-
Medical journals
- Career
Comparision of Histological Types of Primary and Subsequent Relapsing Basal Cell Carcinomas of the Skin
Authors: V. Bartos 1; K. Adamicová 2; M. Kullová 3; M. Pec 4
Authors‘ workplace: Department of Pathological Anatomy, Faculty Hospital in Zilina, Zilina, Slovak Republic 1; Institute of Pathological Anatomy, Jessenius faculty of Medicine in Martin, Martin, Slovak Republic 2; Department of Dermatovenerology, Faculty Hospital in Zilina, Zilina, Slovak Republic 3; Institute of Medical Biology, Jessenius faculty of Medicine in Martin, Martin, Slovak Republic 4
Published in: Klin Onkol 2012; 25(4): 262-266
Category: Original Articles
Overview
Background:
Basal cell carcinoma (BCC) recurrences are relatively frequent event in a routine dermatologic practice. One of the most important factor which impacts risk of their development is a histomorphological appearance of tumor.Design:
The purpose of our study was to compare histological types of primary and corresponding relapsing BCCs of the skin.Material and methods:
The study included 36 cases of BCC recurrences from 34 patients, 17 women and 17 men. The patients ranged in age from 32 to 97 years, with a mean age of 67.1 years at the time of (the first) recurrence.Results:
Both tumor groups generally exhibited the same proportion of indolent and aggressive histological phenotype. In 21 cases (58.4%), we found an identical histological BCC type in primary and subsequent relapsing lesion. In 3 cases (8.3%), primary lesion showed indolent histological features without aggressive-growth component, while recurrent tumor already manifested it. Conversely, in next 3 cases (8.3%) primary tumor exhibited focal infiltrative-growth features and corresponding relapsing lesion did not. Of the remainig 9 cases (25%), histomorpological phenotype was not identical, but it showed the same prognostic histological tumor variant.Conclusion:
Based on the results of our study it can be assumed that a BCC recurrence is a dynamic histogenetic process, during which the phenotypic transformation and the changes in histomorphological picture of lesions occur, probably as a result of the interactions between cancer cells and re-modulated surrounding stroma.Key words:
basal cell carcinoma – tumor recurrenceIntroduction
Basal cell carcinoma (BCC) of the skin is currently the most common malignancy in humans [1–3]. This cancer usually occurs in the elderly people, but there are also known some rare hereditary disorders, in which cutaneous BCCs develop at an early age, such as Gorlin--Goltz syndrome or xeroderma pigmentosum [4,5]. In contrast to the other malignancies, cutaneous BCC is generally characterized by an indolent clinical course, favourable prognosis and minimal metastatic potential [1,2]. However, one of the most negative event in routine clinical practice are relatively frequent tumor recurrences. Although their reliable prediction on the basis of common demographical, clinical and histopathological parameters is very limited [6], some may indicate an increased likelihood of their development. Among them, histomorphological appearance of tumor represents one of the most important prognostic indicator. The results of several studies [6–12] have shown that BCC recurrences were mostly associated with aggressive--growth histological variants (i.e. infiltrative, morpheic BCC, and metatypical carcinoma). These variants are microscopically characterized by irregular, invasive growing tumor nests with deeper infiltration into the soft tissue causing their difficult surgical removal. Since there are not uncommon recurrences of the indolent (superficial and nodular) BCC variants as well, according to some authors [13], no definite correlation could be established between BCC subtypes and recurrence, and histopathological criteria are limited for prognostification. Better understanding of pathogenesis and histogenetic evolution of BCC relapses may be obtained by comparing the individual morphological features and biological characteristics of primary and subsequent relapsing lesions. However, there have been only a few such relevant data published in the literature to date [9,14–16], including our previous recent paper [17]. Herein, we focused our study to compare histological types of primary and corresponding recurrent BCCs of the skin in biopsy specimens.
