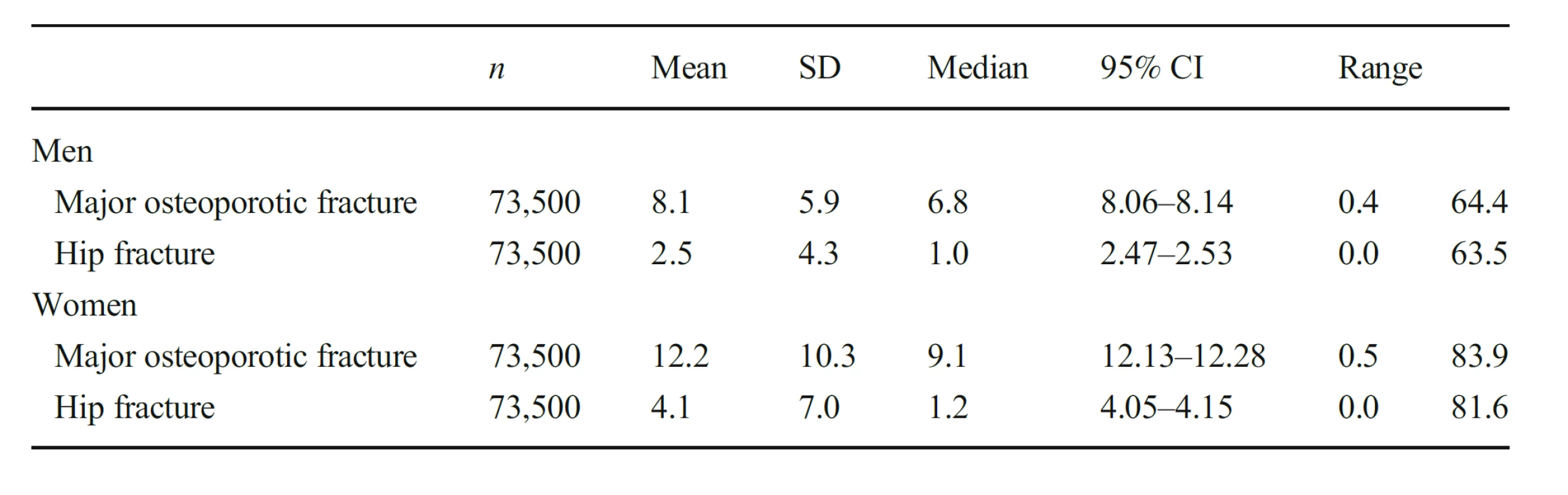
The 10-year probability of a major osteoporotic fracture in women with and without a prior fracture by age is shown in Fig. 1. Probabilities rose with age though the probability ratio (prior fracture/no prior fracture) remained relatively stable ranging from 2.38 in women age 40–44 years to 1.48 at age 85 – 89 years. Probabilities are also shown using the Icelandic FRAX model. The FRAX model yielded slightly lower probabilities since the prior fracture and no prior fracture scenarios with FRAX are calculated in the absence of other clinical risk factors. The probability ratio (prior fracture/no prior fracture)was very similar to that calculated for the present study ranging from 2.35 in women age 40–44 years to 1.55 at age 85–89 years, respectively. Findings were similar for men (data not shown).
1. 10-year probability ofMOF (%) in women with a history of a prior fragility fracture (solid symbols) and no prior fracture (open symbols). The square symbols denote probabilities calculated using the current FRAX tool for Iceland (i.e. no other clinical risk factors and BMI set at 25 kg/m2) and circles denote probabilities together with other clinical risk factors where present 
Follow-up data were available for 2074 individuals following a hip fracture, 1365 cases of clinical vertebral fracture, 2364 following a distal forearm fracture and 1092 cases of fracture at the proximal humerus. Ten-year probabilities of a major osteoporotic fracture and hip fracture in men and women with a prior fragility fracture (any site irrespective of its recency), men and women with a recent sentinel fracture (within 2 years) and the ratio between 10-year probabilities by age are given in the appendix (Tables 4, 5, 6 and 7). Figure 2 shows the probability ratios of major osteoporotic fracture by age following a recent vertebra, hip, humerus, or forearm fracture. Ratios were age dependent, decreasing with age. Thus, the adjustment of conventional probabilities needs to take age into account. For example, a woman age 60 years from Iceland with a prior fracture, no other clinical risk factors and a body mass index of 25 kg/m2 has a 10-year probability of a major osteoporotic fracture of 17% using the FRAX website. If the woman had sustained a clinical vertebral fracture within the past 2 years, the estimate should be uplifted by a factor of 1.84 to 31.3% (17 × 1.84). Had the fracture been a recent hip fracture, the adjusted probability would be 27.2% (17 × 1.60). At the age of 80 years, the same clinical scenario would yield a probability of 32% to be upward adjusted by 1.23 or downward adjusted by 0.95 for a recent vertebral or hip fracture, respectively. Note that the adjustment ratio fell slightly below unity at the age of 90 years, with the exception of vertebral and humerus fracture in women. The phenomenon arises due to the competing death risk immediately following a fracture.
In the case of hip fracture probabilities, adjustment ratios for a recent fracture were also age dependent with the exception of a recent forearm fracture (Fig. 3). The age dependency (or gradient of risk) was, as might be expected, particularly marked for a recent hip fracture. Probability ratios were qualitatively similar in men and women for each recent fracture site.
