-
Medical journals
- Career
Modified harvesting technique for pedicled pectoralis major muscle flap after extended manubrial resection in case of recurrent cervicothoracic junction tumors
Authors: Girotti N. C. P. 1; Djedovic G. 2; Elsaesser W. 3; Tschann P. 1; Königsrainer I. 1
Authors‘ workplace: General and Thoracic Surgery Department, Landeskrankenhaus Feldkirch, Austria 1; Department of Plastic, Reconstructive and Aesthetic Surgery, Landeskrankenhaus Feldkirch, Austria 2; Head and Neck Surgery Department, Landeskrankenhaus Feldkirch, Austria 3
Published in: ACTA CHIRURGIAE PLASTICAE, 64, 2, 2022, pp. 76-81
doi: https://doi.org/10.48095/ccachp202276Introduction
An extended bilateral cervicothoracic dissection and associated manubrial bilateral resection in case of recurrent tumors located at the upper thoracic inlet and involving the manubrium is still considered a surgical challenge for thoracic and head and neck surgeons. In these complex cases a wide surgical window (bilateral resection) is sometimes necessary to achieve a radical oncological resection and to allow a good control of the upper mediastinal and neck structures, reducing the risk of major intraoperative complications and iatrogenic damages [1,2]. However, the choice how to reconstruct the chest wall defect remains a critical point in terms of the postoperative complications risk. A pedicled pectoralis major muscle flap (PMMF) is the standard surgical strategy used for soft tissue and chest wall reconstruction after sternal resection in case of patients after radio-chemotherapy, high infection risk and recurrent tumors [3–5]. The PMMF is standardly harvested thought an accessory incision along the muscle pectoralis line. Modifications of the PMMF aimed at improving aesthetic appearance have been proposed [6,7] and resulted in various additional harvesting techniques and skin paddle designs such as an inframammary skin paddle [8,9].
We here describe a modified harvesting technique for the PMMF (the same-incision harvesting technique: sPMMF) to replace the chest wall defect formation after bilateral manubrial resection in patients with recurrent tumors following radio-chemotherapy treatment.
Material and methods
Patients
A retrospective single center analysis of the patients with tumors located at the upper thoracic inlet referred to the Departments of Otorhinolaryngology and Thoracic Surgery at the Academic Hospital of Feldkirch, Austria, from 1. January 2017 to 31. December 2018 was conducted.
Written informed consent for surgery, data, and image processing was obtained. The disease was staged by an interdisciplinary tumor board (general and thoracic surgeon, plastic surgeon, otorhinology surgeon, radiotherapist, oncologist, radiologist, and nuclear medicine specialist) according to the 7th edition of the Union for International Cancer Control (UICC) TNM staging system. During the multidisciplinary board nutritional status, comorbidities of the patient, tumor staging, imaging and psychological aspects are presented, and based on this data, the board defines the best treatment plan for each patient.
Patients eligible for the study fulfilled all the following inclusion criteria:
– previous radio-chemotherapy treatment;
– patients requiring a bilateral manubrial resection;
– recurrent histologically confirmed malignant tumor (pharyngeal, laryngeal, thyroid, skin or lung cancer of any TNM stage) or consumption of the sternoclavicular join or manubrium (osteomyelitis) because of the previous radio-chemotherapy.
Application of the sPMMF as a reconstructive treatment option was proposed for all included patients in order to provide vital tissue after salvage surgery in a primarily irradiated surgical area. The sPMMF employing the modified technique proposed in this paper was performed immediately after salvage surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and following Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) check list. The study was approved by regional ethics board of Vorarlberg and individual consent for this retrospective analysis was waived.
The analyzed variables were:
– demographics: age and sex;
– clinical details: histology, patients under chemoradiotherapy, time between chemoradiotherapy and surgery, comorbidities;
– postoperative data: reoperation within 30 days, intraoperative complications, operation time, intensive care unit and hospital stay. Postoperative complications were classified according to Dindo-Clavien criteria [10]. Surgical complications were further defined as reoperation, bleeding requiring blood transfusion and surgical site infection. Medical complications were defined as cardiovascular, pulmonary, renal, sepsis, and other events. Events requiring reoperation, intensive care unit admission, or hospitalization longer than 15 days were defined as major complications (Dindo-Clavien > grade III).