Material and Methods
The study included 36 cases of BCC recurrences, whose primary and subsequent relapsing lesions were histologically diagnosed at the Department of Pathology in Faculty Hospital in Zilina, and whose original tumor and each subsequent recurrence were able to retrospectivelly reviewed. Individual cancers were obtained from 34 subjects. Seventeen patients were women and seventeen were men. The patients ranged in age from 32 to 97 years, with a mean age of 67.1 years at the time of (the first) recurrence. The most common sites of presentation were nose and paranasal regions (n = 11), auricles and periauricular areas (n = 6) and eyelids and periocular parts (n = 5) with other tumors located on temporal region (n = 3), cheeks (n = 3), forhead (n = 2), mandibular region (n = 2), neck (n = 2), scalp (n = 1) and back (n = 1). Three women suffered from a repeated re-recurrence of the same carcinoma. Two men had 2 separate relapsing cancers on the different anatomical site. Of the primary BCCs, a total surgical excision of lesion was attempted in 31 cases, 3 patients underwent a partial (probatory) excision, one patient underwent an excochleation and one woman a partial craniectomy. In excisional biopsies, none of the incompletely removed tumors was immediatelly re-excised. The adjuvant postoperative therapeutic modality (local radiotherapy, photodynamic therapy, administration of Imiquimod or Efudix) were applied when required according to the individual decision of the clinicians. A biopsy material was fixed in buffered formalin, embedded in paraffin blocks, stained with hematoxylin and eosin and slides were reviewed by pathologists in the light miscroscope. Histological BCC types were cathegorized into the aggressive-growth and indolent-growth variants according to conventional classification reported in the literature [1]. Aggressive-growth variants included infiltrative (morpheic) type, micronodular type and metatypical carcinoma, whereas indolent-growth variants comprised superficial type, nodular type, and BCC with adnexal (trichoepithelial) differentiation.
Results
Histologically, there was considerable variation in morphology in both, primary and recurrent BCCc, which shows either homogeneous or mixed pattern. In primary BCCs group, in order of frequency, the histological tumor types were following: nodular type (n = 10), nodular BCC with focal infiltrative-growth features (n = 8), infiltrative type (n = 7), nodular BCC with trichoepithelial features (n = 3) (Fig. 1), morpheic type (n = 2), trichoepithelial BCC (n = 2), nodular BCC with micronodular component (n = 1), „pure“ metatypical carcinoma (n = 1) (Fig. 2), metatypical carcinoma combined with nodular and infiltrative BCC component, and superficial BCC (n = 1). In summary, more than half of all cases (n = 20, 55.5%) demonstrated (at least partial) aggressive-growth histological component. Of the recurrent lesions, histological tumor types were as follows: nodular type (10 cases), nodular BCC with focal infiltrative-growth features (8 cases), infiltrative type (6 cases), „pure“ metatypical carcinoma (3 cases), morpheic type, superficial type, trichoepithelial BCC and nodular BCC with trichoepithelial features (2 cases each), and metatypical carcinoma combined with nodular and infiltrative BCC component (1 case). In general, both groups exhibited the same proportion of indolent and aggressive histologic phenotype. When we compared the corresponding primary and recurrent lesions, there were 21 cases (58.4%) with absolutely identical histological type. In 3 cases (8.3%) primary lesion showed indolent histological features only without aggressive-growth component, while recurrent tumor already manifested it (for example nodular type → nodular-infiltrative type). Conversely, in next 3 other cases (8.3%), primary tumor exhibited focal infiltrative-growth features and corresponding relapsing lesion did not (for example nodular-infiltrative type → nodular type). Of the remaining 9 cases (25%), the histomorphological phenotype of primary and recurrent tumors was not identical, but they displayed some changes only within the same prognostic growth variant (for example nodular → superficial, or morpheic type → metatypical carcinoma). In 3 cases of tumor re-recurrences, all lesions (primary, the first and the second recurrence) had an identical histological BCC type, all of which manifested an aggressive-growth component (Graph 1). A majority of relapsing lesions showed a marked dermal fibroplasia accompanied by a chronic inflammatory infiltration of various intensity, and sometimes by granulomatous giant cell reaction. In all 36 cases assessed, a recurrence interval varied between 2–176 months (mean 25.2 months). When we evaluated a relationship between recurrence interval and histologic types of relapsing BCCs we had found, indolent BCC variants slightly predominated in cases of earlier recurrences within 2 years. However, a further increase of recurrence interval was generally accompanied by decreased percentage of indolent-growth variants and increased percentage of aggressive-growth BCC variants (including BCC with partial aggressive-growth histological component). All cases of late recurrence that developed within 4 years and later after therapy had exhibited aggressive histological phenotype only (Tab. 1).