For illustrative purposes, adjustments to the UK FRAX model are shown in Table 3 for women. For example, at the age of 50 years, a prior fracture using FRAX was associated with a 10-year fracture probability of a major osteoporotic fracture of 7.2%. Where a fracture occurred within the past 2 years, fracture probability was uplifted to 19, 17, 14 and 11% where the recent fracture was at the spine, hip, humerus or forearm, respectively.
Discussion
A prior fracture is a well-documented risk factor for future fracture, and this risk is highest immediately after the initial event and subsequently declines with time [11, 14–21]. The present study provides a method for adjusting conventional estimates of fracture probability using FRAX to take account of the increased risk associated with a recent fracture. The magnitude of the adjustment varies according the site of prior fracture and for most scenarios is highly age dependent, with higher probability ratios the younger the age.
For the present study, we provided FRAX adjustments for prior fractures within a 2-year interval. The choice of 2 years is somewhat arbitrary but covers the approximate period of imminent risk [21]. Other scenarios are equally possible. For example, the probability ratio for a woman age 60 years with a sentinel vertebral fracture is 1.84 (Appendix, Table 4). The probability ratio at the time of fracture (time 0) was 1.96 and at 2 years was 1.75, differing fromthe integral value by 5–6% (data not shown). At the age of 70 years or above, the differences were less than 5%. The small differences suggest that the 2-year integral value sacrifices accuracy modestly for a substantial gain in simplicity. Nonetheless, computer-based algorithms could provide a more granular assessment of fracture recency as a continuous variable. The incorporation of recency into FRAX would demand international confirmation with prospective databases that had more or less complete information on all FRAX risk factors. Such data do not (yet) exist. For this reason, our intent is to develop an ‘add on’ to FRAX in much the same way as is undertaken for Trabecular Bone Score.
FRAX uses eight clinical risk factors. Of these, the strongest risk factors are a history of a prior fragility fracture and parental history of hip fracture. Fracture probabilities are approximately doubled in the presence of a prior fracture depending on age and sex [1, 2]. In this context, the added risk provided by taking fracture recency into account is substantial, particularly in younger individuals. For example, for women at the age of 50 years a recent vertebral fracture was associated with a probability ratio of 2.62 for computations of the probability of a major osteoporotic fracture. For a recent hip, humerus or forearm fracture, the probability ratios were 2.38, 1.96 and 1.46, respectively. It is notable that these ratios apply to FRAX calculations of probability in the presence of a prior fracture. Thus, the contribution of prior recent fracture to probability calculations is very substantial, as further illustrated in Table 3.
2. Ratio of 10-year probabilities of a major osteoporotic fracture by age in men (top panel) and women (lower panel). The ratio is the 10-year probability of a major osteoporotic fracture for a recent fracture (within 2 years) at the sites shown divided by 10-year probability at any site irrespective of its recency 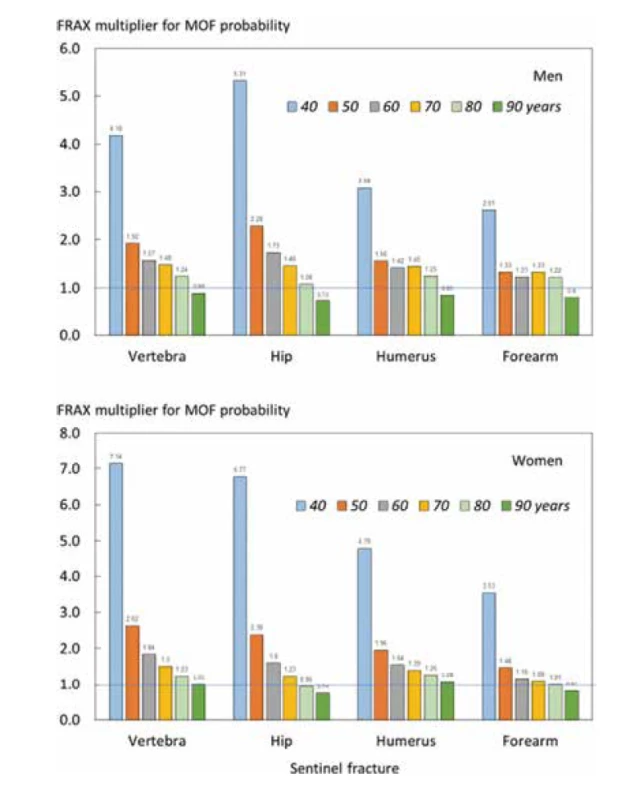
Whereas fracture probabilities are generally upward adjusted, this is not invariably the case, particularly in the elderly. The reason relates to the calculation of fracture probability that integrates the hazards of fracture and the hazards of death [29]. Like the fracture hazard, the death hazard is also increased immediately after a sentinel hip, vertebral or humerus fracture and thereafter wanes over a period of several years [39–41]. Where the incremental death hazard outstrips the incremental fracture hazard, probability ratios fall below unity. The lower fracture probabilities that arise therefrom are relatively small and might be ignored in clinical practice. It should be noted however, that even where these ratios fall below unity, those with a prior recent fracture still have a fracture probability that is higher than in those without a prior fracture (see Table 3).