– follow-up data at the 6th postoperative month: disease survival and clinical status. The data are presented as the median or the average ± standard error for continuous variables and number (percentage) for categorical variables.
Same-incision harvesting technique (sPMMF)
After intubation, the patient was placed in the supine position.
In addition to the usual transverse cervical or apron incision, a median vertical limb of the incision was made down bilateral to below the angle of Louis, following the upper margin of the second ribs (Fig. 1A). If required, a skin area was resected en bloc with the musculoskeletal structures.
Fig. 1. Planned skin incision. A) Usual transverse cervical approach is associated to a vertical incision below the angle of Louis, following the upper margin of the second ribs bilaterally. B) Subsequently both medial ends of the clavicle together with the sternoclavicular joints and the costochondral junction of the first rib and the manubrium were resected. Resection margins are demarcated by the bold line. 
Firstly, otolaryngology surgeon proceeded to expose the trachea, cervical vessels and vagus nerve bilaterally. At the suprasternal notch, both anterior jugular veins were ligated and divided, and the sternal heads of both sternocleidomastoid muscles were released. Afterwards, in the second step, the thoracic surgeon performed the dissection of the anterior thoracic inlet: the clavicular insertions of both pectoralis major muscles were separated from the sternal ones. As described in the Dartevelle´s technique [1], the first cartilages were resected bilaterally and both costoclavicular ligaments were sectioned.
Subsequently, using the sternal saw with a guard, both medial ends of the clavicle together with the sternoclavicular joints, medial ends, and the costochondral junction of the first rib and the manubrium were removed (Fig. 1B).
The plastic surgeon proceeded to cover the defect using the modified unilateral sPMMF from the right thoracic side (Fig. 2). The upper borders of the pectoralis major muscle of the left side were already exposed by the dissection of the clavicles.
Fig. 2. A) Schematic view of the blood supplying pectoral branch of the thoracoacromial artery and the muscles dissection lines are depicted by the dotted line. B) Final set of the right sided pedicled flap after rotation to cover the chest wall defect. 
Skin hooks were placed on the inferior skin edge and the skin flap was elevated to permit a complete pectoralis major muscle exposure from its clavicular and sternocostal aspects, leading laterally toward the deltopectoral groove.
After blunt dissection in the infraclavicular fossa one nutrient vessel of the pectoralis major muscle, the pectoral branch of the thoraco-acromial artery was exposed between the clavicular and the sternocostal aspects of the muscle. Afterwards, the muscle was detached from its abdominal and sternocostal origin by electrocautery. The attachment to the humerus was detached by electrocautery under visual guidance to avoid nerve or vascular lesions in the deltopectoral groove. The clavicular portion of the pectoralis major muscle was particularly incised, to avoid kinking of the pectoral branch and the detached muscle was transposed into the defect. To avoid nerve fasciculation and vascular spasm, the accompanying nerves of the muscle were dissected. Afterwards, two suctions drains were placed along the trachea bilaterally and the flap was fixed to the dissection margins with interrupted 3-0 Vicryl sutures. At the end of the procedure, a split-thickness skin graft was harvested from the right thigh with the thickness of 0.2 mm and the PMMF was finally covered with this unmeshed skin graft (Fig. 3). The skin graft was fixed with skin staples and afterwards with a vacuum assisted wound closure device (pressure: 100 mmHg, continuous suction mode).
Fig. 3. A) Intraoperative picture after manubrial-clavicle-rib resections and extended neck dissection. B) Chest wall defects replacement with the pedicled major muscle flap and the split-thickness skin graft. 
Results
Up to now we have performed this technique in 15 patients with a malignant recurrence and after previous radio-chemo-
therapy. Clinical data are resumed in table 1. All patients required an extended manubrial resection en-bloc with skin and musculoskeletal structures.