1. Histomorphological picture of nodular BCC with trichoepithelial growth features (hematoxylin and eosin staining, magnification 40×). 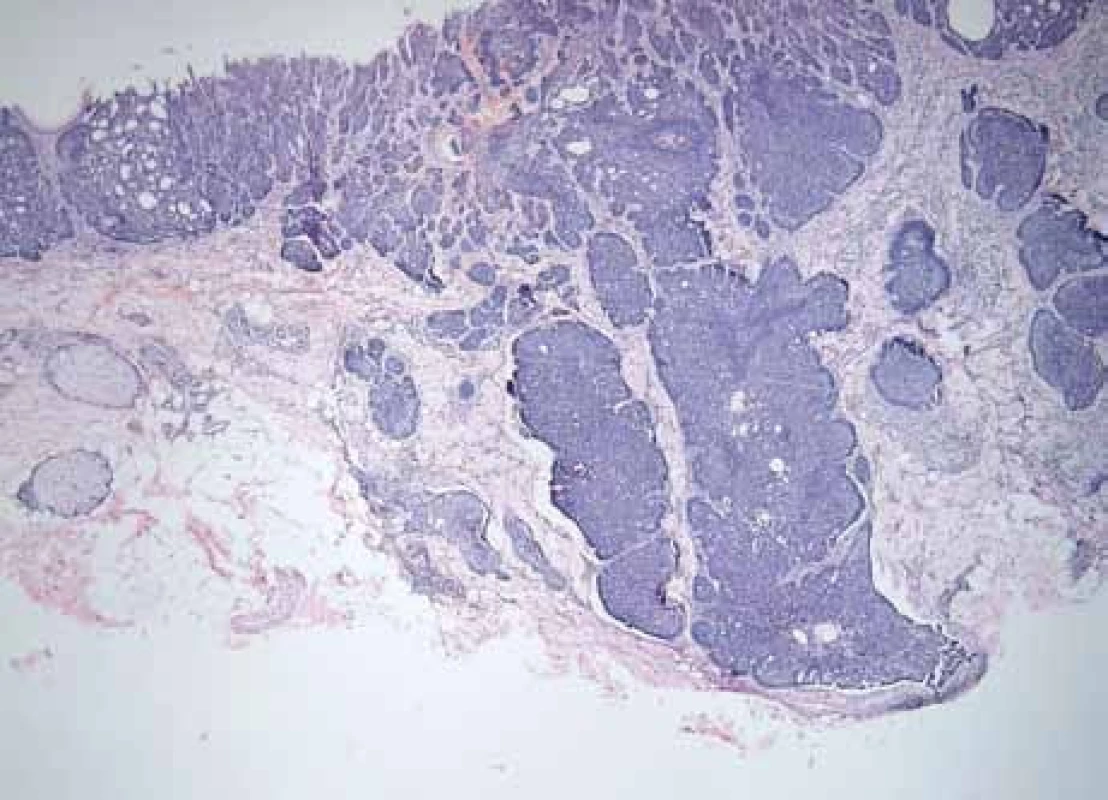
2. Histomorphological picture of metatypical carcinoma (hematoxylin and eosin staining, magnification 120×). 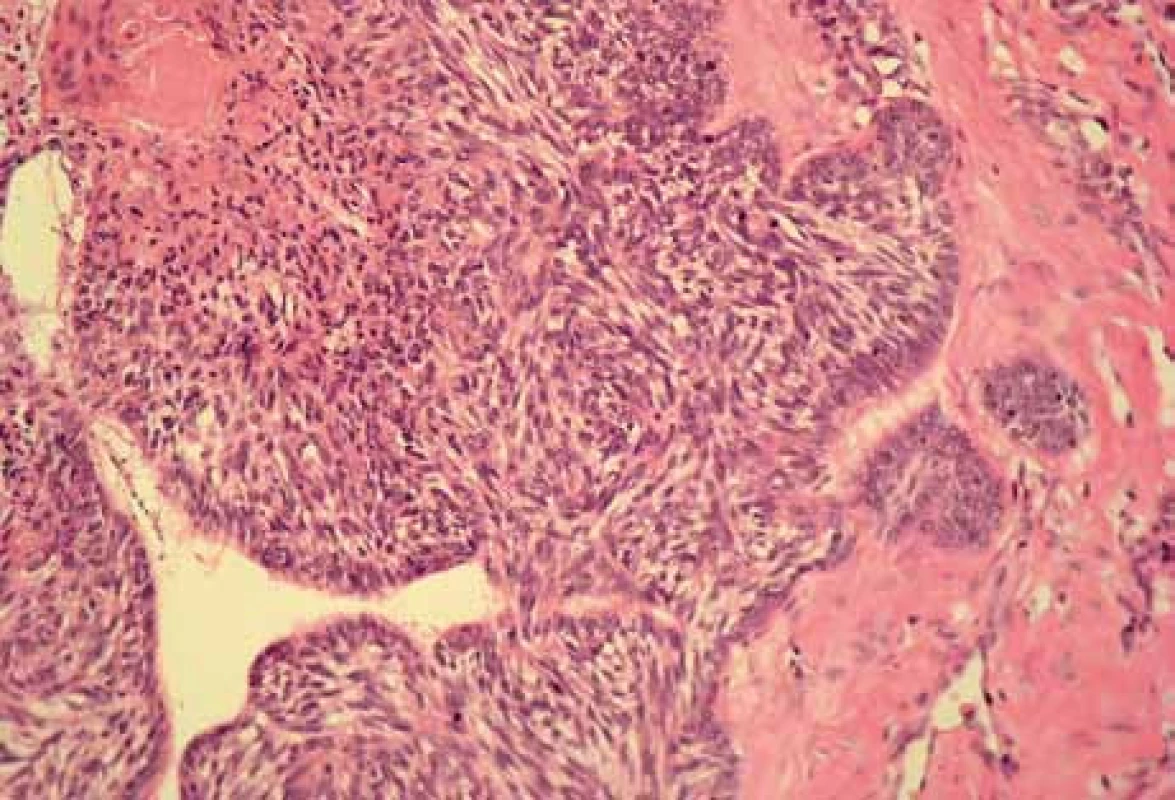
1. Graphical overview of the changes in histomorphological phenotype of tumors (pBCC – primary basal cell carcinoma, rBCC – corresponding recurrent basal cell carcinoma). 
1. Relationship between the interval of (the first) tumor recurrence and percentage of individual histological variants of recurrent basal cell carcinoma (rBCC). Indolent rBCC types with (at least) focal aggressive-growth component were included into the group of aggressive rBCCs. 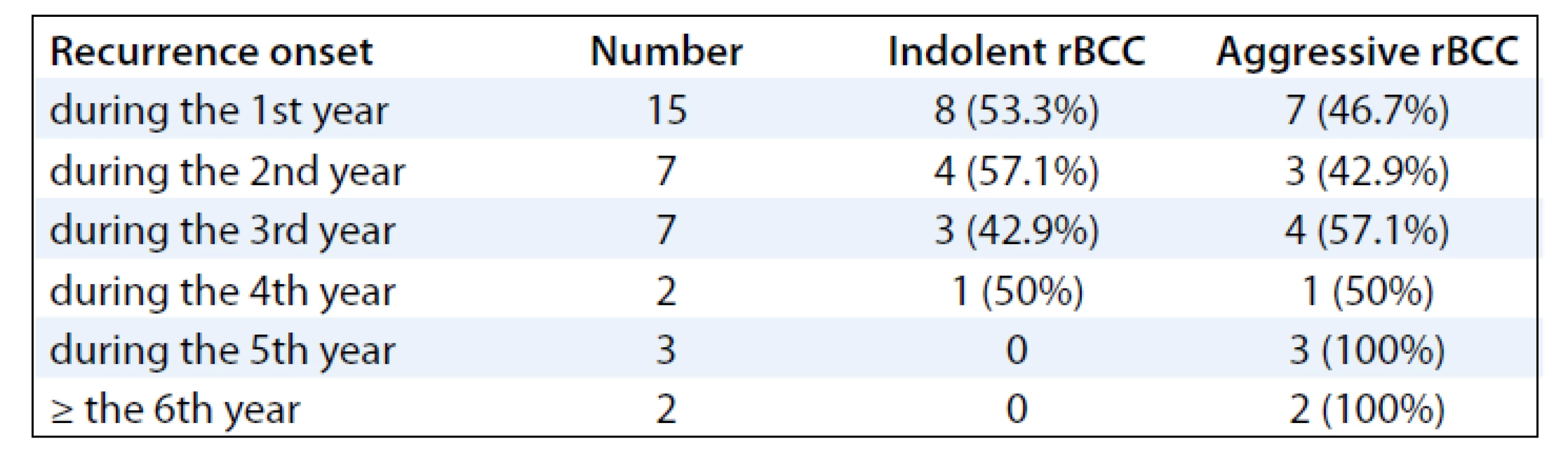
Discussion
Recurrent BCCs may clinically manifest as area of induration, erythema, ulceration or bleeding at a prior operative site for known primary lesion [1]. The vast majority of them develop within 3 years after treatment of primary lesion [7,14]. According to several papers [12,14–16], supported also by our present study it is obvious that BCC recurrence represents a dynamic histogenetic process. Although Başer et al [9] observed an identical histological type in primary and corresponding relapsing BCCs, there was very small number of cases consisting of 5 recurrences only. Boulinquez et al [15] compared 33 primary and subsequent recurrent BCCs and found that 20 of original tumors had exhibited microscopic features of non-aggressive, and remaining 13 lesions of aggressive tumor variant. During recurrence, 20% of originally non-aggressive carcinomas became histologically aggressive, and 31% of originally aggressive BCCs developed even more aggressive component. Similarly, in an earlier study, Lang and Maize [14] observed that 65% of all primary BCCs had demonstrated aggressive histomorphology characterized by poor palisading and an infiltrating and/or micronodular pattern. The subsequent recurrent lesions developed a