Many current clinical guidelines recommend treatment of individuals who have sustained a fragility fracture [42]. It might be argued that, if patients with a prior fracture are to be treated, then adjustment for recency of fracture is not worthwhile. However, several considerations should temper this view. First, some assessment guidelines restrict the indication for treatment to a prior hip fracture alone, prior hip or vertebral fractures, to multiple fractures or to fracture patients only in the presence of densitometric osteoporosis [43–48]. The realisation ofmuch higher risks than those derived from FRAX with prior fractures of uncertain recency might modify these restrictions in future guideline iterations. Second, the International Osteoporosis Foundation (IOF) and the European Society for Clinical and Economic Evaluation of Osteoporosis and Osteoarthritis (ESCEO) recognise the dichotomisation of high risk into high and very high-risk categories [22]. The stimulus arose from the increasing availability of anabolic agents, including new agents such as abaloparatide and romosozumab or established agents such as teriparatide, which have demonstrably more rapid and greater fracture risk reductions than antiresorptive treatments [49–51]. These have the potential to revolutionise treatment strategies, particularly in individuals at very high fracture risk. Thus, categorisation of very high risk, which may incorporate the recency and site of prior fracture amongst other factors, helps to direct interventions and in turn depends on quantifying probabilities. Finally, the discussion of fracture probabilities is of value in the interaction of patients and health care professionals which may in turn promote adherence to medication [52].
3. Ratio of 10-year probabilities of a hip fracture by age in men (top panel) and women (lower panel). The ratio is the 10- year probability of hip fracture for a recent fracture (within 2 years) at the sites shown divided by 10- year probability at any site irrespective of its recency 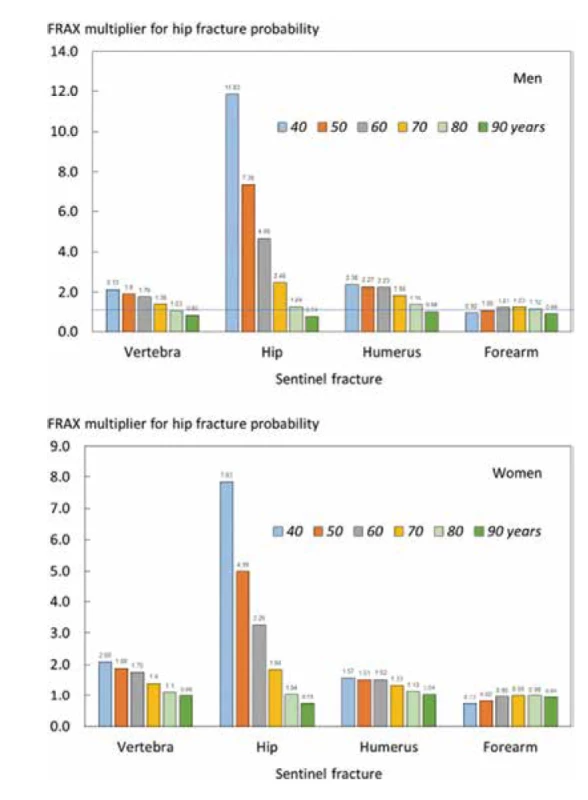
Strengths in this study were the random sampling of a large population, the detail placed on fracture ascertainment, the long duration of observation [23, 24] and the high accuracy for the ascertainment of fractures [26]. However, there were also, some limitations to this study. Despite the extensive information on fracture, age, sex, mortality, dates and sites of fracture, there was no information on other clinical risk factors that contribute to the assessment of fracture probability. For this purpose, data were simulated assuming that the distribution of clinical risk factors was the same in the Icelandic cohort as that of the population-based cohorts used in the synthesis of FRAX. The same assumption is used in all FRAX models and is justified by the low heterogeneity in risk indicators between cohorts [1]. In the present study, the simulated population had very similar FRAX-based probabilities as the population-based AGES study, reinforcing the safety of this assumption. A further limitation is that the probability calculations and the ratios derived therefrom were determined without the inclusion of bone mineral density, an omission that requires further study.
