-
Medical journals
- Career
Comparison of the efficiency of peripheral blood stem cell apheresis on the blood cell separators
Authors: K. Filonenko 1; V. Zarutska 2; A. Martynchyk 3; Y. Kushchevyi 2; Y. Stepanishyna 2; I. Kriachok 4
Authors‘ workplace: Department of Hematology and Transplantology, University Clinical Center, Gdansk, Poland 1; Bone Marrow Transplantation Department, National Cancer Institute, Kyiv, Ukraine 2; Immunology and Molecular Oncology, Epworth Healthcare, East Melbourne, VIC, Australia 3; Department of Chemotherapy of Hemoblastoses, National Cancer Institute, Kyiv, Ukraine 4
Published in: Klin Onkol 2022; 35(6): 448-453
Category: Original Articles
doi: https://doi.org/10.48095/ccko2022448Overview
Background: Stem cells apheresis is a key step in the process of the autologous stem cell transplantation. Available blood cell separators (BCS) have different efficiency due to the technical characteristics and influence of the operator. Materials and methods: Retrospectively, data were collected of the peripheral blood stem cells apheresis performed using available BCS manufactured by Fresenius (ComTec and Amicus) in the National Cancer Institute Ukraine from 2017 to 2020. The collection efficiency coefficient (CEC) was calculated, the formula for predicting the total volume of processed blood (TVPB) was adapted for each separator. Results: The analysis included data from 60 patients (total of 92 apheresis procedures). The mean CEC was established at the level of (53.8 ± 36.6) % for the Amicus device and (44.2 ± 37.3) % for the ComTec device; P = 0.22. The lower product volume was obtained using the Amicus device compared to the ComTec device; P = 2×10–7. The amount of collected stem cells was comparable in both groups (5.8 ± 5.7) ×106/kg and (4.1 ± 3.1) ×106/kg, respectively; P = 0.064. The adaptation of the formula for predicting the TVPB to achieve the optimum amount of stem cells was performed. Conclusion: The CEC for each device was within the generally accepted limits of 30–50%, and did not differ significantly. Nevertheless, using of the Amicus BCS allowed to collect lower volumes of the product, maintaining other characteristics of the product competitive.
Keywords:
Stem cells – Leukapheresis – efficacy – autologous transplantation – blood cell separation – cell separation – Amicus – ComTec
Introduction
High dose chemotherapy (HDCT) followed by autologous stem cell transplantation (ASCT) is utilized as a standard treatment for multiple hematological malignancies and some solid tumors [1] and can significantly improve the survival of patients [2–6]. To ensure the effectiveness of graft function and timely recovery of hematopoiesis after ASCT, a sufficient number of a patient’s stem cells need to be collected prior to starting the HDCT (conditioning regimen). Hematopoietic stem cells are usually mobilized into the peripheral blood by a short course of subcutaneous injections of granulocyte-colony-stimulating factor (G-CSF, filgrastim), and then collected with one or multiple leukapheresis procedures. According to the European Group for Blood and Marrow Transplantation (EBMT) recommendations, in order to guarantee a quick recovery of blood counts after transplant, the collected peripheral blood stem cells (PBSC) should contain ≥ 2×106 CD34+ cells/kg of patient’s body weight [7–9]. A number of factors can influence the effectiveness and the duration of PBSC apheresis, including the concentration of hematopoietic stem cells in the peripheral blood on the day of collection, platelet count, hematocrit, blood cell separator (BCS) specifications, type of software used, the operator’s experience etc. [7,9–11]. Before starting the apheresis, the total volume of processed blood (TVPB), a key parameter, needs to be chosen as it can affect both the duration and the efficiency of the procedure. In fact, the choice of an insufficient TVPB can lead to the unsuccessful collection, whereas opting for an excessive TVPB can affect an already weak patient by: prolonging patient’s stay in a forced position, and increasing the risk of hypocalcemia/hypomagnesemia induced tetany (without appropriate replacement therapy), or increasing the risk of bleeding due to a decrease in the platelet count at the end of the apheresis procedure [9].
