-
Medical journals
- Career
Demineralization and Hydrogen Peroxide Penetration in Teeth with Incipient Lesions
Authors: André Luiz Fraga Briso 1; Rafael Simões Gonçalves 1; Fernanda Bernardi Da Costa 1; Marjorie De Oliveira Gallinari 1; Luciano Tavares Angelo Cintra 1; Paulo; Henrique Dos Santos 2
Authors‘ workplace: Department of Restorative Dentistry, Araçatuba Dental School, UNESP - Univ Estadual Paulista, Araçatuba, SP, Brazil 1; Department of Dental Materials and Prosthodontics, Araçatuba Dental School, UNESP - Univ Estadual Paulista, Araçatuba, SP, Brazil 2
Published in: Brazilian Dental Journal (2015) 26(2)
Category: Original articles
doi: https://doi.org/10.1590/0103-6440201300225This is an open-access article distributed under the terms of the Creative Commons Attribution License.
The electronic version of this article is the complete one and can be found online at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-64402015000200135Overview
The aim of this study was to evaluate the demineralization and hydrogen peroxide (HP) penetration in teeth with incipient lesions submitted to bleaching treatment. For analysis of HP penetration, sound and demineralized enamel/dentin discs were placed in artificial pulp chambers containing acetate buffer solution. After bleaching treatment, this solution was subjected for analysis of optical density by spectrophotometry and the disc surfaces were analyzed with scanning electron microscopy (SEM) and polarized light microscopy (PLM). The remaining discs were subjected for cross-sectional hardness analysis at different depths. Data were analyzed by repeated measures ANOVA and PLSD Fisher test (a=0.05). It was observed that previously demineralized teeth showed greater HP penetration (p<0.05). The bleaching treatment caused changes to a depth of 20 µm in sound enamel and up to 90 µm in demineralized enamel. SEM and PLM images revealed that the bleaching treatment caused superficial changes that were considerably more accentuated in previously demineralized teeth. It may be concluded that the enamel mineralization level influences HP penetration and the bleaching agent contributed to increase the demineralization depth.
Key words:
bleaching; hydrogen peroxide; tooth demineralization; cariesINTRODUCTION
Tooth bleaching is one of the most popular treatments to achieve esthetic whitening and is considered a conservative, easily performed and relatively low-cost procedure (1). At-home bleaching is the most widely used bleaching technique for vital teeth, with recognized efficacy and biosafety (2). The patient uses carbamide peroxide gel at low concentrations under the supervision of a dentist. (3).
Another alternative for vital tooth bleaching is the in-office technique, characterized by the use of products based on hydrogen peroxide (HP) in concentrations ranging from 20% to 38%. Obtaining fast results with immediate lightening effects through observation is the main marketing appeal that anchors the success of this technique (4). It is known that whatever the used bleaching agent, the active ingredient that will act in tooth structure is hydrogen peroxide, which should penetrate into the enamel and reach the dentin to produce the desired effect.
Despite the success of bleaching techniques, it has been demonstrated that application of HP gel on the enamel can result in its partial dissolution, deproteinization, and increased permeability of the tissue ( 5 , 6 , 7 ). This fact becomes worrisome because the presence of incipient lesions, often at sub-clinical stage, may suffer interference of bleaching treatment and progress to more advanced lesions, allowing more penetration of peroxides during the treatment.
Several studies in vitro show that the HP present in bleaching agents, even at low concentrations, readily crosses enamel and dentin, reaching the pulp tissue (8), which may respond with lipid peroxidation, fragmentation of proteins and result in cell membrane injury (9). In this way, it is believed that the bleaching treatment performed in teeth with incipient caries lesions could result in higher post-treatment sensitivity. Thus, the aim of this study was to evaluate the trans-enamel and trans-dentinal penetrations of hydrogen peroxide in a previously demineralized enamel and, at the same time, evaluate the HP influence in the progress of the lesion.