1. Clinical data of the patients. 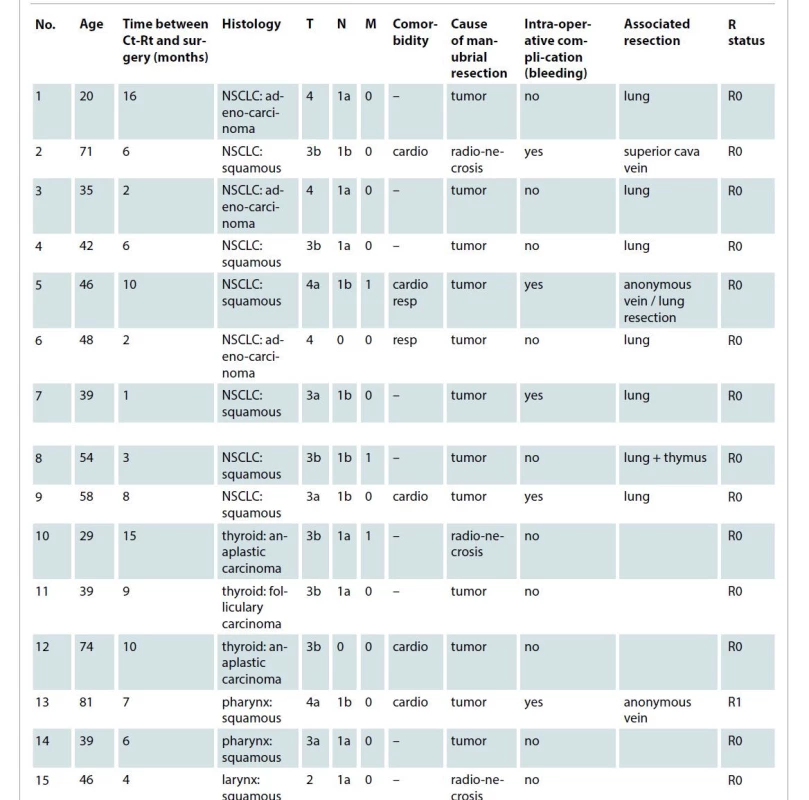
cardio – cardiologic, Ct-Rt – chemoradiotherapy, M – metastases, N – nodes, NSCLC – non-small cell lung cancer, resp – respiratory, T – tumor The following different types of recurrences were detected: three squamous cell skin carcinomas, ten non-small lung cancers and three thyroid anaplastic carcinomas. The patients required the resection because of following reasons:
– recurrence involving the internal cortical layer of the sternum or the sternoclavicular join;
– consumption of the sternoclavicular join or manubrium (osteomyelitis) because of the previous radiochemotherapy.
In one patient, a combined laryngectomy was performed. The mean overall operation time was 175 ± 51.6 min. The majority (N = 10; 66.6%) of patients had an uneventful course (no surgery related complications) of recovery and showed satisfying aesthetic results and no donor site morbidity. Four (26%) patients had major complications that required surgical revision (Dindo-Clavien > grade III). In two cases, due to a postoperative bleeding associated with a significant Hb reduction (more than 3 mg/dl in 12 hours), and in two cases with a surgical site infection, a reoperation was required. In two cases with bleeding, a subcutaneous hematoma evacuation was necessary. In one patient with surgical site infection a partial necrectomy (less than 10% of the surface) of the skin graft was required and in the other case, evacuation of an infected hematoma, located under the muscle flap, with a subsequent new vacuum assisted wound closure device was necessary. The postoperative data are resumed in Tab. 2.