more aggressive histologic feature in 23.5%, but in 13.7%, microscopic appearence became „more benign“. Lee et al [16] studied 21 recurrent BCCs and found that 6 cases (28.5%) of primary nodular BCCs had transformed to aggressive histologic variant when they recurred. In study of Pieh et al [12], repeated recurrences were related to increasing percentage of sclerosing BCC type. While this type was found in 14.4% of all primary tumors (which later recur), it was diagnosed in 35.3% of the first recurrence, in 70.0% of the second recurrence and finally, all third and fourth recurrences represented only sclerosing histological type. In our study, we have shown that during the recurrence, 8.3% of all cancers had developed a „more aggressive“ histologic features, and the same percentage became microscopically „more indolent“. Moreover, about one quarter of all cases displayed the changes within the same prognostic growth variant. These results (as well as the results of the papers mentioned above) confirmed a fact, primary and subsequent relapsing tumors have not always manifest the same histomorphology, and hence, they may also exhibit a different biological behaviour. On this occasion it is worth to mention a stepwise model of BCC progression previously postulated by Kaur et al [18]. This theory supposes that various BCCs exhibit distinct epithelial-stromal-inflammatory patterns that correlate with the individual BCC subtypes, tumor progression and neoplastic evolution from low risk to high risk tumor for local recurrence and implicates a histologic continuum reflecting dynamic host-BCC interactions. If we consider the individual morphological types of BCC being the „levels“ in their (usually slow) developmental process, this may explain, why some recurrent tumors did not manifest identical histological type in comparision to their primary lesions. It is most likely that this diversity in microscopical picture reflects a different evolutional stage of „re-carcinogenesis“. In such cases, tumor histomorphology (and hence biological