MATERIAL AND METHODS
Collection and Standardization of Specimens
One hundred sound permanent bovine incisors obtained from steers aged between 24 and 30 months were selected and the teeth were mounted in a cutting machine using a 8-mm-diameter cylindrical diamond tip (Dinser Ferramentas Diamantadas; São Paulo, SP, Brazil). From the middle third of buccal surface of each tooth were cut 5.7-mm diameter discs containing enamel and dentin. The dentin surface was polished with wet 400 - and 600-grit aluminum oxide paper (Saint-Gobain Abrasivos, Jundiaí, SP, Brazil) until the specimens reached a thickness of 3.5 mm, approximately 1.3 mm and 2.2 mm (±0.1 mm) for enamel and dentin, respectively, measured by a digital caliper (Mitutoyo Sul Americana Ltda, São Paulo, SP, Brazil). A 0.5 mol/L EDTA solution, pH 7.2, was applied on dentin surface for 30 s for removal of the smear layer, and subsequently rinsed with sterile deionized water.
Sample Selection
The remaining tooth structure was used for microhardness analysis to standardize the initial enamel conditions. For this, new fragments were obtained from the region adjacent to the one employed for obtaining the discs. The fragments were fixed on an acrylic base and their surface was polished in a polishing machine (Arotec SA Ind. e Com., Cotia, SP, Brazil), using aluminum oxide sandpaper (Buehler Ltd., Lake Bluff, IL, USA) with water cooling at low speed (90 rpm) - 600 - and 800-grit for 2 min and 1200-grit for 4 min The final polishing was performed with felt disks moistened with diamond paste to 1 μm (Extec Corp., Enfield, CT, USA) for 5 min.
The enamel surface hardness was evaluated in a microhardness tester (HMV-2000; Shimadzu Corp, Tokyo, Japan) equipped with a Knoop indenter type that worked with a static load of 25 g for 5 s. This was performed at 3 indentations in the center of buccal enamel surface fragment, with a distance between them of 100 μm. A total of 60 enamel/dentin discs were selected, with hardness values closer to the mean value (KHN 351).
Experimental Groups
The selected 60 discs were divided into two groups: discs in Group I presented sound enamel (control); discs in Group II were exposed to a demineralizing solution, pH=5.0 for 24 h ( 11 , 12 ) followed by thorough washing with deionized water to produce white spot lesions on enamel.
Preparation of Artificial Pulp Chamber (APC)
The discs were adapted into a stainless steel device developed at the Laboratory of Experimental Pathology and Biomaterials of Araraquara Dental School/UNESP, Brazil (13). The discs were positioned in the APC between silicone rings, which cooperated to stabilize them and create a perfect seal for an APC.
Whitening Procedure
The bleaching treatment was performed on 15 discs from each group with the product based on hydrogen peroxide at 35% (Whiteness HP Maxx; FGM Produtos Odontológicos, Joinville, SC, Brazil) according to the manufacturer's recommendations. After mixing, the final product was inserted into a 1 mL graduated, disposable syringe with 0.04 mL applied to each specimen, and remaining in contact with the dental tissue for 15 min. Subsequently, the product was removed by sucking and two further applications were made, totalizing 45 min of exposure to bleach. The procedure was performed 3 times with one-week intervals between sessions.
Quantification of HP Penetration
For quantification of the HP that penetrated the enamel/dentin discs, the APCs were individually placed in wells of acrylic plates for cell culture. Each well was filled with 1 mL of acetate buffer solution and subsequently received APCs, already containing the dental fragments. Thus, the dentin surface remained in contact with the acetate solution during all bleaching procedures and the diffused hydrogen peroxide became part of it.
After the whitening procedure had been completed, 25 µL of acetate buffer solution was removed. It was then mixed with 2.750 µL of distilled water, 100 mL of leucocrystal violet (0.5 mg/mL) and 50 µL of peroxidase (1 mg/mL) (Sigma Chemical Co., St. Louis, MO, USA) and the solution was diluted to a final volume of 3 mL with distilled water ( 1 , 13 , 14 ).
Readings were done using ultraviolet visible reflectance spectrophotometer equipment UV-2450 (Shimadzu), 30 min after each bleaching session. To obtain the CF (calibration factor) equivalent to the ratio of the concentration of the standard solution of hydrogen peroxide to its absorbance, the following equation was used:
Parametric tests were performed using ANOVA repeated measure and PLSD Fisher, using the statistical program StatView (StatView Inc., Nesbit, MS, USA), at a significance level of 0.05.
