-
Medical journals
- Career
NUMERICAL EVALUATION OF SCAR AFTER BREAST RECONSTRUCTION WITH ABDOMINAL ADVANCEMENT FLAP
Authors: M. Kovář 1; L. Čapek 1; L. Vítová 2; M. Molitor 2
Authors‘ workplace: Technical University of Liberec, Department of Applied Mechanics, Liberec, Czech Republic 1; University Hospital Bulovka, Department of Plastic Surgery, Prague, Czech Republic 2
Published in: ACTA CHIRURGIAE PLASTICAE, 58, 1, 2016, pp. 12-17
INTRODUCTION
The treatment of breast cancer has developed a lot during the last decade; nevertheless it still remains a considerable social and economical problem all over the world. It is estimated that 89.2% of treated patients survive. However, this number still represents 22.2 deaths per 100,000 patients a year in raw data in the USA1. To complete the patient´s treatment, mastectomy should be followed by breast reconstruction. There are several surgical techniques used for breast reconstruction. Based on the surgeon´s suggestion, the patient can have a newly shaped breast with the use of a breast implant, his or her own tissue, or use the combination of both methods. The choice of the surgery depends on a patient´s protocol and the surgeon´s preferences. One of the used techniques is the advancement of an upper abdominal flap, skin and fat tissue in so called abdominal advancement flap (AAF). This flap pulls the elevated abdominal skin upward; this enhances the inferior portion of the breast and creates a new inframammary fold. This flap has been used for breast reconstruction in a combination with breast prosthesis for more than 40 years2. It can be used directly after previous expansion. It can also be called the lower thoracic advancement flap3.
The numerical analyses, mainly finite element method, are standardized tools in biomechanics. It is commonly used for stress prediction and deformation of human tissues or implant. In breast reconstruction the finite element method (FEM) is used for surgical intervention planning. Roose et al. proposed FEM as a pre-operative simulation tool for breast reconstruction4. They used the CT-MR data for the breast reconstruction and linear materials models used for soft tissue. They obtained promising results, according to the authors, with the mean geometrical error below 4 mm. The material of the implant was not specified. Palomar et al. used the FEM approach to predict the real breast deformation caused by gravity forces5. Azar et al. proposed a new method for guiding clinical breast biopsy6. The results showed that it is possible to create a deformable model of the breast based on finite elements with non-linear material properties, capable of modeling and predicting breast deformations in a clinically useful amount of time. Cardaso et al. developed a methodology and software that would allow estimating the form of the breast after surgery by knowing its form before the surgery7.
In all works mentioned above, surgical approach was not considered to be important, only the resulting deformation of the breast was analyzed. However, the aesthetical aspect of the resulting scar is of the same importance as the breast deformation itself. Moreover, faster recovery of the patient can be achieved8. The aim of the following study is to evaluate the stress concentration around the sutured wound and forces on stitches after using abdominal advanced flap approach for breast reconstruction. The following null hypotheses were tested:
- The anatomical and round implant resulted in the same loading of the wound sutures;
- The anatomical breast implant provides better aesthetical results.
MATERIAL AND METHODS
Modeling of the sutured skin flap used for breast implant placement was divided into the following two steps. At first, the material model of the selected silicone implant was identified. Secondly, the FEM model of the breast and implant was performed. The experiments were approved by the ethical committee at the University Hospital Bulovka, Czech Republic (file No. 2012/6456/EK-Z).
Experimental part – validation
Proposed standardized implant is based on the geometry of a round implant type POLYTECH 20725-285, size M. The experiment consisted of the compression tests of the real breast implant. The implant was placed into the testing machine Instron E3000 (Instron, USA) and pushed down up to 10 mm. The loading speed was 30 mm/min. After the load the implant was released and it was left to relax for one hour, the experiment was repeated four times. For a material model evaluation the following FEM model was performed. The geometry of the breast implants was scanned by NextEngine scanner (Next Engine, USA) and the geometry was cleaned in GeomagicStudio software (3D Systems, USA). The models were imported into MSC.MARC software (MSC.Software, USA). The breast implant was meshed using solid four-node elements. The lower surface of the implant was fully constrained and the upper spherical cup was loaded by a rigid surface towards the base. The material parameters of the breast implant were updated according to the experimental data.
