-
Medical journals
- Career
Use of licap and ltap flaps for breast reconstruction
Authors: L. Martellani; M. Manara; N. Renzi; G. Papa; V. Ramella; Z. Arnež
Authors‘ workplace: ASUITS Trieste, Italy
Published in: ACTA CHIRURGIAE PLASTICAE, 60, 1, 2018, pp. 4-8
INTRODUCTION
Breast conserving surgery (BCS) followed by radiotherapy is considered the gold standard treatment for early breast cancer. Survival rates after BCS are similar to those of mastectomy. Associated to those are considerable aesthetic and psychological benefits for the patient.1
The main issue in BCS is accurate selection of patients as, this surgical technique is addressed to patients with a positive ratio between breast size and tumor volume. Residual breast parenchyma is redistributed according to breast reduction patterns or rotation/advancement flaps (volume displacement) and usually a contralateral breast reduction or mastopexy is required to achieve symmetry and a pleasant aesthetic result.2,3,4
The introduction of local flaps from the lateral thoracic region for volume replacement reconstruction has widened the indications for BCS by allowing surgeons to perform wider excision (even more than 20% of breast parenchyma) to ensure tumor-free margins and an immediate good aesthetic result with no need for contralateral surgery.5,6
As described by Hamdi et al., the costal segment of the intercostal vessels provides several perforators to the skin by means of the intercostal, serratus anterior and latissimus dorsi muscles. The lateral intercostal artery perforator (LICAP) flap can be harvested based on these perforators.7,8
Intercostal perforators can be found predominantly between the fifth and eighth intercostal spaces. They emerge deep to the intercostal muscles, from the subcostal groove, run into the intercostal space deep to the internal and external intercostal muscles and then obliquely under a slip of the origin of the serratus anterior muscle.
Moreover, vascular connections link the intercostal perforators to the serratus anterior branch, enabling a safer harvesting of the flap.
Flaps based on the lateral thoracic artery are another valuable option. They can be raised as perforator flaps, as described by McMillan et al.9, or as an axial flap based on the principal (lateral thoracic) vessel allowing the reconstruction of more medial defects or a total breast reconstruction as the vascular pedicle and consequently the arc of rotation are longer.
MATERIALS AND METHODS
Perforator flaps from the lateral thoracic region have been described for reconstruction of partial thoracic defects, axillary contractures6,10 and defects after BCS.11-15 In our case series we used LICAP and flaps harvested on the lateral thoracic artery perforator (LTAP) and lateral thoracic artery axial flap.
From May 2015 to October 2016, 19 patients were treated, with a total of 20 flaps harvested. In 15 patients the perforator flaps from the lateral thoracic region were used after quadrantectomy or wide local excision (in one patient also the contralateral breast was shaped for better symmetry after a previous quadrantectomy without reconstruction by the same technique), in 3 patients as volume replacement after mastectomy and in 1 patient the flap was used to reinforce the lower breast pole instead of an acellular dermal matrix (ADM), after mastectomy following a previous breast augmentation. All patients who underwent reconstruction by this technique had medium to small breasts, adequate subcutaneous tissue and skin laxity in the lateral thoracic region as well as an unfavorable tumor/breast size ratio. The average patient age was 54 years (range 43-64years).
In 17 patients lateral intercostal artery perforator (LICAP) flaps were harvested. In 8 patients we took advantage of the vascular connections between the intercostal perforators and the lateral thoracic system for a more reliable vascular supply, and in 2 patients the flap was raised solely on the perforators of the lateral thoracic artery (LTAP flap).
In the OR the patients were positioned on the back with an inflatable bag placed behind the shoulder. After that quadrantectomy, wide tumor excision or mastectomy and axillary clearance were performed. Following tumor excision, the specimen was weighted and then sent for histology. After completing the oncologic part of the procedure, the inflatable device was inflated, which exposed the lateral thoracic region for a comfortable harvesting of the flap. The size of the flap was determined preoperatively taking in consideration the location of the defect (lateral quadrants of the breast) and the estimated volume to be replaced by the flap. Flap harvesting and breast reconstruction were thus performed without the need to reposition the patient. All our flaps were harvested on several perforators. The perforator that offered the best swing of the flap with minimal rotation of the perforator was finally selected; all the others were clipped. The flap was then de-epithelialized, folded, when necessary, and positioned into the defect. Two suction drains were positioned (one drained the breast and one the donor site). (Fig. 1–4.)