Cross-Sectional Hardness
After hydrogen peroxide quantification, 10 specimens of each group, including those that were not bleached, were longitudinally sectioned, using a double-sided floppy diamond disk Ø 22 mm (KG Sorensen Ind. and Com., Cotia, SP, Brazil) at low speed and under intense water cooling. Each disc was embedded in acrylic resin using an embedding machine Pre 30 (Arotec). The inner surface was polished with 320-, 600 - and 1200-grit aluminum oxide sandpaper and 1 μm diamond paste. The cross-sectional hardness was determined by a microhardness tester with Knoop indenter type that worked with a static load of 5 g for 10 s. The indentations were made at the center of the block at distances of 10, 20, 30, 60, 90 and 120 µm from the enamel surface. Other two indentations rows were made; 200 µm on the right and left from the indentations made at the center of the discs.
Scanning Electron Microscopy
Three specimens from each group, including those not submitted to bleaching treatments, were prepared for surface analysis by scanning electron microscopy - EVO HD LS-15 (Carl Zeiss, Oberkochen, Germany). Therefore, the specimens were cleaned, dehydrated, sputter-coated with gold and enamel SEM micrographs in different surface conditions were obtained at 3,000× magnification.
Polarized Light Microscopy Analysis
Two specimens of each group, including those that did not undergo the bleaching procedure, were intended for analysis by PLM . The specimens were embedded and serially cut longitudinally in the metallographic cutter Isomet 2000 (Buehler Ltd.). The obtained slices were polished with aluminum oxide sandpaper 600 - and 1200-grit until the samples presented a thickness of 100 µm.
The analysis was performed using a polarized light microscope Axiophot Zeiss - DSM 940 A (Carl Zeiss Meditec, Dublin, CA, USA) at 25× magnification. For analysis, the software AxioVision 4 (Carl Zeiss Meditec) was used.
Statistical Analysis
Data were analyzed statistically using repeated-measures ANOVA and PLSD Fisher's test at 5% significance level.
RESULTS
The application of repeated-measures ANOVA showed that HP penetration, as far as cross-sectional hardness is concerned, was different in groups with sound and demineralized enamel (p<0.0001). The application of the PLSD Fisher test at 5% significance level showed that the condition of enamel influenced the intensity of the hydrogen peroxide, presenting the highest values in specimens subjected to cariogenic challenge, regardless of bleaching session (Table 1).
1. Mean (SD) peroxide concentrations (μg/mL) recovered from artificial pulp chambers after tooth whitening in sound and demineralized enamel, according to the bleaching sessions 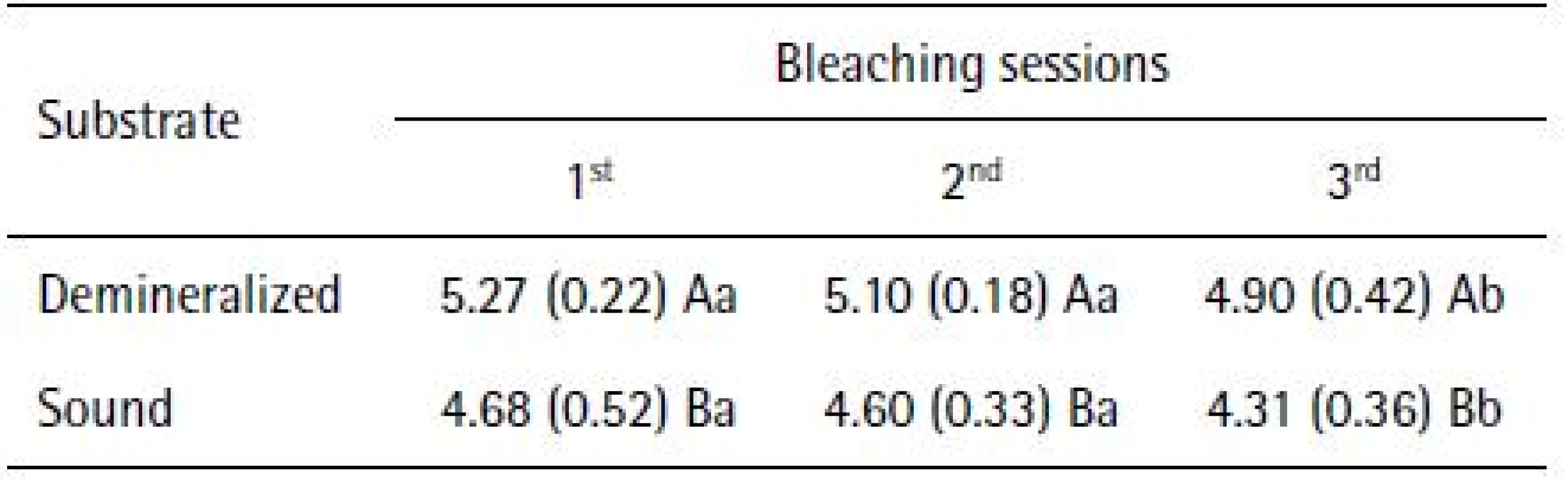
Different uppercase letters in columns and lowercase letters in rows indicate statistically significant difference (p<0.05). Different uppercase letters in columns and lowercase letters in rows indicate statistically significant difference (p<0.05).