behaviour) may be significantly influenced by local reparative and inflammatory changes of tissue after previous surgery, during which a remodulation of stromal component become. In addition, even a form of adjuvant therapy probably plays an important role in transformation of tumor phenotype. For example, two decades ago Lang and Maize [14] supposed that development of the infiltrative and more invasive recurrent BCC types could be directly related to squamous differentiation of malignant cells induced by previous radiotherapy.
Conclusion
Based on the results of our study it can be concluded that like the normal mechanisms of BCC carcinogenesis, even BCC recurrence is a dynamic histogenetical process, during which the phenotypic transformation and the changes (at least to some extent) in histomorphology of lesions and their biological characteristics occur. This transformation is probably a result of the interactions between neoplastic cells and re-modulated surrounding microenvironment.
Acknowledgements
We would like to thank Dr. Pokorny Dusan, Dr. Zacharova Olga, Dr. Haluska Pavel and Dr. Doboszova Jana for their practical and educational participation in diagnostic process of tumors presented in this study.
The authors declare they have no potential conflicts of interest concerning drugs, pruducts, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Vladimir Bartos, MD
Department of Pathological Anatomy
Faculty Hospital in Zilina
V. Spanyola 43
012 07 Zilina
Slovak Republic
e-mail: bartos@jfmed.uniba.sk
Submitted: 16. 12. 2011
Accepted: 29. 1. 2012
Sources
1. Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol 2006; 19 (Suppl 2): S127–S147.
2. Bartoš V, Adamicová K, Kullová M et al. Bazocelulárny karcinóm kože – biologické správanie nádoru a prehľad najvýznamnejších molekulových ukazovateľov progresie ochorenia v praxi patológa. Klin Onkol 2011; 24(1): 8–17.
3. Adamkov M, Halasova E, Rajcani J et al. Relation between expression pattern of p53 and survivin in cutaneous basal cell carcinomas. Med Sci Monit 2011; 17(3): BR74–BR80.