3. The 10-year probability of major osteoporotic fracture (MOF) and hip fracture in women from the UK (BMI set at 25 kg/ m2). The probabilities ‘no prior fracture’ and ‘prior fracture’ are calculated using FRAX in the absence of other clinical risk factors. The remaining probabilities are for recent (0–2 years) sentinel fractures adjusted according to the present study 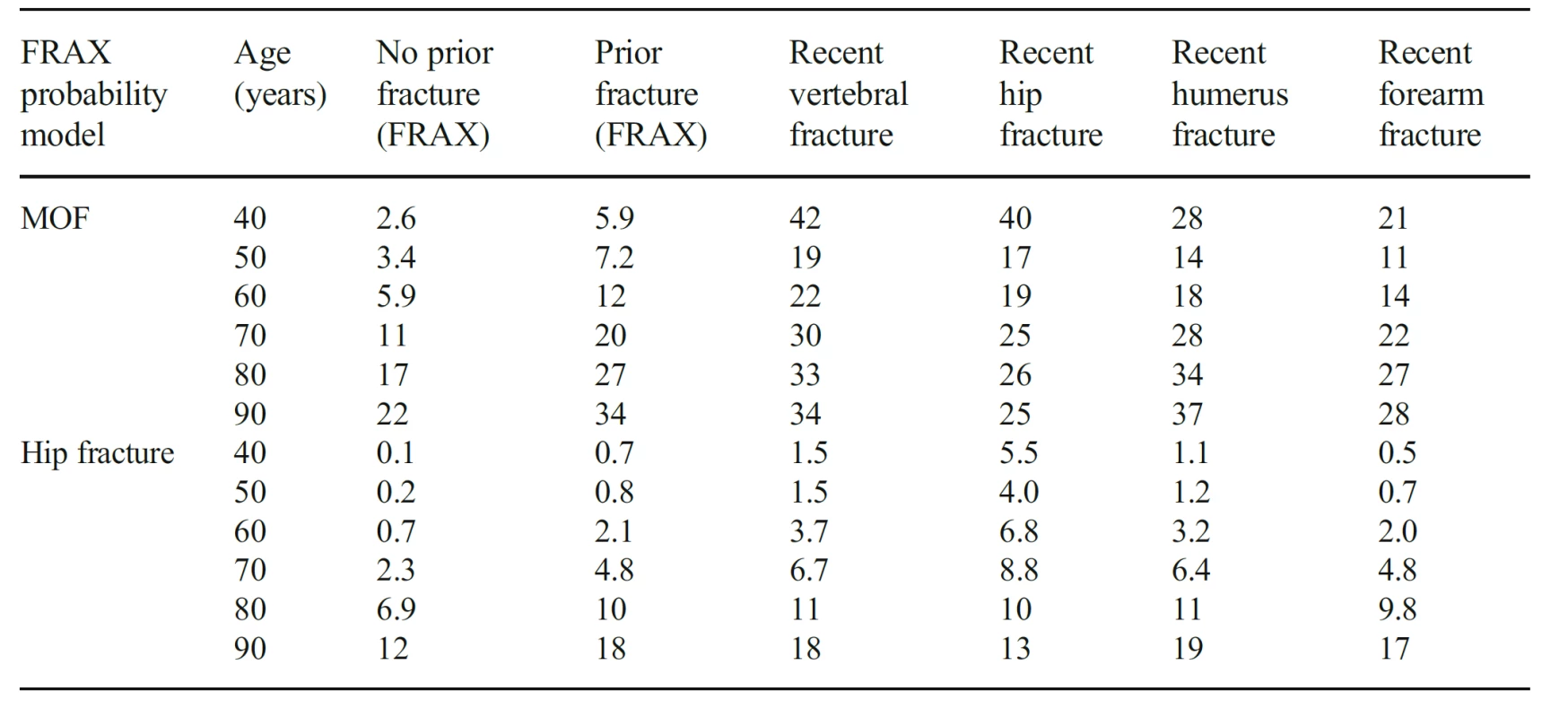
For the present study, we relied on within-cohort probability calculations rather than on FRAX. This was required because a proportion of individuals with a prior fracture would have other risk factors whereas FRAX, developed for individual rather than population assessment, does not accommodate proportional exposure to clinical risk factors. It is expected, therefore, that estimates of fracture probability would be somewhat higher in the within-cohort sample with a prior fracture than in a calculation based on FRAX. This proved to be the case. Despite the differences in absolute probabilities, the risk ratio for a prior fracture versus no prior fracture was near identical in the present study to that of FRAX.
There are known to be substantial differences in age - and sex-specific fracture incidence in different regions of the world [3]. Thus, the absolute probability values we observed will not be representative of other populations. For this reason, we computed probability ratios and there is no reason to suppose that there would be differences in the probability ratios with time. This assumption has not been extensively tested. However, probability ratios following a sentinel vertebral fracture in the present study were very similar to those calculated for the UK. For women age 50, 60, 70, 80 and 90 years, probability ratios were 2.62, 1.84, 1.50, 1.23 and 1.01, respectively, and were 2.47, 1.86, 1.52, 1.24 and 1.04 when modelled on the UK distribution of age and BMI [22]. This suggests that the probability ratios derived in the present study can be applied to adjust FRAX estimates of fracture probability in all FRAX models.
The very high immediate risk following a sentinel fracture has a marked impact on the 10-year probability of fracture and in younger men and women is substantially higher than that which FRAX would predict in the presence of a prior fracture. It is anticipated that the results of this work will be applied to conventional FRAX estimates with appropriate programmes on a web-based platform. The further refinement of risk stratification within FRAX-based guidelines is likely to aid the identification of individuals at very high fracture risk for treatment with anabolic first regimens. Additionally, the functions could be used to populate health economic models that incorporate imminent risk.
Acknowledgements We thank the participants in the Reykjavik Study for their valuable contribution. We are particularly grateful to the Committee of Scientific Advisors and the Committee of National Societies of the International Osteoporosis Foundation for their review, constructive comments and endorsement of this position paper.
Compliance with ethical standards
The study was approved by the National Bioethics Committee and the Data Protection Authority in Iceland. All participants gave informed written consent.
Conflicts of interest VGudnason,GSigurdsson, KSiggeirsdottir, E Liu, L Vandenput and H Johansson have no competing interests to declare. N. Harvey has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Kyowa Kirin, Consilient Healthcare, Radius Health and Internis Pharma.