1. Flap harvested on three perforators of the lateral intercostal artery 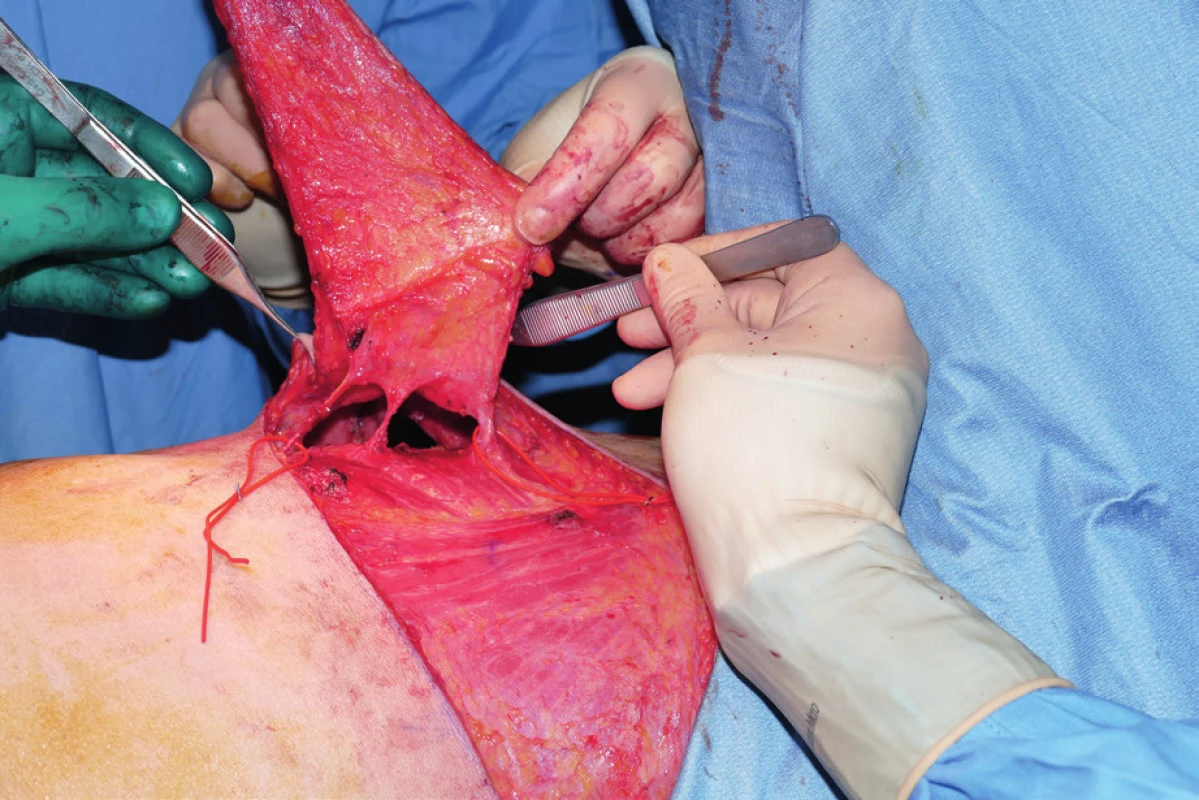
2. Flap harvested on one lateral intercostal artery perforator and one lateral thoracic artery perforator. The distal one (LICAP) was later clipped 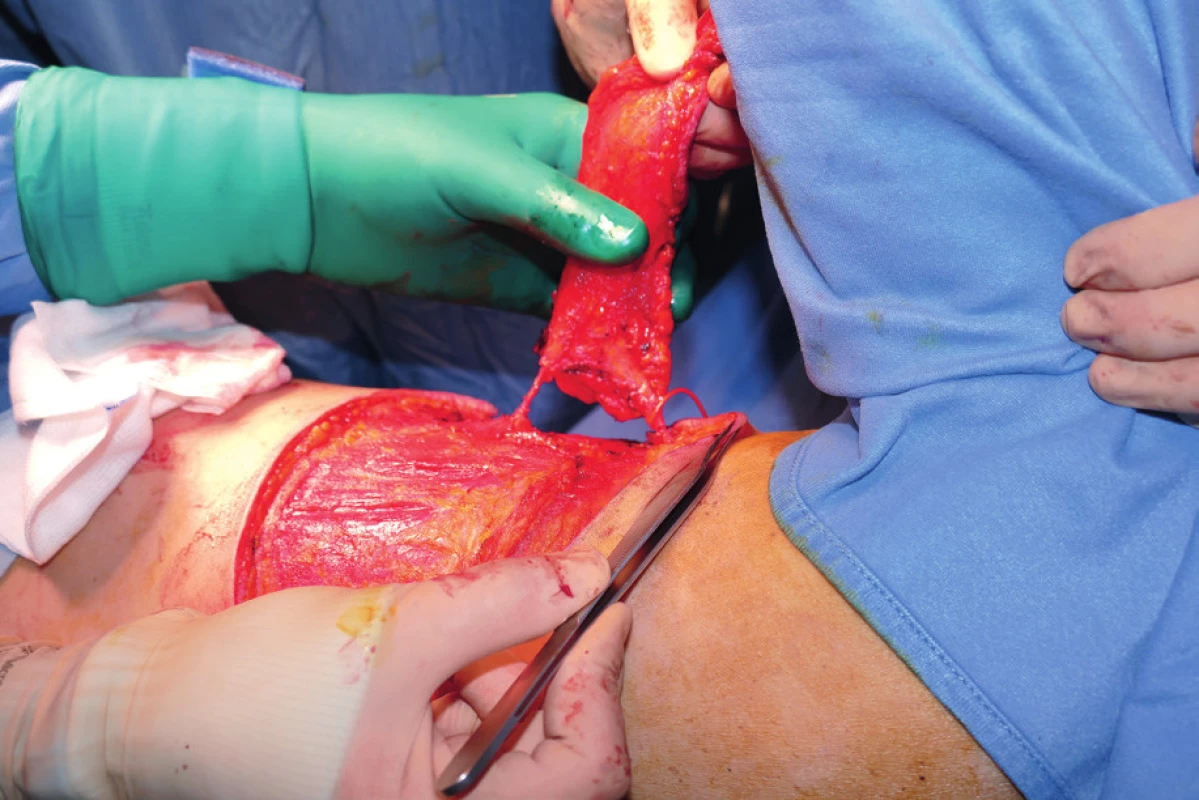
3. Patient with lower outer quadrant cancer of the right breast. Quadrantectomy, sentinel lymph node biopsy and reconstruction with LICAP flap were performed. A: Preoperative view. B: One moth postoperative view. C: Result after radiotherapy (loss of volume, shrinkage of the flap and radiodermatitis) 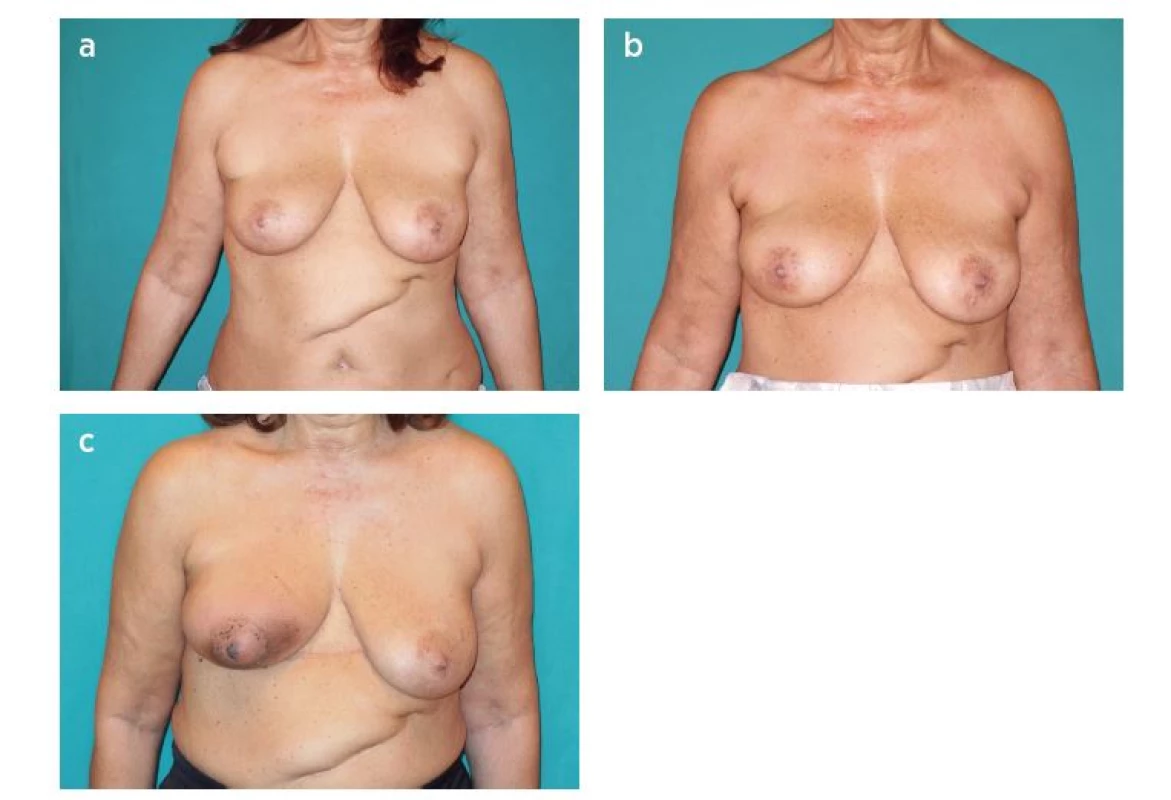
4. Patient with upper outer quadrant cancer of the right breast. Quadrantectomy, sentinel lymph node biopsy and reconstruction with LICAP flap were performed. A: Preoperative view. B: One month postoperative view 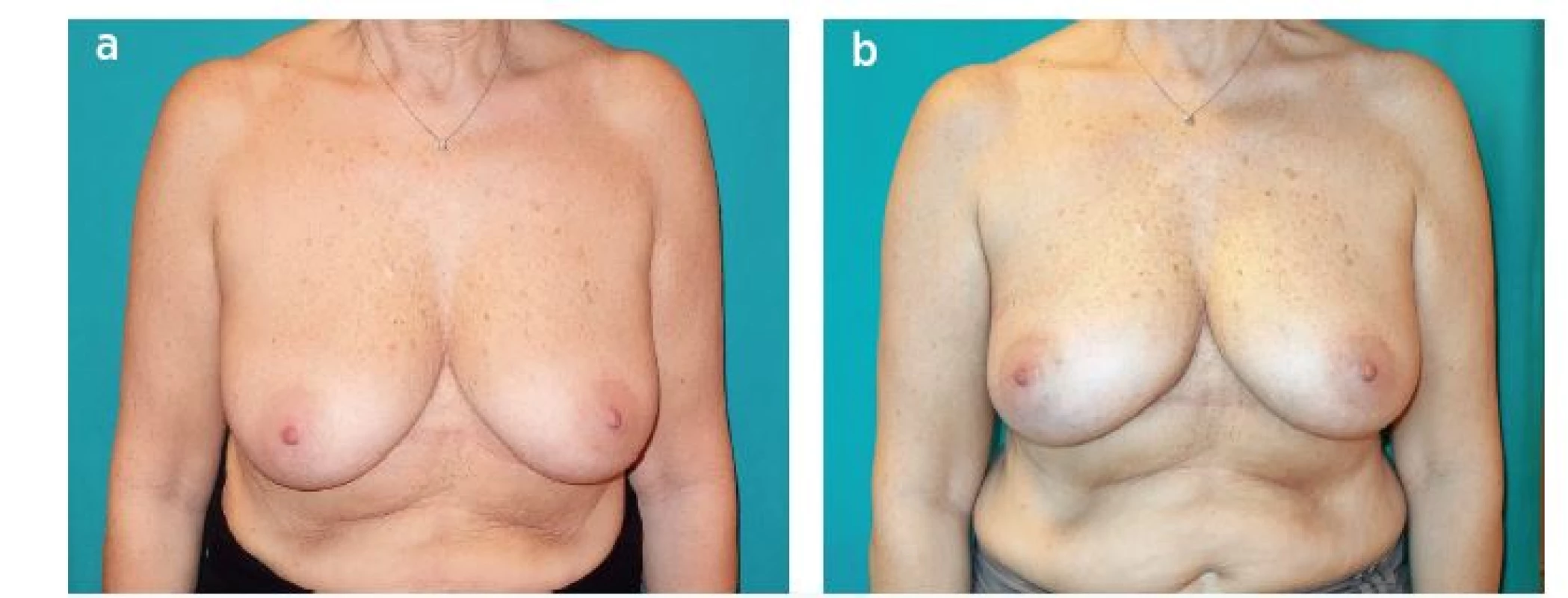
RESULTS
All our flaps survived. We encountered no flap loss (neither partial nor total). The total number of complications was 8: three seromas on the back and four small superficial wound dehiscences. One patient required return to the operating theatre on the first postoperative day for evacuation of a hematoma.