The purpose of our work was to determine the collection efficiency coefficient (CEC) of the PBSC using BCS available at the Department of Oncohematology of the National Cancer Institute (NCI), Ukraine, manufactured by Fresenius (ComTec and Amicus), as well as to adapt the formula for predicting the TVPB for each of the devices.
Materials and methods
The data on the procedure of PBSC apheresis in patients of the Department of Oncohematology of the NCI from 2017 to 2020 on the ComTec BCS and the Amicus BCS manufactured by Fresenius were collected retrospectively. We analyzed the complex impact of the multiple factors on efficiency of the collection. These included: demographic (age, gender) and disease (diagnosis, disease status at the time of collection) characteristics, the TVPB adopted, blood cell counts (hematocrit, platelet and leukocyte level) and the level of circulating CD34+ cells at the beginning of collection, and the use of plerixafor [“Mosifer”, 1.2 mL] prior to collections. The prognostic risk factors for poor mobilization that were analyzed were: age > 60 years, advanced stage of the underlying disease, number of previous therapies, prior treatment with fludarabine, melphalan and lenalidomide, low CD34+ cells level before apheresis, and low platelet count prior to it [7].
The parameter for starting the apheresis was the presence of at least 20 circulating CD34+ cells/μL, detected in the patient’s blood by standard cytofluorimetric methods on the day of collection. The additional use of plerixafor was indicated in patients who had previously failed to mobilize sufficient numbers of stem cells with standard regimens, or collected insufficient numbers of PBSC, or in whom the number of circulating CD34+ cells/μL after 4 days of mobilization with G-CSF was >10 but < 20 [7]. In addition, some patients who initially had high risk of poor mobilization were offered the use of plerixafor at the first attempt to mobilize.
The definition of ‘poor mobilization’ included either the inability to perform apheresis due to low CD34+ cells in the peripheral blood after mobilization (< 20 cells/μL) or the inability to collect at least 2×106/kg CD34+ cells for a maximum of four consecutive days of apheresis.
The TVPB was chosen independently by an experienced operator of a BCS based on a standard approach. For patients who did not have risk factors for poor mobilization and had a sufficient amount of the PBSC (≥ 20 cells/μL) on the day of collection, apheresis was used from the TVPB amounting to 2 or 3 volumes of circulating blood (VCB) of a patient. For patients with a high risk of unsuccessful mobilization apheresis with a larger TVPB was used, equal to 4 or 5 the patient’s VCB [12–14]. To objectify the choice of the TVPB by taking into account the parameters of the BCS available in the Department of Oncohematology, the CEC was analyzed retrospectively.
The level of stem cells (SC) of the product was determined by flow cytometry. The result of the collection was defined as the amount of SC in the product per kg of patient’s body weight.
For both BCS, the CEC was calculated according to the standard formula 1 [15], and the CEC of both separators were compared.
Formula 1. CEC – collection efficiency coefficient, SC – stem cells, TVPB – total volume of processed blood, PBSC – peripheral blood stem cells 
The TVPB forecasting formula has been adapted for each of the BCS available in the department from the standard formula 2 [16]
Formula 2. PBSC – peripheral blood stem cells, CEC– collection efficiency coefficient, TVPB –total volume of processed blood 
Statistical data processing was performed using EZR v.1.35 (Saitama Medical Center, Jichi Medical University, Saitama, Japan, 2017). Descriptive statistics methods were used to present the results of the study. The arithmetic mean is (X), the error of the mean is SX, and the standard deviation is σ. The differences between samples were evaluated by Student‘s parametric criterion. In all cases, the critical level of significance is taken at the level 0.05. In the analysis of discrete indicators, the comparison between the groups was carried out by constructing conjugation tables and using the exact Fisher or X2-Pearson criteria. The pairwise intergroup analysis was performed using the Bonferroni amendment.