EV McCloskey has received consultancy/lecture fees/grant funding/honoraria from AgNovos, Amgen, AstraZeneca, Consilient Healthcare, Fresenius Kabi, Gilead, GSK, Hologic, Internis, Lilly, Merck, Novartis, Pfizer, Radius Health, Redx Oncology, Roche, SanofiAventis, Servier, Synexus, UCB, Viiv, Warner Chilcott, I3 Innovus and Unilever.
JA Kanis reports grants from Amgen, Eli Lilly and Radius Health and consulting fees from Theramex. JAK is the architect of FRAX® but has no financial interest.
M Lorentzon has received lecture fees from Amgen, Lilly, Meda, Renapharma, and UCB Pharma and consulting fees from Amgen, Radius Health, UCB Pharma, Renapharma and Consilient Health, all outside the presented work.
Appendix
4. Ten-year probability of a major osteoporotic fracture and hip fracture (%) in men and women with a prior fragility fracture (any site irrespective of its recency), probabilities for a recent clinical vertebral fracture (within 2 years) and the ratio between 10-year probabilities by age 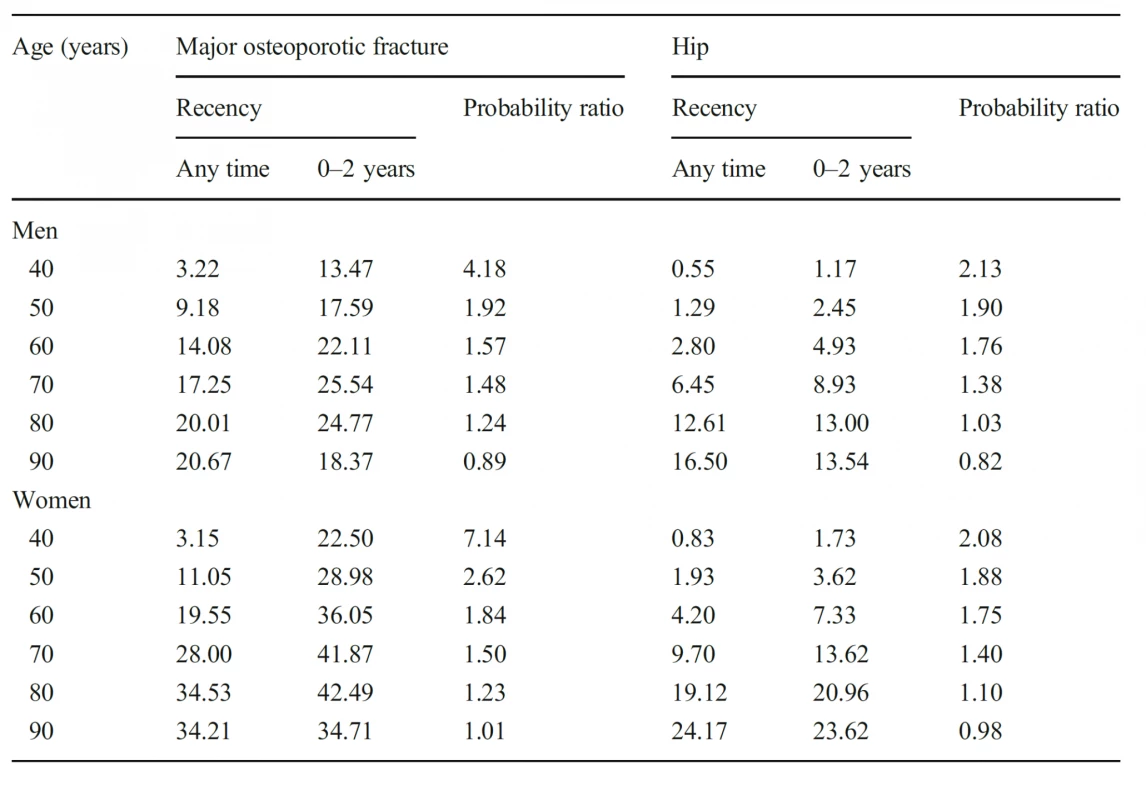
5. Ten-year probability of a major osteoporotic fracture and hip fracture (%) in men and women with a prior fragility fracture (any site irrespective of its recency), probabilities for a recent clinical hip fracture (within 2 years) and the ratio between 10- year probabilities by age 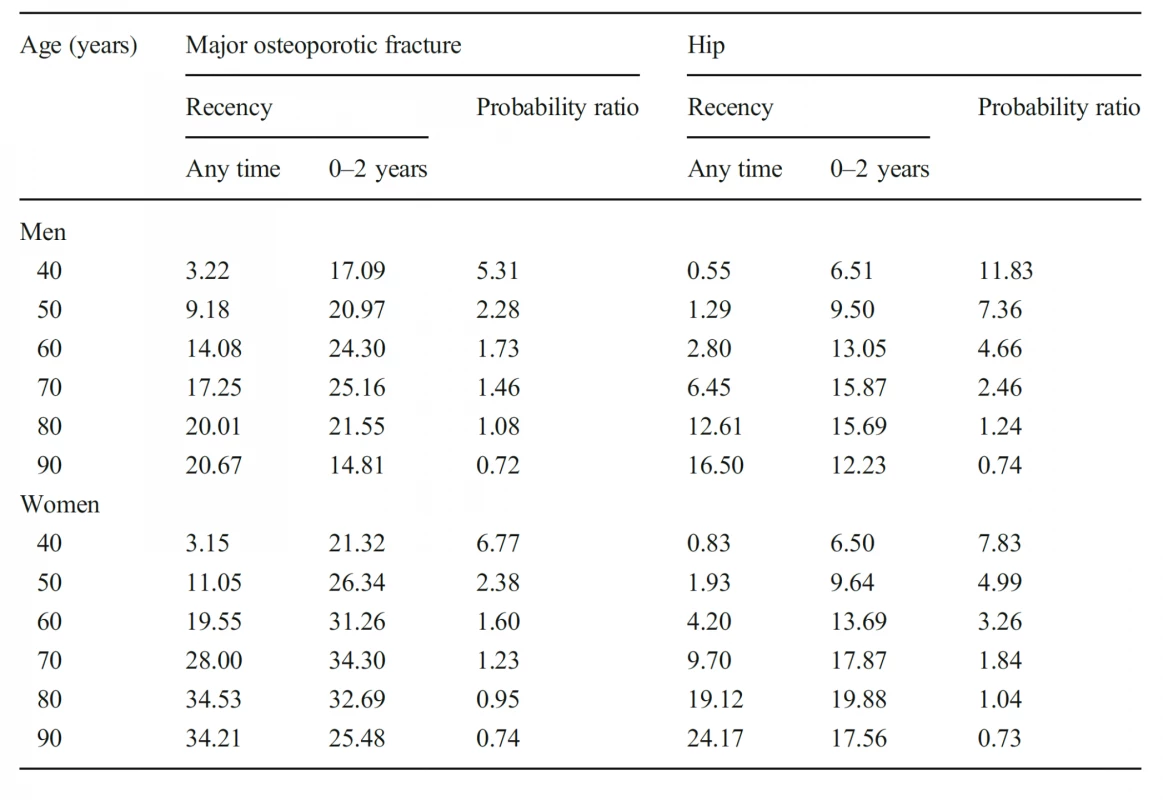
6. Ten-year probability of a major osteoporotic fracture and hip fracture (%) in men and women with a prior fragility fracture (any site irrespective of its recency), probabilities for a recent clinical humeral fracture (within 2 years) and the ratio between 10-year probabilities by age 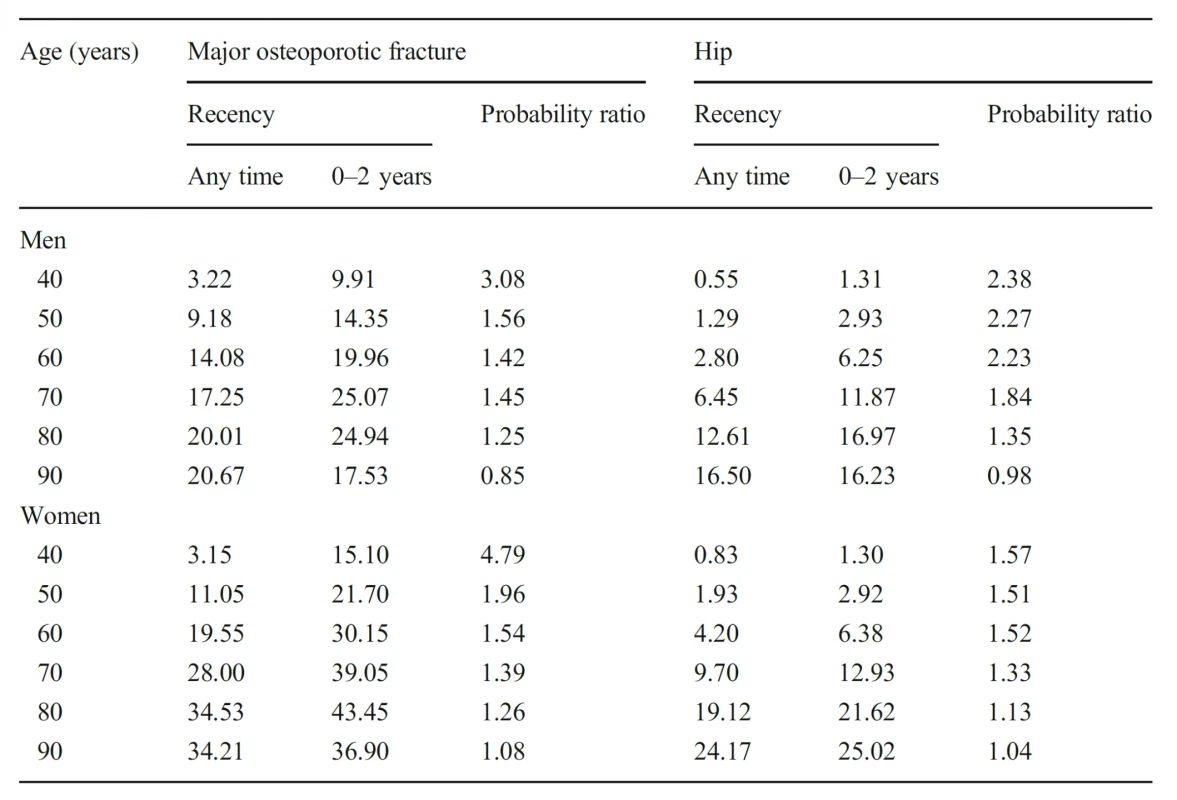
7. Ten-year probability of a major osteoporotic fracture and hip fracture (%) in men and women with a prior fragility fracture (any site irrespective of its recency), probabilities for a recent clinical forearm fracture (within 2 years) and the ratio between 10-year probabilities by age 
J. A. Kanis
w.j.pontefract@shef.ac.ukReceived: 8 May 2020 / Accepted: 1 June 2020
Sources
1. Kanis JA on behalf of the World Health Organization Scientific Group (2008a) Assessment of osteoporosis at the primary health-care level. Technical report. WHO Collaborating Centre, University of Sheffield, UK. Available at http://www.shef.ac.uk/FRAX/index.htm. Accessed 25 March 2020
2. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey EV (2008b) FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19 : 385–397
3. Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl D, Cyrus Cooper C, on behalf of the IOF Working Group on Epidemiology and Quality of Life (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23 : 2239–2256
4. Kanis JA, Johansson H, Oden A, Cooper C, McCloskey EV, the Epidemiology and Quality of Life Working Group of IOF (2014) Worldwide uptake of FRAX. Arch Osteoporos 9 : 166. https://doi. org/10.1007/s11657-013-0166-8
5. National Institute for Health and Care Excellence (2012) CG146: osteoporosis: fragility fracture risk. Short clinical guideline - evidence and recommendation. National Clinical Guideline Centre, London