In all cases a good aesthetic result, with good symmetry, was achieved.
14 patients underwent radiotherapy 6 to 8 weeks after surgery. The average duration of the reconstruction was 63 minutes (range 50-80min). The average weight of anatomical specimens after quadrantectomy or wide local excision was 81.89 g (range 29-200g) (Table 1.)
1. The table shows patients’ age, type of surgery and location of the defect, specimen weight, type of flap harvested and operating time. ( * ) In these cases the connections between lateral intercostal artery perforator and the lateral thoracic perforator have been preserved 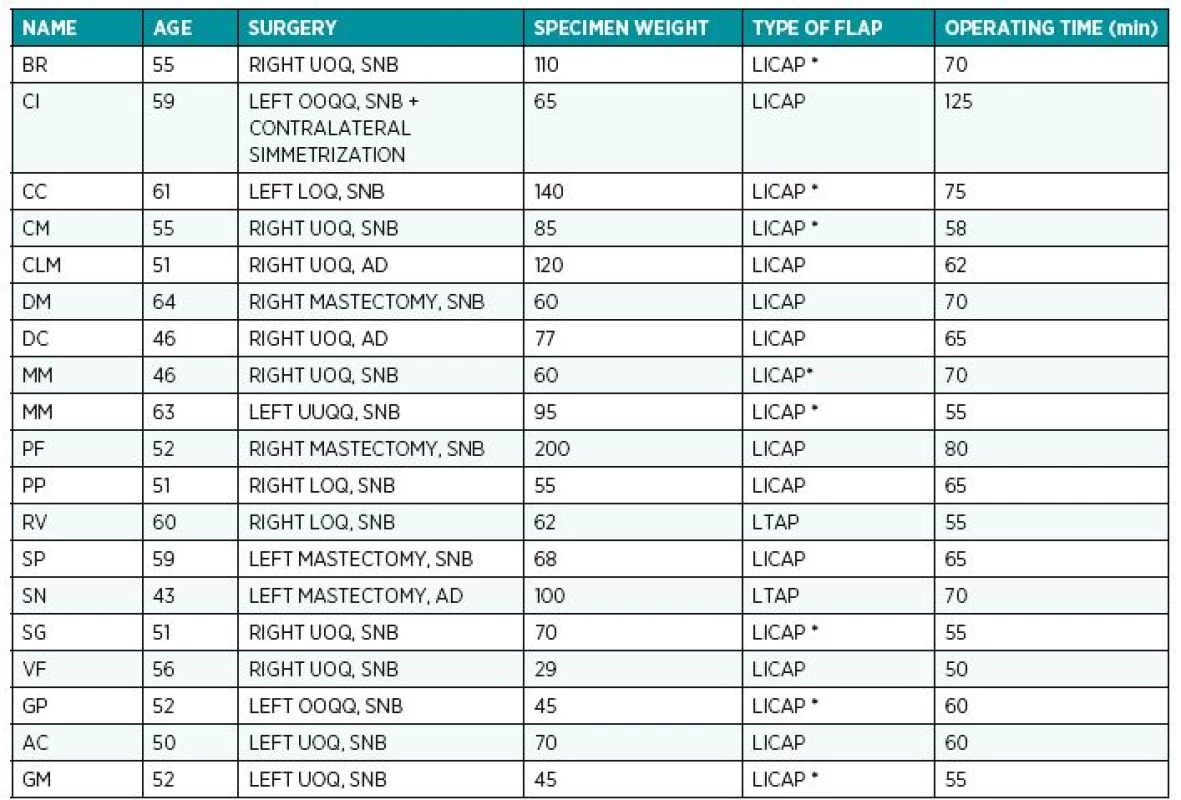
DISCUSSION
The pedicle perforator flaps from the lateral thoracic region for reconstruction of partial breast defects after BCS are more and more popular as they allow breast reconstruction with minimal donor site morbidity. Even after wide resections, an adequate volume replacement can be achieved with no need to sacrifice latissimus