Results
In this study, we analyzed 92 stem cell apheresis procedures from 60 patients (28 males and 32 females). The mean age of patients was 36.7 ± 12.0 years. The apheresis procedures were performed using the ComTec (Fresenius Kabi, France) device in 32 patients, with a total of 48 procedures performed; and the Amicus (Fresenius Kabi, France) device in 30 patients, with a total of 43 procedures. The data on patient demographic characteristics, key hematological parameters, the distribution of diagnoses and the status of the achieved response at the time of apheresis in the two groups are presented in Tab. 1; the differences were not statistically significant.
1. Distribution by demographic characteristics, hemogram parameters, diagnosis and disease status. 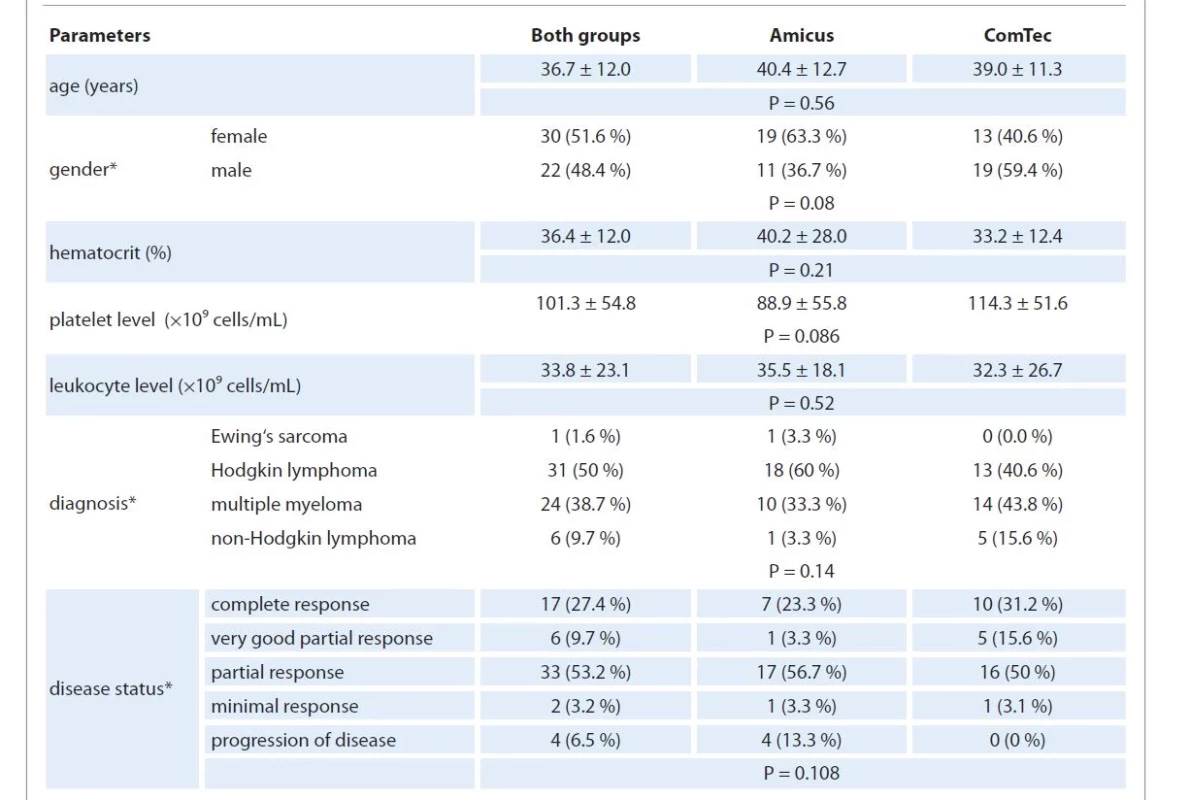
* The following factors like gender, diagnosis, and disease status were calculated not by the number of collection attempts but by the number of patients. If one patient passed the collection on both separators, he was considered in both groups. We then compared the two groups for indicators directly related to the mobilization procedure and collection, including the number of patients at risk of failed mobilization; the use of plerixafor; the selected TVPB and the circulating CD34+ cell number on the day of apheresis. The data are presented in Tab. 2. The two groups did not differ in the selected TVPB, the level of CD34+ cells on the day of apheresis and the frequency of the use of plerixafor. However, the group of patients who were collected using the Amicus BCS included a higher number of patients with factors associated with unsuccessful mobilization: 48.8% vs. 27.1% (P = 0.05).