The application of the PLSD Fisher test for cross-sectional hardness analysis data showed that, in general, the cross-sectional hardness (CSH) values were increased in all the experimental conditions, since the depth was 10 µm to 120 µm. Sound enamel showed higher microhardness values to a depth of 90 μm, equated with demineralized specimens at 120 μm in depth (Table 2).
2. Mean (SD) cross-sectional hardness before and after tooth whitening in sound and demineralized enamel, according to different depths 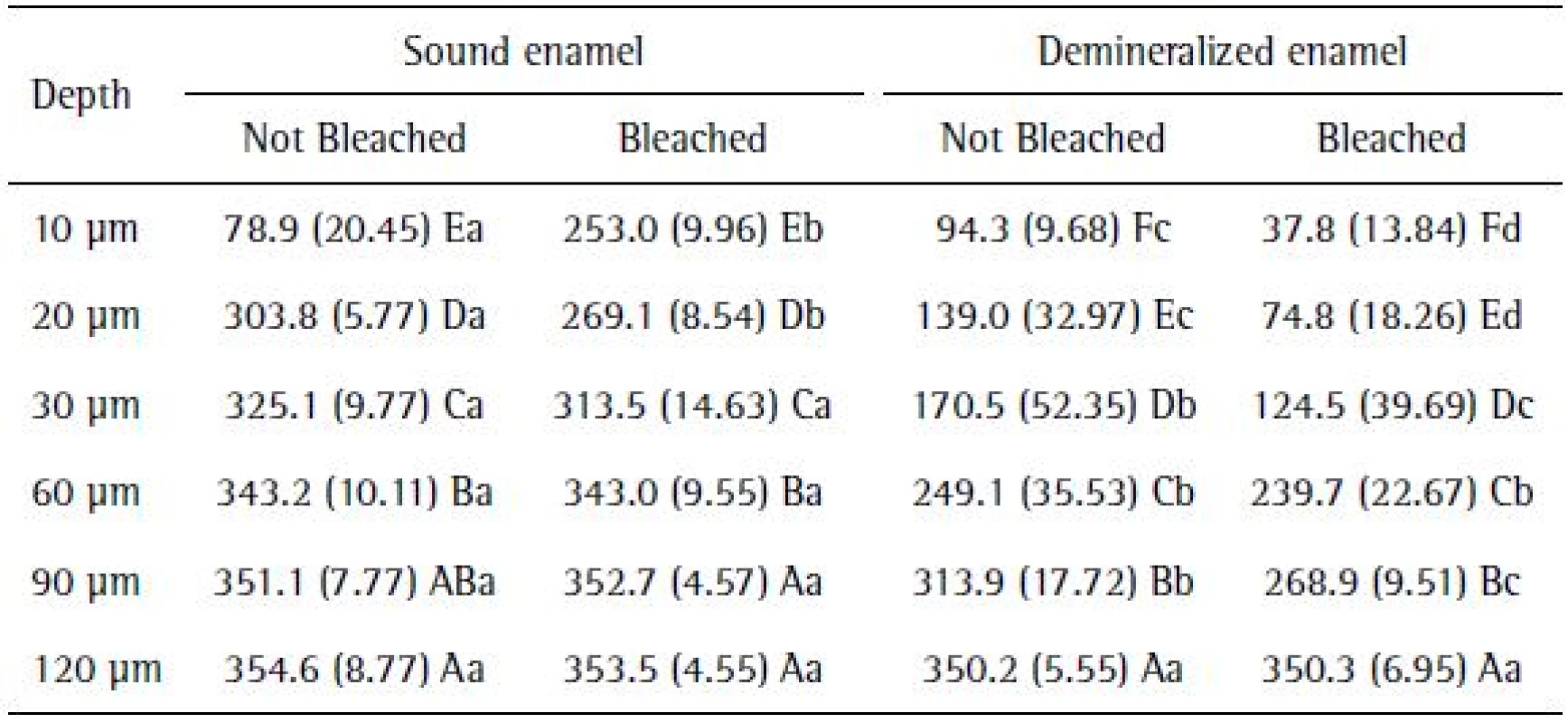
Different uppercase letters in columns and lowercase letters in rows indicate statistically significant difference (p<0.05). Different uppercase letters in columns and lowercase letters in rows indicate statistically significant difference (p<0.05)
Only the effect of the bleaching agents on the sound enamel led to changes in the microhardness to a depth of 20 µm. Moreover, the action of bleaching in demineralized enamel influenced the microhardness values, which showed more pronounced changes than the group that was not bleached to a depth of 90 µm (Table 2).