2. Postoperative stay and complications. 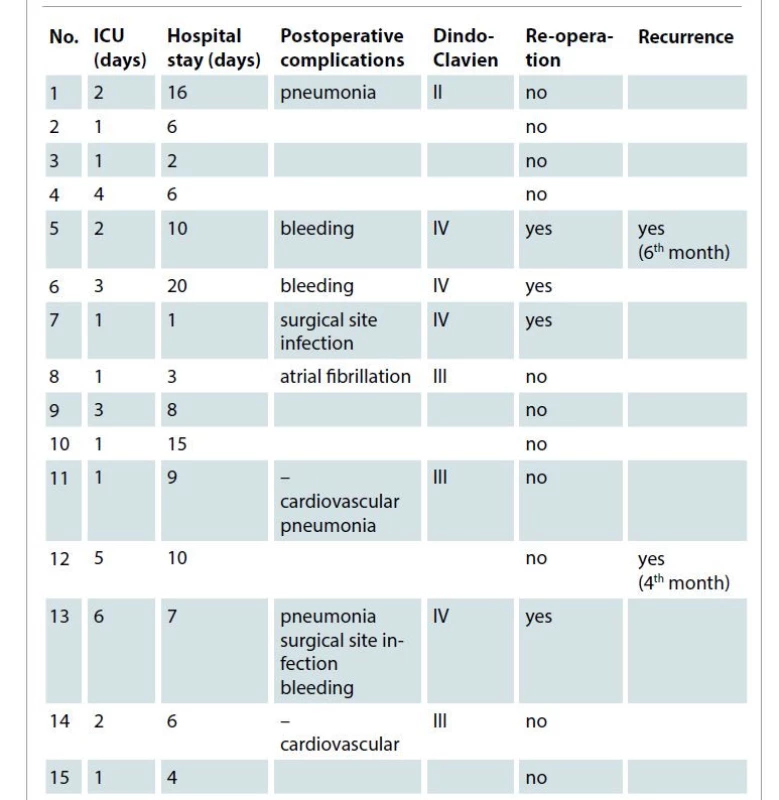
ICU – intensive care unit All patients had early postoperative shoulder mobilization without functional deficit or aesthetic deformity. At the last follow up (chest computer tomography scan 6 months after surgery), all patients were in good performance status and only two patients have developed a local recurrence (one anaplastic carcinoma at the 4th month and one non-small cell lung cancer (adenocarcinoma) at the 6th month). No anatomical deformities or functional alteration of necks and upper thoracic muscles were reported at 3 months after surgery.
Discussion
The PMMF has been a well-established procedure in reconstructive surgery since 1968 [11]. Although more sophisticated microsurgical reconstructive techniques exist, available data suggest that the PMMF is still a favorable treatment option due to its reliability and harvest simplicity [12,13]. In many critical cases associated with a high risk of postoperative complications, the PMMF remains the reference flap [14–16].
Since the preceding therapies in this patient group include radiotherapy or radiochemotherapy, the risk of postoperative wound healing complications such as local infection is quite high. For this reason, the PMMF has been reported by several authors to be an effective reinforcement option for the chest wall by transferring vital tissue to a previously irradiated surgical field [17–20]. Also in case of sternal infection after cardiac surgery, the PMMF still remains the gold standard to cover an anterior chest wall defect. In any cases, the PMMF is standardly harvested through a large accessory lateral incision; therefore, in addition the main medial incision used for the sternal resection, the patients have two different high risk wounds in the same anatomical field previously treated with radiochemotherapy. The next problem that arises in this type of surgery is the speed of execution of the muscle flap. However, alternative techniques requiring extensive vessel anastomosis with microsurgical equipment have been reported to approach 9 hours [21]. The long operating time is the main risk factor for intraoperative and postoperative complications. Some authors reported that the risk of morbidity increases significantly above 3 hours [22].
Therefore, with our approach fewer perioperative and postoperative complications are expected, thus resulting in a shorter postoperative recovery period [8].
Our simple harvesting technique, using only one incision, seems to reduce the risk of wound complications and moreover it shows to reduce drastically the time for free tissue transfer [21].
Moreover, our technique aims to preserve functionality by sparing the most of the pectoralis major muscle with a subsequent lower impact on the arm mobility.