Fig. 1. Position of scar on a virtual model 
Numerical model
In this study a three dimensional torso of a woman (29 years old) was used for the experiment. In the place of a removed breast an incision for implant insertion was made (Fig. 1). The implant models include round implant size M, 285 ml (Polytech, USA) and the anatomical implant size MX, 290 ml (McGhan, USA). The model was discretized by four-node shell elements used for the torso and four-node solid elements for the implant in the MSC.MARC software. The model consists of 32762 elements, with an average aspect ratio quality of 0.7. The material model of breast implant was assumed to be isotropic and homogenous, defined by Young’s moduli and Poisson’s ratio gained from previous experiments (E=0.26 MPa, ν = 0.1). The large strain isotropic model was used to represent the human skin. This constitutive model is based on Ogden energy strain function. The Ogden model is one of various strain energy functions and is very often used to describe soft tissues9,10. The strain energy function is written in terms of the principal stretches as
where μn and αn are material constants, K is the initial bulk modulus and li is the principal stretch.
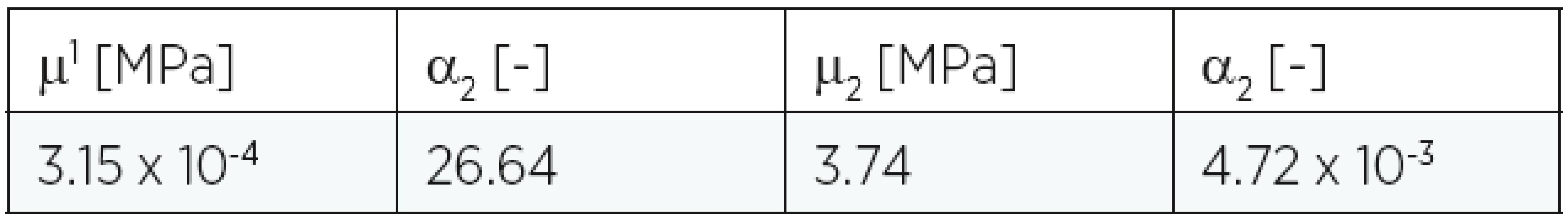
The material constants are summarized in Table 1, and they correspond to those mentioned in the literature11. For numerical purposes the real clinical situation was simplified:
- The thickness of human skin was 1 mm.
- The size of elements was chosen according to convergence of results and distance between stitches in a real surgery.
- The boundary conditions consisted of the breast implant movement towards the 10 mm torso and constraining (zero xyz movement) the lateral nodes of the torso.
- The contact between the implant and skin was supposed to be without friction.
- The pre-stress of human skin was neglected.
1. Material constants for Ogden model 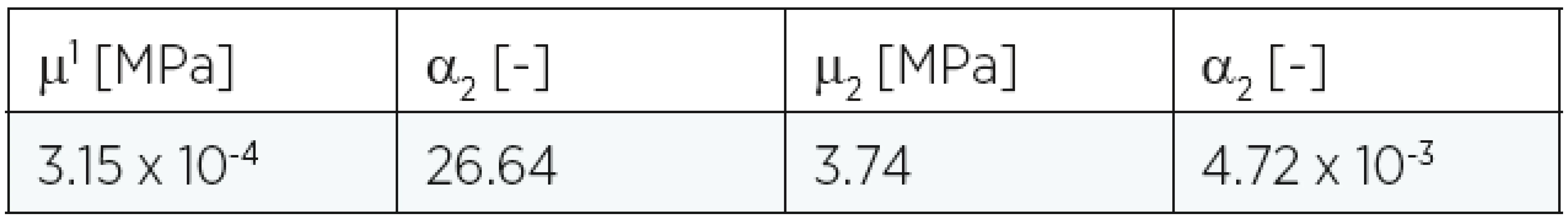
After having the breast shape completed a final opened wound appeared and its raw dimensions were measured (Fig. 2). Next step consisted in virtual suturing of the wound. The real stitches were substituted by trust elements put into their positions. The negative coefficient of thermal expansion (-0.1 K-1) was assigned to all trust elements. This step has no physical meaning but ensures the suturing procedure. The suturing procedure was performed by thermal treatment of the trust elements, so that the opposite nodes of the wound were pushed together. After suturing the whole wound, the mesh model was frozen and exported into the stereolithographic format. The mesh of healthy and reconstructed breast was compared and analyzed by means of inspection tools of the Geomagic software. The maximal and minimal geometrical difference was found.