2. Parameters of the PBSC mobilization and collection 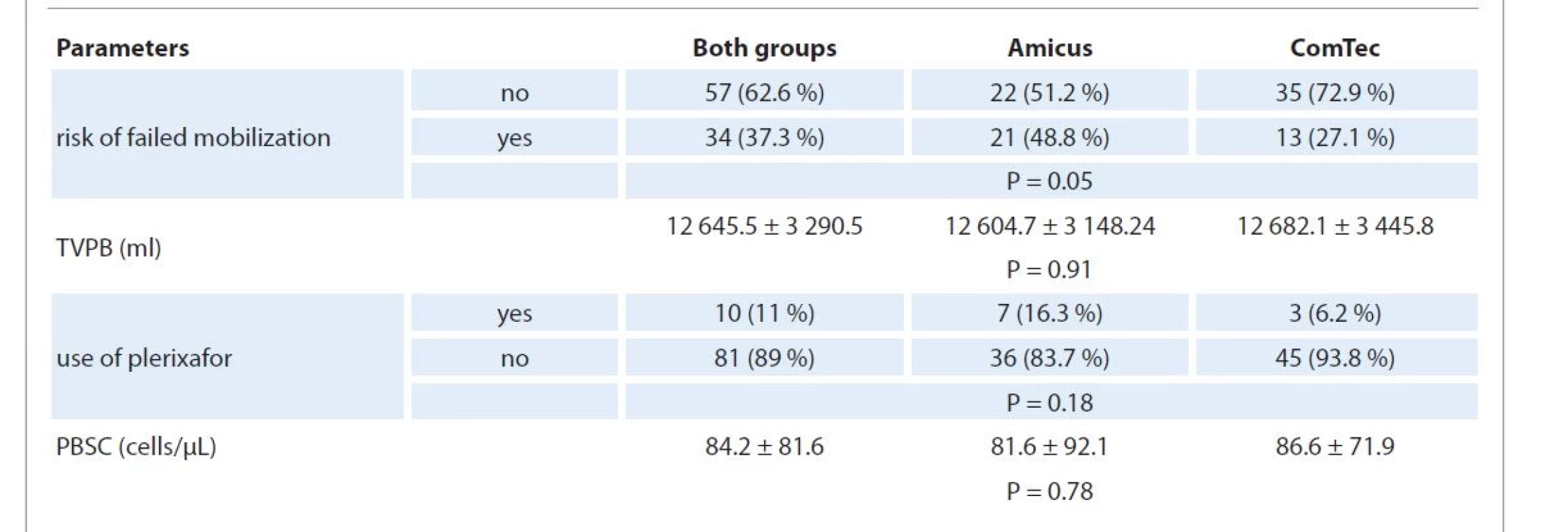
TVPB – total volume of processed blood, PBSC – peripheral blood stem cells Nevertheless, the analysis of grafts from each group showed that the average numbers of the CD34+ cells obtained from both separators were satisfactory and comparable: (5.8 ± 5.7) ×106/kg for Amicus BCS and (4.1 ± 3.1) ×106/kg for ComTec BCS (P = 0.064) (Fig. 1, Tab. 3), respectively. However, significant differences in the volume of the obtained product and the concentration of PBSC per unit volume of the product were revealed.