dorsi muscle and major vessels (e.g. thoracodorsal artery) since even big flaps can be reliably raised on minor vessels, or a single perforator.8,9,14,15 Moreover in case of local relapse of the tumor, the reconstruction after mastectomy is still feasible as all major vascular pedicles have been preserved by previous surgery.
LICAP flap has also been used as an ADM in a patient who underwent dual plane breast augmentation first and then a mastectomy for breast cancer. Wishing to preserve the implant, we harvested the flap and used it to protect and support the lower pole of the prosthesis, while in the upper pole it was already covered by the pectoralis major muscle. Such reconstruction proved to be strong and stable, with no ptosis or mal-positioning of the implant with time.
The vascular anatomy of the region is constant, the dominant perforators of the intercostal artery are typically located in the fourth to eighth intercostal spaces, with a higher concentration in the sixth and seventh intercostal spaces, 3.5 cm medial from the anterior border of the latissimus dorsi.8 Pedicle length was always adequate, also in cases of total breast reconstruction (dissection of the main pedicle was performed to the costal groove if a longer arch of rotation was required) and we did not face problems of venous stasis even after a rotation of 180°. In our practice, while searching for the intercostal perforators, we noticed that the course of the lateral thoracic permitted harvesting of the flap on a perforator vessel from it (LTAP flap) or on the main vessel itself, as an axial flap, enhancing the arc of rotation.
Harvesting of local pedicle perforator flaps from the lateral thoracic region, if compared to the TDAP, does not prolong operating time due to the more constant anatomy, the less tedious harvesting technique and because the repositioning of the patient is not necessary. Moreover, the learning curve for the surgeons is significantly shorter when compared to other reconstructive techniques.
The main problem that we outlined in this case series has been the changes that the flaps underwent after radiotherapy in terms of pliability and volume.16 The early post-operative results were all excellent (see fig. 4) but in almost all cases we could appreciate a variable degree of stiffness and loss of volume of the flap after radiotherapy if compared to the immediate postoperative results (see fig. 3). We partially attributed this effect to the ratio between dermis and subcutaneous tissue of the flaps harvested. In majority of patients the BMI was normal to slightly underweight, so that scarce subcutaneous fat could have made flaps, mainly consisting of dermis, more sensitive to radiation damage.
We did not use any secondary lipostructure to improve tissue pliability and correct volume deficiencies due to lack of adequate studies about its oncologic safety.
CONCLUSIONS
Perforator flaps of the lateral thoracic region should become the gold standard for reconstructions after BCS involving 20% of the breast volume. It is a safe procedure, that allows good aesthetic results, it spares the LD muscle and minimizes donor site morbidity. It does not prolong the operating time significantly due to constant anatomy, an easy harvesting without repositioning of the patient.