4. Plevová P, Krutílková V, Puchmajerová A et al. Gorlinův syndrom. Klin Onkol 2009; 22 (Suppl): S34–S35.
5. Plevová P, Šilhánová E, Foretová L. Vzácné hereditární syndromy s vyšším rizikem vzniku nádorů. Klin Onkol 2006; 19 (Suppl): S68–S75.
6. Kyrgidis A, Vahtsevanos S, Tzellos TG et al. Clinical, histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma. A 1,062 patient-cohort study from a tertiary cancer referral hospital. Eur J Dermatol 2010; 20(3): 276–282.
7. Santiago F, Serra, D, Vieira R et al. Incidence and factors associated with recurrence after incomplete excision of basal cell carcinomas: a study of 90 cases. J Eur Acad Dermatol Venereol 2010; 24(12): 1421–1424.
8. Zimmermann AC, Klauss V. Predictors of recurrent basalioma of the eyelids and periorbital region. Ophthalmologe 2001; 98(6): 555–559.
9. Başer TN, Bulutoğlu R, Barutcu AY et al. Evaluation of basal cell carcinomas with positive surgical margins: A five year retrospective analysis. Turk J Cancer 2008; 38(1): 16–19.
10. Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1,039 consecutive neoplasms. J Am Acad Dermatol 1990; 23(6 Pt 1): 1118–1126.
11. Bumpous JM, Padhya TA, Barnett SN. Basal cell carcinoma of the head and neck: identification of predictors of recurrence. Ear Nose Throat J 2000; 79(3): 200–204.
12. Pieh S, Kuchar A, Novak P et al. Long-term results after surgical basal cell carcinoma excision in the eyelid region. Br J Ophthalmol 1999; 83(1): 85–88.
13. Thomas J, McKiernan M, Rao GS. Basal cell carcinoma: how long a follow-up is needed? A surgical audit. Eur J Plast Surg 1996; 19 : 318–319.
14. Lang PG Jr, Maize JC. Histologic evolution of recurrent basal cell carcinoma and treatment implications. J Am Acad Dermatol 1986; 14(2 Pt 1): 186–196.
15. Boulinquez S, Grison-Tabone C, Lamant L et al. Histological evolution of reccurent basal cell carcinoma and therapeutic implications for incompletely excised lesions. Br J Dermatol 2004; 151(3): 623–626.
16. Lee JS, Yi JH, Yun SK et al. Clinicohistopathological study of recurrent basal cell carcinomas after surgical excision. Korean J Dermatol 2010; 48(6): 453–459.
17. Bartos V, Pokorný D, Zacharová O et al. Recurrent basal cell carcinoma: a clinicopathological study and evaluation of histomorphological findings in primary and recurrent lesions. Acta Dermatovenerol Alp Panonica Adriat 2011; 20(2): 67–75.
18. Kaur P, Mulvaney M, Carlson A. Basal cell carcinoma progression correlates with host immune response and stromal alterations: a histological analysis. Am J Dermatopathol 2006; 28(4): 293–307.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue 4-
All articles in this issue
- Implication of Bone Marrow Microenvironment in Pathogenesis of Multiple Myeloma
- CT Colonography – Evolution of Methodology and Indications
- Long Non-Coding RNAs And Their Relevance in Cancer
- Treatment of Langerhans Cells Histiocytosis by Cladribin Reached Long-Term Complete Remission in 9 out of 10 Adult Patients
- EGFR Mutations in Patients with Advanced NSCLC
- HARDROCK Project: Parametric Data Collection and Analysis of Patients with Head and Neck Cancer in the Comprehensive Cancer Centre of Ostrava – Role of Fractionation and Target Volume Definition in Radiotherapy
- What is the Prognostic Importance of Molecular Genetic Factors in Endometrial Carcinoma?
- Hepatocellular Carcinoma – Long-Term Treatable Disease
- Treatment For Volume Upgrading of the Low-Grade Supratentorial Glioma After the Subtotal Neurosurgical Resection
- Comparision of Histological Types of Primary and Subsequent Relapsing Basal Cell Carcinomas of the Skin
- Paratesticular Mesothelioma in Young Age. Case Report
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Hepatocellular Carcinoma – Long-Term Treatable Disease
- Treatment For Volume Upgrading of the Low-Grade Supratentorial Glioma After the Subtotal Neurosurgical Resection
- EGFR Mutations in Patients with Advanced NSCLC
- CT Colonography – Evolution of Methodology and Indications
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