Fig. 1. Number of CD34+ cells collected per kilogram of body weight of patients for each device. 
3. Results of apheresis. 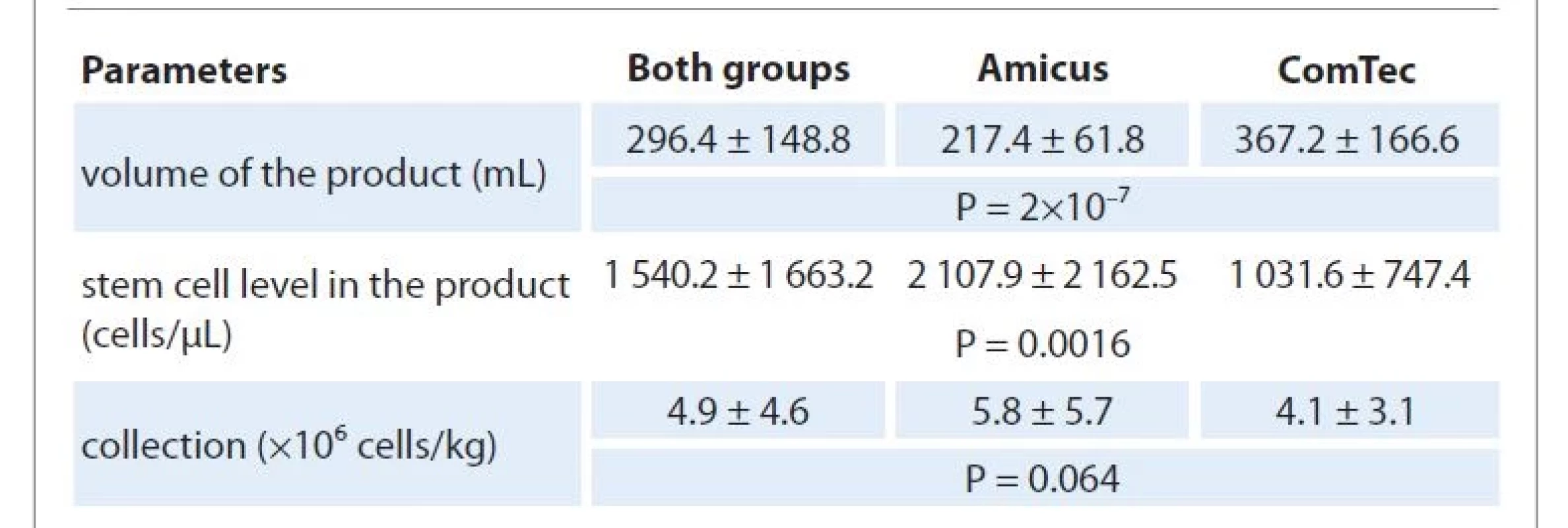
TVPB – total volume of processed blood, PBSC – peripheral blood stem cells We observed that using the Amicus device, the final product had a smaller volume (217.4 ± 61.8 mL) compared to the ComTec device (367.2 ± 166.6 mL) (P = 0.000). Therefore, the CD34+ cell concentrations in the product of the two groups were: 2 107.9 ± 2 162.5 cells /μL (Amicus) and 1 031.6 ± 747.4 cells/μL, (ComTec), respectively (P = 0.0016).
The next part of the analysis was the CEC calculation for both separators. For the Amicus BCS, the mean CEC was 53.8 ± 36.6%, and for ComTec BCS, it was 44.2 ± 37.3%, P = 0.22 (Fig. 2). Thus, the CEC indicators for both separators were comparable and were not lower than the acceptable range 30–50% [15].
Fig. 2. Collection efficiency coefficient for each device. 
Further, we adapted the formula used to predict expected TVPB that must be processed to achieve the optimal number of PBSC for transplantation; this was performed for each of the devices.