Microscopic Observations
The images obtained from SEM (scanning electron microscopy) showed that both the demineralized and sound surfaces exhibit superficial changes (Figs. 1A and 1B), which are considerably more pronounced in teeth that were previously demineralized (Fig. 1B) when compared with Group I, which were only bleached (Fig. 1A).
1. SEM analysis. A: Specimen subjected to cariogenic challenge. The enamel porous surface aspect presents partial dissolution of the aprismatic layer (arrow) (3,000×). B: Specimen subjected to cariogenic challenge and bleaching procedure. The substantial dissolution of the aprismatic layer and increased porosity can be observed, allowing visualization of the enamel prism mouths, forming new channels for dissemination (3,000×). 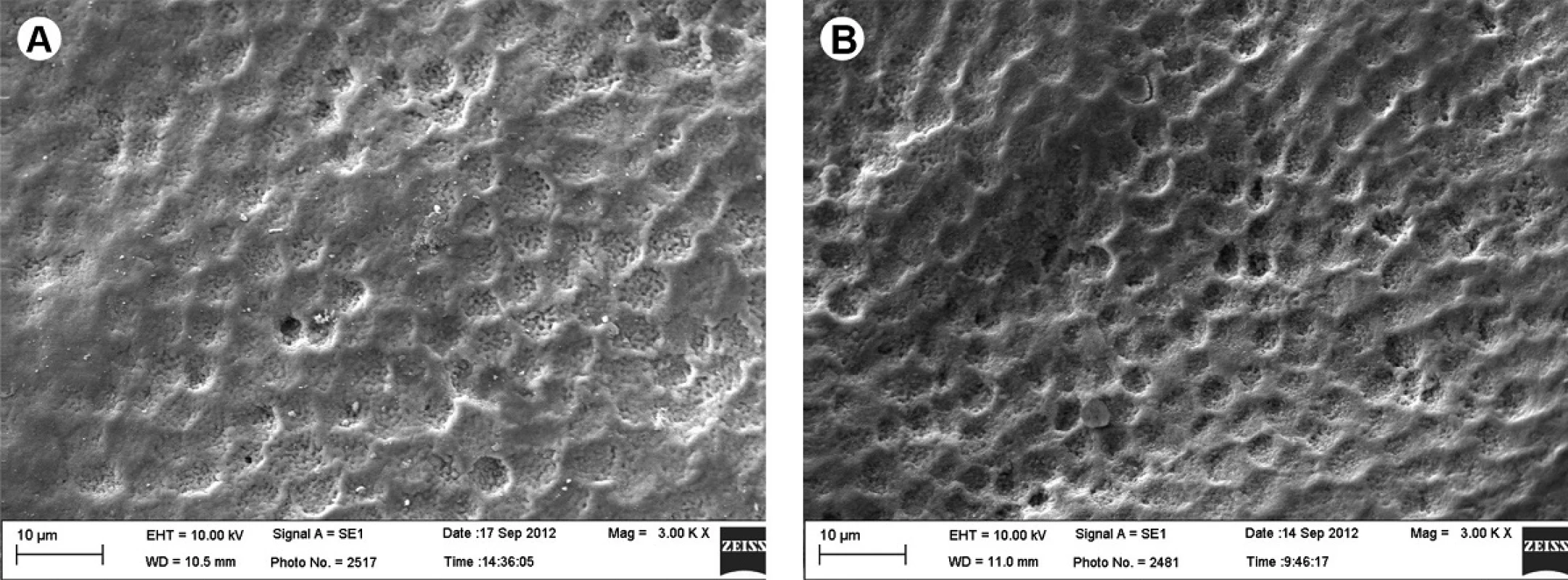
The PLM analyses showed that the specimens of Group I (Fig. 2A - arrow) as well as those of Group II (Fig. 2B - arrow) presented negative birefringence areas on the enamel surface, which are considerably more pronounced and thicker in previously demineralized teeth (Fig. 2B).