In skinnier patients the aesthetic result is less pleasant, particularly after radiotherapy because of fibrosis and shrinkage of the flap, but this reconstructive technique has still to be preferred to prosthetic reconstruction.
Further studies and longer outcome measurements are necessary to evaluate the stability of the aesthetic results with time and to improve our knowledge about the feasibility of axial pattern flaps based on the lateral thoracic artery.
Conflict of interest statement: The authors do not have any potential conflicting interests to declare.
Corresponding author:
Zoran Marij Arnež, M.D.
ASUITS Trieste
strada di Fiume 447 34137 Trieste, Italy
E-mail: zoran.arnez@asuits.sanita.fvg.it
Sources
1. Veronesi U, Cascinelli N, Mariani L et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002 Oct 17;347(16):1227-32.
2. Doridot V, Nos C, Aucouturier JS, Sigal-Zafrani B, Fourquet A, Clough KB. Breast-conserving therapy of breast cancer. Cancer Radiother. 2004 Feb;8(1):21-8.
3. Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003 Jan;237(1):26-34.
4. Hamdi M. Oncoplastic and reconstructive surgery of the breast. Breast. 2013 Aug;22 Suppl 2:S100-5.
5. Dixon JM, Venizelos B, Chan P. Latissimus dorsi mini-flap: a technique for extending breast conservation. Breast. 2002 Feb;11(1):58-65.
6. Stillaert FB, Casaer B, Roche N et al. The inframammary extending lateral intercostal artery perforator flap for reconstruction of axillary contractures: a case report. J Plast Reconstr Aesthet Surg. 2008 Dec;61(12):e7-11.
7. Taylor GI, Daniel RK. The anatomy of several free flap donor sites. Plast Reconstr Surg. 1975 Sep;56(3):243-53.
8. Hamdi M, Spano A, Van Landuyt K, D’Herde K, Blondeel P, Monstrey S. The lateral intercostal artery perforators: anatomical study and clinical application in breast surgery. Plast Reconstr Surg. 2008 Feb;121(2):389-96.
9. McCulley SJ, Schaverien MV, Tan VK, Macmillan RD. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J Plast Reconstr Aesthet Surg. 2015 May;68(5):686-91.
10. Schwabegger AH, Herczeg E, Piza H. The lateral thoracic fasciocutaneous island flap for treatment of recurrent hidradenitis axillaris suppurativa and other axillary skin defects. Br J Plast Surg. 2000 Dec;53(8):676-8.
11. Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg. 1986 Jun;77(6):933-43.
12. Hamdi M, Wolfli J, Van Landuyt K. Partial mastectomy reconstruction. Clin Plast Surg. 2007 Jan;34(1):51-62
13. Losken A, Hamdi M. Partial breast reconstruction: current perspectives.Plast Reconstr Surg. 2009 Sep;124(3):722-36.
14. Hamdi M, Van Landuyt K, Monstrey S, Blondeel P. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg. 2004 Sep;57(6):531-9.
15. Hamdi M, Van Landuyt K, de Frene B, Roche N, Blondeel P, Monstrey S. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg. 2006;59(6):644-52.
16. Albino FP, Koltz PF, Ling MN, Langstein HN. Irradiated autologous breast reconstructions: effects of patient factors and treatment variables. Plast Reconstr Surg. 2010 Jul;126(1):12-6
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2018 Issue 1-
All articles in this issue
- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Securing intraoral skin grafts to the floor of the mouth: case report and technique desription
- Intraosseous haemangioma od the zygoma - case report and literature review
- Editorial
- Pedicled pectoralis major flap in head and neck reconstruction - our experience
- In memoriam: Professor Rajko Doleček
- Commemoration of professor Miroslav Fára, M.D., DSc.
- Use of licap and ltap flaps for breast reconstruction
- Image Possibilities of the Peripheral Nerve Tumor Using Magnetic Resonance Imaging - Case Report
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pedicled pectoralis major flap in head and neck reconstruction - technique and overview
- Use of licap and ltap flaps for breast reconstruction
- Editorial
- Intraosseous haemangioma od the zygoma - case report and literature review
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