Thus, having calculated the CEC for each of the devices and the target result of the collection and patients’ data (body weight and number of CD34+ cells on the day of apheresis), one can predict the required for this TVPB procedure.
To use the formula when planning apheresis in each of the patients in Excel is convenient, a spreadsheet editor was created to be used as an algorithm to predict the required TVPB for this procedure. According to this algorithm, to perform calculations in the appropriate columns for a given separator that will be used, it is necessary to enter the PBSC level on the day of collection (cells/μL) and the patient‘s weight (kg). The table will automatically calculate the TVPB, which is required for SC collection of 2×106/kg, 3×106/kg or 4×106/kg (Fig. 3).
Fig. 3. The view of the spreadsheet editor created to predict the required total volume of processed blood before apheresis procedure. 
CEC – collection efficiency coefficient, PBSC – peripheral blood stem cells, TVPB –total volume of processed blood Discussion
Our analysis showed that when using the ComTec and Amicus BCS used in the Department of Oncohematology of the NCI, the CEC was not less than the generally accepted range of 30–50% and did not differ significantly between the two devices. It was noted that in the group of patients whose collections were performed on the Amicus BCS, there was a higher number of those who had risk factors for unsuccessful collection, with other comparable indicators and comparability of the collection. Such data may indicate the feasibility of using this device during apheresis in patients at higher risk of unsuccessful collection.
The characteristics of the PBSC obtained as a result of apheresis on both devices of the product also did not differ in most parameters. However, it is noteworthy that the volume of the product, obtained using the Amicus device was smaller, which in turn led to the reduced use of dimethylsulfoxide (DMSO), which is a necessary reagent during the PBSC cryopreservation procedure and represents 5–10% of the total volume of cryopreserved product [17]. Thus, in some patients, the risks associated with the DMSO transfusion during the SC transfusion were reduced and the number of SC transfusion days was reduced [17–19].
In the routine practice of our department before the conducted analysis, the TVPB was chosen by the operator based on the standard approach and experience of the operator. Now, the calculation in the Excel spreadsheet editor is available to determine and standardize this parameter prior to the procedure.
Adapting the formula for predicting the total blood volume that needs to be processed to achieve the optimal number of stem cells for transplantation requires validation in a prospective study and will allow us to determine more accurately the parameters of stem cell apheresis in the future.
Thus, after performing calculations as a local practice for performing the PBSC apheresis in our department, the Amicus device is preferred over the ComTec device. A prospective evaluation of the obtained data is carried out to further validate the TVPB calculations, as well as to optimize current collections.
Conclusion
The CEC for each device was within the generally accepted limits of 30–50%, and did not differ significantly. Nevertheless, using of the Amicus BCS allowed to collect lower volumes of the product, maintaining other characteristics of the product competitive.
Kateryna Filonenko
University Clinical Center
Department of Hematology and
Transplantology
Smoluchowskiego str., 17
80 952 Gdansk
Poland
e-mail: ksfi lonenko@yahoo.com,
kfi lonenko@uck.gda.pl.
Submitted/Obdrženo: 22. 11. 2021
Accepted/Přijato: 20. 5. 2022
Sources
1. Duarte RF, Labopin M, Bader P et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant 2019; 54 (10): 1525–1552. doi: 10.1038/s41409-019-0516-2.
2. Child JA, Morgan GJ, Davies FE et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348 (19): 1875–1883. doi: 10.1056/NEJMoa022340.
3. Attal M, Harousseau JL, Stoppa AM et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996; 335 (2): 91–97. doi: 10.1056/NEJM199607113350 204.
4. Mounier N, Canals C, Gisselbrecht C et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant 2012; 18 (5): 788–793. doi: 10.1016/j.bbmt.2011.10.010.
5. Linch DC, Winfield D, Goldstone AH et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin‘s disease: results of a BNLI randomised trial. Lancet 1993; 341 (8852): 1051–1054. doi: 10.1016/0140-6736 (93) 92411 - l.