In our opinion, the modified harvesting technique specifically reduces the donor site morbidity by sparing the clavicular and the superior sternocostal aspects of the muscle as the cranial aspect of the muscle. These muscle segments are responsible for internal rotation of the shoulder and horizontal adduction of the arm [23,24]. However, it was not possible to compare the sPMMF with other harvesting techniques, performed in our hospital before 2017. No previous surgical data were available.
Although previous PMMF modifications have attempted to improve an aesthetic outcome, this process is continuing, and additional surgical treatment options are warranted. The importance of aesthetic reconstruction should not be underestimated as an aesthetic outcome has been shown to be a crucial component in patient’s satisfaction following an oncological surgery [25].
In comparison, the conventional method of harvesting the pectoral muscle leaves the patient with extensive scarring and, hence, a less appealing aesthetic outcome.
Limitations of this study include limited follow-up due to the retrospective study design and the fact that more detailed results are missing with regard to functional outcome and postoperative quality of life. Thus, a prospective study including standardized testing of functionality and strength pre - vs. postoperatively is planned by our group.
Conclusions
Advantages of the sPMMF include the simplicity of the technique and the lower morbidity as compared to microsurgical alternatives or standard PMMF harvesting. Moreover, the sPMMF provides, like the standard PMMF, an adequate defects coverage with excellent aesthetic and functional results.
Role of authors: Paolo N.C. Girotti: data search, data setting, writing, draft of manuscript, last revision
Gabriel Djedovic: data search, data setting, writing, draft of manuscript, last revision
Wolfgang Elsaesser: draft of manuscript, last revision
Peter Tschann: draft of manuscript, last revision
Ingmar Königsrainer: draft of manuscript, last revision
Disclosure statement: Funding: all authors did not receive any type of funding
Conflicts of interest: all authors have no conflict of interest
Ethics approval: approved in accordance with the Vorarlberg Ethic Commission rules, Austria
Consent to participate: in this retrospective study informed consent was not required.
Standardized report guidelines: STROBE
Paolo N.C. Girotti, MD
General and Thoracic Surgery Department
Landeskrankenhaus Feldkirch
Carinagasse 47
6800 Feldkirch
Austria
e-mail: paolo.girotti@vlkh.net
Submitted: 21. 4. 2022
Accepted: 25. 7. 2022
Sources
1. Dartevelle P., Chapelier AR., Macchiarini P., et al. Anterior transcervical-thoracic approach for radical resection of lung tumours invading the thoracic outlet. J Thorac Cardiovasc Surg. 1993, 105(6): 1025–1034.
2. Grunenwald D., Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg. 1997, 63(2): 12–19.
3. Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg. 1979, 63(1): 73–81.
4. Ariyan S. Further experiences with the pectoralis major myocutaneous flap for the immediate repair of defects from excisions of head and neck cancers. Plast Reconstr Surg. 1979, 64(5): 605–612.
5. Hueston JT., McConchie IH. A compound pectoral flap. Aust N Z J Surg. 1968, 38(1): 61–63.
6. Rauchenwald T., Dejaco D., Morandi EM., et al. The pectoralis major island flap: short scar modified muscle-sparing harvesting technique improves aesthetic outcome in reconstructive head and neck surgery. ORL J Otorhinolaryngol Relat Spec. 2019, 81(5–6): 327–337.
7. Ariyan S. The donor site of the pectoralis major myocutaneous flap. Plast Reconstr Surg. 1980, 66(1): 165–166.
8. McLean JN., Carlson GW., Losken A. The pectoralis major myocutaneous flap revisited: a reliable technique for head and neck reconstruction. Ann Plast Surg. 2010, 64(5): 570–573.
9. Miller LE., Stubbs VC., Silberthau KB., et al. Pectoralis major muscle flap use in a modern head and neck free flap practice. Am J Otolaryngol. 2020, 41(4): 102475.
10. Crosher R., Llewelyn J., Mitchell R. A modification of the pectoralis major myocutaneous flap that reduces the defect at the donor site. Ann R Coll Surg Engl. 1995, 77(5): 389–391.