6. National Institute for Health and Care Excellence (2017) TA 464: bisphosphonates for treating osteoporosis. Technology appraisal guidance 464. National Institute for Health and Care Excellence, London nice.org.uk/guidance/ta464. Accessed 25 March 2020
7. Kanis JA, Cooper C, Rizzoli R, Reginster J-Y, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30 : 3–44
8. Committee forMedicinal Products for Human Use (CHMP) (2006) Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis. CHMP, London
9. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15 : 721–739
10. Haentjens P, Johnell O, Kanis JA, Bouillon R, Cooper C, Lamraski G, Vanderschuren D, Kauffman J-M, Boonen S (2004) Genderrelated differences in short and long-term absolute risk of hip fracture after Colles’ or spine fracture: Colles’ fracture as an early and sensitive marker of skeletal fragility in men. J Bone Miner Res 19 : 1933–1944
11. Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Pettersen C, De Laet C, Jonsson B (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15 : 175–179
12. Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ III, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35 : 375–382
13. Hansen L, Petersen KD, Eriksen SA, Langdahl BL, Eiken PA, Brixen K, Abrahamsen B, Jensen JE, Harslof T, Vestergaard P (2015) Subsequent fracture rates in a nationwide population-based cohort study with a 10-year perspective. Osteoporos Int 26 : 513–519
14. Johnell O, Oden A, Caulin F, Kanis JA (2001) Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int 12 : 207–214
15. Giangregorio LM, Leslie WD (2010) Manitoba bone density program. Time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res 25 : 1400–1405
16. Dretakis KE, Dretakis EK, Papakitsou EF, Psarakis S, Steriopoulos K (1998) Possible predisposing factors for the second hip fracture. Calcif Tissue Int 62 : 366–369
17. Nymark T, Lauritsen JM, Ovesen O, Röck ND, Jeune B (2006) Short time-frame from first to second hip fracture in the Funen County Hip Fracture Study. Osteoporos Int 17(9):1353–1357
18. Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285 : 320–323
19. Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res 24 : 1299–1307
20. van Geel TACM, van Helden S, Geusens PP, Winkens B, Dinant G-J (2016) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68 : 99–102
21. Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28 : 775–780
22. Kanis JA, Harvey NC, McCloskey E, Bruyère O, Veronese N, Lorentzon M, Cooper C, Rizzoli R, Adib G, Al-Daghri N, Campusano C, Chandran M, Dawson-Hughes B, Javaid K, Jiwa F, Johansson H, Lee JK, Liu E, Messina D, Mkinsi O, Pinto D, Prieto-Alhambra D, Saag K, Xia W, Zakraoui L, Reginster J-Y (2020) Algorithm for the management of patients at low/middle/ high risk of osteoporotic fracture: a global perspective. Osteoporos Int 31 : 1–12
23. Bjornsson G, Bjornsson OJ, Davidsson D et al (1982) Report abc XXIV. Health survey in the Reykjavik Area. –Women. Stages I-III, 1968–1969, 1971–1972 and 1976-1978. Participants, invitation, response etc. The Icelandic Heart Association, Reykjavík