6. Philip T, Guglielmi C, Hagenbeek A et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin‘s lymphoma. N Engl J Med 1995; 333 (23): 1540–1545. doi: 10.1056/NEJM199512073332305.
7. Mohty M, Hübel K, Kröger N et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2014; 49 (7): 865–872. doi: 10.1038/bmt.2014.39.
8. Carreras E, Dufour C, Mohty M et al. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. Springer 2019 : 117–121. doi: 10.1007/978-3-030-02278-5.
9. Giralt S, Costa L, Schriber J et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 2014; 20 (3): 295–308. doi: 10.1016/j.bbmt.2013.10.013.
10. Mohty M, Hübel K, Kröger N et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2014; 49 (7): 865–872. doi: 10.1038/bmt.2014.39.
11. Gertz MA. Current status of stem cell mobilization. Br J Haematol 2010; 150 (6): 647–662. doi: 10.1111/j.1365-2141.2010.08313.x.
12. Abrahamsen JF, Stamnesfet S, Liseth K et al. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion 2005; 45 (2): 248–253. doi: 10.1111/j.1537-2995.2004.04210.x.
13. Bojanic I, Mazic S, Rajic L et al. Large volume leukapheresis is efficient and safe even in small children up to 15 kg body weight. Blood Transfus 2017; 15 (1): 85–92. doi: 10.2450/2016.0151-15.
14. Duong HK, Savani BN, Copelan E et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2014; 20 (9): 1262–1273. doi: 10.1016/j.bbmt.2014.05. 003.
15. Almeida-Neto C, Rocha V, Moreira FR et al. Validation of a formula predictive of peripheral blood stem cell yield and successful collection in healthy allogeneic donors. Hematol Transfus Cell Ther 2020; 42 (2): 164–165.e5. doi: 10.1016/j.htct.2019.04.004.
16. Rosenbaum ER, O‘Connell B, Cottler-Fox M. Validation of a formula for predicting daily CD34 (+) cell collection by leukapheresis. Cytotherapy 2012; 14 (4): 461–466. doi: 10.3109/14653249.2011.652733.
17. Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant 2014; 49 (4): 469–476. doi: 10.1038/bmt.2013.152.
18. Júnior AM, Arrais CA, Saboya R et al. Neurotoxicity associated with dimethylsulfoxide-preserved hematopoietic progenitor cell infusion. Bone Marrow Transplant 2008; 41 (1): 95–96. doi: 10.1038/sj.bmt.1705883.
19. Donmez A, Tombuloglu M, Gungor A et al. Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci 2007; 36 (1): 95–101. doi: 10.1016/j.transci.2006.05.019.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2022 Issue 6-
All articles in this issue
- Editorial
- Fecal microbiota transplantation – new possibility to influence the results of therapy of cancer patients
- Prognostic and predictive factors of brain meningiomas
- Informace z České onkologické společnosti
- Cardiovascular complications among hematopoietic cell transplantation survivors – the role of cardiomarkers
- Aktuality z odborného tisku
- Souhra klinické onkologie, radiační onkologie a chirurgie v léčbě pacientů s nádory GIT
- Tebentafusp
- Comparison of the efficiency of peripheral blood stem cell apheresis on the blood cell separators
- Reconstruction and analysis of circRNA-miRNA-mRNA network in the pathology of lung cancer
- Advantages and limitations of 3D organoids and ex vivo tumor tissue culture in personalized medicine for prostate cancer
- Benign lymphoid hyperplasia mimicking oligometastasis from non-small cell lung cancer after stereotactic ablative radiotherapy
- Fatal myocarditis after the first dose of nivolumab
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Prognostic and predictive factors of brain meningiomas
- Advantages and limitations of 3D organoids and ex vivo tumor tissue culture in personalized medicine for prostate cancer
- Fecal microbiota transplantation – new possibility to influence the results of therapy of cancer patients
- Fatal myocarditis after the first dose of nivolumab
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