2. Polarized light microscopy analysis. Image obtained by polarized light microscopy (25×). A: Specimen subjected to cariogenic challenge. Uniform demineralization of the enamel surface (arrow) may be observed. B: Group II specimen subjected to cariogenic challenge and bleaching procedure, arrow indicates a thicker area of negative birefringence, to an average depth of up to 83 μm, without apparent cavitation. 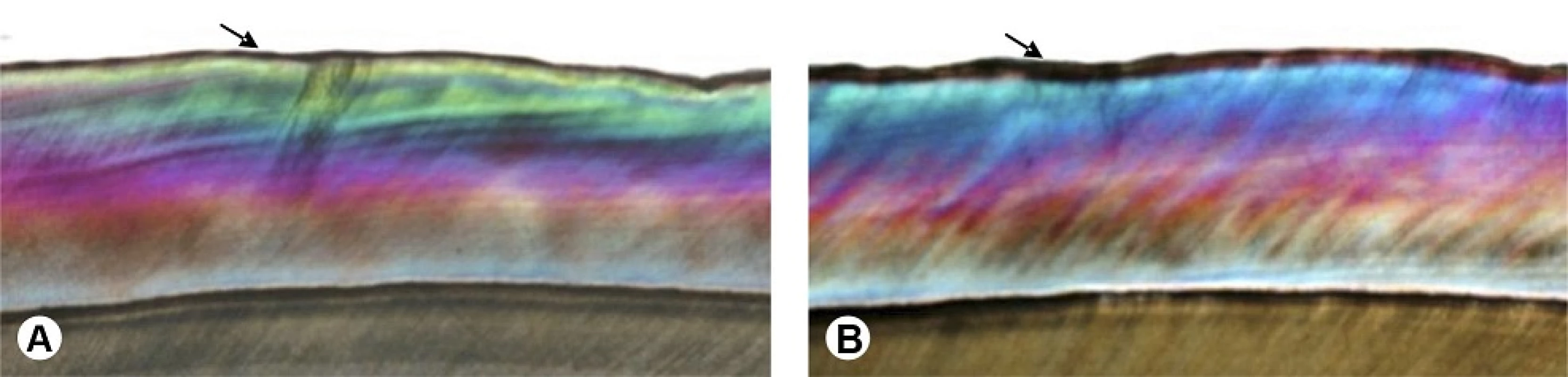
DISCUSSION
Bovine teeth have been widely used for in vitro research (15). Specifically in the used methodology, its use allows obtaining experimental units with suitable dimensions for creation of APCs, as it is possible to simulate clinical application of bleaching agents on the enamel. Furthermore, it is a chemically uniform substrate, and allows less variation in response to cariogenic challenges ( 15 ), being an interesting option for this study.
The results showed that the HP penetration in demineralized enamel was significantly greater than in sound enamel. It is believed that increasing diffusion has significant correlation with the histomorphological changes produced by cariogenic challenge, since the control group possibly offered the greatest challenge for the penetration of peroxide. In this context, the microscopic images showed that exposure of enamel to the demineralizing solution modified the surface characteristics of enamel and the bleaching treatment accentuated these changes.