11. Clavien PA., Barkun J., de Oliveira ML., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009, 250(2): 187–196.
12. Hueston JT., McConchie IH. A compound pectoral flap. Aust N Z J Surg. 1968, 38(1): 61–63.
13. Teo KG., Rozen WM., Acosta R. The pectoralis major myocutaneous flap. J Reconstr Microsurg. 2013, 29(7): 449–456.
14. Bussu F., Gallus R., Navach V., et al. Contemporary role of pectoralis major regional flaps in head and neck surgery. Acta Otorhinolaryngol Ital. 2014, 34(5): 327–341.
15. Schneider DS., Wu V., Wax MK. Indications for pedicled pectoralis major flap in a free tissue transfer practice. Head Neck. 2012, 34(8): 1106–1110.
16. Liu M., Liu W., Yang X., et al. Pectoralis major myocutaneous flap for head and neck defects in the era of free flaps: harvesting technique and indications. Sci Rep. 2017, 7(1): 46256.
17. Del Campo C. Pectoralis major muscle flap reconstruction after clavicular-manubrial resection. Ann Thorac Surg. 2000, 69(2): 668–670.
18. Gilbert MR., Sturm JJ., Gooding WE., et al. Pectoralis major myofascial onlay and myocutaneous flaps and pharyngocutaneous fistula in salvage laryngectomy. Laryngoscope. 2014, 124(12): 2680–2686.
19. Patel UA., Moore BA., Wax M., et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013, 139(11): 1156–1162.
20. Oosthuizen JC., Leonard DS., Kinsella JB. The role of pectoralis major myofascial flap in salvage laryngectomy: a single surgeon experience. Acta Otolaryngol. 2012, 132(9): 1002–1005.
21. Guimarães AV., Aires FT., Dedivitis RA., et al. Efficacy of pectoralis major muscle flap for pharyngocutaneous fistula prevention in salvage total laryngectomy: a systematic review. Head Neck. 2016, 38(S1 Suppl 1): E2317–2321.
22. McMahon JD., MacIver C., Smith M., et al. Postoperative complications after major head and neck surgery with free flap repair – prevalence, patterns, and determinants: a prospective cohort study. Br J Oral Maxillofac Surg. 2013, 51(8): 689–695.
23. Hardy KL., Davis KE., Constantine RS., et al. The impact of operative time on complications after plastic surgery: a multivariate regression analysis of 1753 cases. Aesthet Surg J. 2014, 34(4): 614–622.
24. Peterson Kendall F., Kendal McCreary E., Geise Provance P. Muskeln – funktionen und tests. Lübeck: Gustav Fischer Verlag 1998.
25. Platzer W. Taschenatlas anatomie bewegungsapparat. Stuttgart: Thieme 2018.
26. Enajat M., Smit JM., Rozen WM., et al. Aesthetic refinements and reoperative procedures following 370 consecutive DIEP and SIEA flap breast reconstructions: important considerations for patient consent. Aesthetic Plast Surg. 2010, 34(3): 306–312.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2022 Issue 2-
All articles in this issue
- Editorial
- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Temporary skin closure in extremity soft tissue sarcoma – our indications
- Modified harvesting technique for pedicled pectoralis major muscle flap after extended manubrial resection in case of recurrent cervicothoracic junction tumors
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Subcutaneous shoulder hibernoma presenting as an atypical lipomatous tumor – a case report
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Single-stage reconstruction of a large lower eyelid defect using a full-thickness bilamellar autograft
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Single-incision endoscopic-assisted temporoparietal fascia harvest for single stage auricular reconstruction
- Salvage of a large exposed cranial implant on irradiated necrosed scalp using free latissimus dorsi and forehead flaps – a case report
- Bilateral latissimus dorsi for breast reconstruction in one stage
- Hook nail treatment – a bulky palmar flap as an alternative to the “antenna” procedure and to the thenar flap for fingertip coverage
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