24. Bjornsson OJ, Davidsson. D., Olafsson H et al (1979) Report XVIII. Health Survey in the Reykjavik Area. — Men. Stages I – III, 1967–1968, 1970–1971 and 1974–1975. Participants, invitation, response etc. The Icelandic Heart Association, Reykjavík
25. Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12 : 417–427
26. Siggeirsdottir K, Aspelund T, Sigurdsson G, Mogensen B, Chang M, Jonsdottir B, Eiriksdottir G, Launer LJ, Harris TB, Jonsson BY, Gudnason V (2007) Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol 22 : 631–639
27. Breslow NE, Day NE (1987) Statistical methods in cancer research, vol 2. IARC scientific publications, no 32, Lyon, pp 131–135
28. Albertsson-Wikland K, Martensson A, Savendahl L, Niklasson A, Bang P, Dahlgren J, Gustafsson J, Kriström B, Norgren S, Pehrsson NG, Odén A (2016) Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab 101 : 2149–2159
29. Kanis JA, Johnell O, Oden A, Sernbo I, Redlund-Johnell I, Dawson A, de Laet C, Jonsson B (2000) Long-term risk of osteoporotic fractures in Malmo. Osteoporisis Int 11 : 669–674
30. Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Oglesby A (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Mineral Res 17 : 1237–1244
31. United Nations (2019) Department of Economic and Social Affairs. World Population Prospects 2019 https://populationunorg/wpp/ Download/Standard/Population/ accessed 15 Feb 2020
32. Johansson H, Kanis JA, Ode’n A, Johnell O, McCloskey E (2009) BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporos Int 20 : 1675–1682
33. Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ 3rd, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18 : 1033–1046
34. Sekikawa A, Steingrimsdottir L, Ueshima H, Shin C, Curb JD, Evans RW, Hauksdottir AM, Kadota A, Choo J, Masaki K, Thorsson B, Launer LJ, Garcia ME, Maegawa H, Willcox BJ, Eiriksdottir G, Fujiyoshi A, Miura K, Harris TB, Kuller LH, Gudnason V (2012) Serum levels of marine-derived n-3 fatty acids in Icelanders, Japanese, Koreans, and Americans–a descriptive epidemiologic study. Prostaglandins Leukot Essent Fatty Acids 87 : 11–16
35. Kanis JA, Johansson H, Odén A, McCloskey EV (2012b) The distribution of FRAX® based probabilities in women from Japan. J Bone Miner Metab 30 : 700–705
36. Dawson-Hughes B, Looker AC, Tosteson ANA, Johansson H, Kanis JA, Melton LJ III (2010) The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int 21 : 41–52
37. Johansson H, Kanis JA, Oden A, Compston J, McCloskey E (2012) A comparison of case-finding strategies in the UK for the management of hip fractures. Osteoporos Int 23 : 907–915
38. Thordardottir M, Lindqvist EK, Lund SH et al (2018) Dietary intake is associated with risk of multiple myeloma and its precursor disease. PLoS One 13(11):e0206047. https://doi.org/10.1371/ journal.pone.0206047
39. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32 : 468–473
40. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fractures. Osteoporos Int 15 : 108–112
41. Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Pettersen C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15 : 38–42
42. Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV (2016) A systematic review of intervention thresholds based on FRAX. Arch Osteoporos 11(1):25