The bleaching effects modifying the characteristics of the enamel surface are well documented ( 5 , 6 , 7 , 16 , 17, 18 ). In this study was observed that the dental tissues degree of mineralization may have important influence on the HP penetration. This is alarming because it is known that uncontrolled penetration of HP in the pulp tissue has been associated with varying degrees of cell aggression ( 9 , 18 , 19 , 20 , 21 ). Thus, it is important that the dentists perform a thorough clinical examination and proper exams before starting the bleaching treatment, since inadvertent application of whitening gel on incipient lesions may compromise the safety of the procedure(6).
Regarding the CSH analysis, the results showed that bleaching agents were able to increase the depth of incipient lesions demineralization. The CSH data together with images of microscopic observations, makes it possible to consider that under the used experimental conditions, tooth whitening can influence the demineralization progress, also rejecting the second hypothesis of the study. In addition, other studies provide information that sound enamel is more resistant to demineralization than carious enamel when submitted to a bleaching procedure (20).
It was found that in sound enamel, the changes caused by the bleaching treatment were not limited to the surface of the tissue, producing deleterious effects up to 20 µm in depth. These data agree with previous studies(16) where microcomputerized tomography evaluation was used, proving the existence of mineral loss in the subsurface tooth enamel submitted to bleaching procedures.
When the bleaching was performed on previously demineralized enamel, the CSH results showed that the bleaching agents produced changes to a depth of 90 µm. These observations are opposed to some studies (6), where it was found that the action of the bleaching is most evident on the surface of this tissue. However, it should be emphasized that the researchers conducted an in situ study and possibly the oral environment conditions, especially the action of saliva (21), may explain the difference between the studies.
It is worth noting that from a depth of 120 µm, the bleaching agent, as well as the demineralizing solution, had no influence on CSH, possibly because of the distance to the outer surface (22). Another important factor to be observed is the change of the product pH. During its application, the whitening gel had a pH change from 7 to 5, which might have caused significant histomorphological changes in the enamel surface (23) and influenced the HP diffusion through the tooth structure.
Since this is an in vitro study, the results cannot be extrapolated directly to clinical situations, as the presence of intrapulpal pressure, the presence of saliva and odontoblast cytoplasmic processes may decrease the peroxide diffusion (24). In addition, fluoride dilution by saliva, the availability of the product and clearance from oral cavity cannot be taken into consideration in in vitro models too. However, in vitro models can offer important initial information to provide the investigators with confidence to appropriately design clinical trials(25). Otherwise it offers valuable information on the importance of the enamel integrity on the influence of HP penetration during the bleaching treatment, because bleaching is a cosmetic treatment and therefore should be performed in patients without any pathology in the hard and soft oral tissues.
It may be concluded that the application of bleaching agents in previously demineralized enamel favors HP penetration through the tooth structure and the bleaching treatment can increase the demineralization depth of incipient lesions.
ACKNOWLEDGEMENTS
Financial support from The São Paulo State Research Foundation - FAPESP (No. 2012/07086-5) is greatly acknowledged.
Received: September 14, 2014
Accepted: December 01, 2014Correspondence:
Dr. André Luiz Fraga Briso
Rua José Bonifácio, 1193
16015-050 Araçatuba
SP, BrasilTel: +55-18-3636-3348
e-mail: alfbriso@foa.unesp.br
Sources
1. Gokay O, Yilmaz F, Akin S, Tuncbilek M, Ertan R. Penetration of the pulp chamber by bleaching agents in teeth restored with various restorative materials. J Endod 2000;26 : 92-94.
2. Buchalla W, Attin T. External bleaching therapy with activation by heat, light or laser--a systematic review. Dent Mater 2007;23 : 586-596.
3. Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int 1992;23 : 471-488.
4. Guan YH, Lath DL, Lilley TH, Willmot DR, Marlow I, Brook AH. The measurement of tooth whiteness by image analysis and spectrophotometry: a comparison. J Oral Rehabil 2005;32 : 7-15.
5. Jiang T, Ma X, Wang Y, Zhu Z, Tong H, Hu J. Effects of hydrogen peroxide on human dentin structure. J Dent Res 2007;86 : 1040-1045.