43. Chakhtoura M, Baddoura R , El-Hajj Fuleihan G (2013) Lebanese FRAX-based osteoporosis guidelines. http://www.osteos.org.lb/ admin/uploads/Full%20document.pdf accessed 19 Oct 2015
44. Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, FavusMJ, Harris ST, Jan de Beur SM, Khosla S, Lane NE, Lindsay R, Nana AD, Orwoll ES, Saag K, Silverman S, Watts NB (2014) The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 25 : 1439–1443
45. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25 : 2359–2381
46. Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD, Scientific Advisory Council of Osteoporosis Canada (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182 : 1864–1873
47. Scottish Intercollegiate Guidelines Network (SIGN) (2015) Management of osteoporosis and the prevention of fragility fractures. Edinburgh: SIGN; 2015 (SIGN publication no 142)[March 2015] Available from URL: http://wwwsignacuk accessed 11 May 2015
48. Agency for care effectiveness (2018) Osteoporosis – identification and management in primary care. Available at http://wwwacehtagovsg/ our-guidance/osteoporosis-identification-andmanagement - in-primary-carehtml Accessed 22 Dec 2019
49. Cosman F, Nieves JW, Dempster DW (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 32 : 198–202
50. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377 : 1417–1427
51. Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner - Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Möricke R, López-Romero P (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, doubledummy, randomised controlled trial. Lancet 391 : 230–240
52. Parsons CM, Harvey N, Shepstone L, Kanis JA, Lenaghan E, Clarke S, Fordham R, Gittoes N, Harvey I, Holland R, Redmond NM, Howe A, Marshall T, Peters TJ, Torgerson D, O'Neill TW, McCloskey E, Cooper C, SCOOP Trial Group (2020) Systematic screening using FRAX leads to increased use of, and adherence to, anti-osteoporosis medications: the Uk SCOOP Trial. Osteoporosis Int 2018 31 : 67–75
Labels
Clinical biochemistry Paediatric gynaecology Paediatric radiology Paediatric rheumatology Endocrinology Gynaecology and obstetrics Internal medicine Orthopaedics General practitioner for adults Radiodiagnostics Rehabilitation Rheumatology Traumatology Osteology
Article was published inClinical Osteology

2020 Issue 4-
All articles in this issue
- Mnohostranné dopady pandemie
- FRAX bring greater benefit
- Diagnosis and treatment of Paget´s disease of bone: update 2020
- Bone and immune system – osteoimmunology
- Vitamin D and bone quality
- Nutritional (vitamin D deficient) rickets – a neglected diagnosis? Case report
- Adjusting conventional FRAX estimates of fracture probability according to the recency of sentinel fractures
- Clinical Osteology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnosis and treatment of Paget´s disease of bone: update 2020
- Nutritional (vitamin D deficient) rickets – a neglected diagnosis? Case report
- FRAX bring greater benefit
- Bone and immune system – osteoimmunology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