6. De Arruda A, Santos PD, Sundfeld R, Berger S, Briso A. Effect of hydrogen peroxide at 35% on the morphology of enamel and interference in the de-remineralization process: an in situ study. Oper Dent 2012;37 : 518-525.
7. Soares DG, Ribeiro AP, Sacono NT, Loguércio AD, Hebling J, Costa CA. Mineral loss and morphological changes in dental enamel induced by a 16% carbamide peroxide bleaching gel. Braz Dent J 2013;24 : 517-21
8. Gokay O, Mujdeci A, Algn E. Peroxide penetration into the pulp from whitening strips. J Endod 2004;30 : 887-889.
9. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 2002;192 : 1-15.
10. Queiroz CS, Hara AT, Paes Leme AF, Cury JA. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de - and remineralization. Braz Dent J 2008;19 : 21-27.
11. Pinto CF, Paes Leme AF, Cavalli V, Giannini M. Effect of 10% carbamide peroxide bleaching on sound and artificial enamel carious lesions. Braz Dent J 2009;20 : 48-53
12. Dias Ribeiro AP, Sacono NT, Lessa FC, Nogueira I, Coldebella CR, Hebling J, et al.. Cytotoxic effect of a 35% hydrogen peroxide bleaching gel on odontoblast-like MDPC-23 cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108 : 458-464.
13. Mottola HA, Simpson BE, Gorin G. Absorptiometric determination of hydrogen peroxide in submicrogram amounts with leuco crystal violet and peroxidase as catalyst. Anal Chem 1970;42 : 410-411.
14. Palo R, Bonetti-Filho I, Valera M, Camargo C, Camargo S, Moura-Netto C, et al. Quantification of peroxide ion passage in dentin, enamel, and cementum after internal bleaching with hydrogen peroxide. Oper Dent 2012;37 : 660-664.
15. Mellberg JR. Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J Dent Res 1992;71 : 913-919.
16. Efeoglu N, Wood DJ, Efeoglu C. Thirty-five percent carbamide peroxide application causes in vitro demineralization of enamel. Dent Mater 2007;23 : 900-904.
17. Efeoglu N, Wood D, Efeoglu C. Microcomputerised tomography evaluation of 10% carbamide peroxide applied to enamel. J Dent 2005;33 : 561-567.
18. Soares DG, Ribeiro AP, Sacono NT, Hebling J. de Souza Costa CA. Effect of fluoride-treated enamel on indirect cytotoxicity of a 16% carbamide peroxide bleaching gel to pulp cells. Braz Dent J 2013;24 : 121-127
19. Coldebella CR, Ribeiro AP, Sacono NT, Trindade FZ, Hebling J, Costa CA. Indirect cytotoxicity of a 35% hydrogen peroxide bleaching gel on cultured odontoblast-like cells. Braz Dent J. 2009;20 : 267-74.
20. Berger SB, Pavan S, Dos Santos PH, Giannini M, Bedran-Russo AK. Effect
of bleaching on sound enamel and with early artificial caries lesions using confocal laser microscopy. Braz Dent J 2012;23 : 110-5.
21. Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent 2005;33 : 223-233.
22. Soares DN, Valinoti AC, Pierro VS, Antonio AG, Maia LC. Cross-sectional microhardness of bovine enamel subjected to three paediatric liquid oral medicines: an in vitro study. Eur Arch Paediatr Dent 2012;13 : 261-265.
23. Sa Y, Sun L, Wang Z, Ma X, Liang S, Xing W, et al. Effects of two inoffice bleaching agents with different pH on the structure of human enamel: an in situ and in vitro study. Oper Dent 2013;38 : 100-110.
24. Karlinsey RL, Mackey AC, Blanken DD, Schwandt CS. Remineralization of eroded enamel lesions by simulated saliva in vitro. Open Dent J 2012;6 : 170-176.
25. Rana R, Itthagarun A, King NM. Effects of dentifrices on artificial caries like lesions: an in vitro pH cycling study. Int Dent J 2007;57 : 243-8.
Labels
Dental medicine
Article was published inBrazilian Dental Journal

2015 Issue 2
Most read in this issue
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