Fig. 2. Dimensions of resulted wound after placing breast implants (anatomical implant – left, round implant – right) 
RESULTS
Experimental part
The force displacement curves of compressed implant are nonlinear for all the cases. The maximal force of 120 N was reached at 10 mm of compression. The fitted material model to the measured data can be seen in (Fig. 3).
Fig. 3. Force displacement curve of uniaxial compression experiment 
Simulation part
Evaluation of geometrical accuracy was performed by measuring the Euclid distance between each node on the deformed and non-deformed mesh of the breast. The resulted color map of the deviation is shown in Fig. 4 and Fig. 5. The maximal deviation for anatomical and round implant is on the lower surface of the breast and upper surface of the breast, while in the region of lateral geometry and the area around nipple the agreement reaches very high level. The maximal and mean deviation for both models are shown in Table 2.
Fig. 4. Geometric deviation after breast reconstruction for anatomical implant 
Fig. 5. Geometric deviation after breast reconstruction for round implant 
The geometry of opened wounds resemble an elliptical shape. The dimensions are 97 x 16.8 mm after application of the anatomical implant and 95.4 x 18.7 mm after application of the round one. The Cauchy equivalent stress distribution around the sutured wound is presented. In case of anatomical breast implant the maximal equivalent of Cauchy stress is located around the nipple more medially and reaches the value of 329 kPa (Fig. 6). All stiches are loaded by tension. The maximal tension is located in two median stitches (Fig. 7). The maximal force reaches 0.025 N. In case of the round breast implant the equivalent Cauchy stress is also located around the nipple more medially and reaches the value of 380 kPa (Fig. 8). The maximal tension is located in two median stitches (Fig. 9). The maximal force reaches 0.0213 N.
Fig. 6. Equivalent of Cauchy stress around scar after breast reconstruction by anatomical implant 
Fig. 7. Resultant forces in stiches for anatomical implant 
Fig. 8. Equivalent of Cauchy stress around scar after breast reconstruction by round implant 
Fig. 9. Resultant forces in stiches for round implant 
DISCUSSION
Clinical practice shows that there are still many gaps in knowledge to be sorted with regards to patients’ satisfaction with breast reconstruction. Much attention was put on the research of factors such as smoking, obesity, hypertension and age12–15. Nevertheless, prediction of the resulting scar after breast reconstruction has not been studied yet. There are several studies dealing with numerical analyses of skin wound suturing16–23. The common practice consisting in prescribing the displacement into opposite nodes of the skin wounds is used in these studies. The aim of this study was to evaluate the stress concentration around the sutured wound of the skin flap used for breast implant placement. In our models the new approach of modeling the stiches was used.
From our results it can be seen, that the anatomical and round breast implants did not result into the same scar loading. The differences are in the distribution of equivalent Cauchy stress around the resulted scar and its values. The maximal value difference reaches 13.4% between the stress values for these two breast implants, while the round implant resulted in higher loaded scar than the anatomical one. The first null hypothesis was not proved. Numerical analyses show that the maximum tension is concentrated in two stitches medially oriented in the sutured wound for both cases. Generally, this corresponds with surgeons´ experience. There is a lack of information regarding forces acting on stiches in different skin wounds. The force values correspond with those mentioned by Flynn on the planar models24, nevertheless in these models the natural pre-stress of human skin was not considered. Capek et al. demonstrated that the difference between numerical models with zero pre-stress and maximal pre-stress can reach up to 44%11. Nelson et al. developed a model to predict risk complications in wound healing after breast reconstruction25. Werfully et al demonstrated that tensile forces depend on the stability of the blood clot and subsequently on the biochemical and mechanical properties of the wound bed26. According to our results the skin tension as an implant shape function could be another risk factor in wound healing.
The evaluation of aesthetical results from these two implants showed that the anatomical implant provides better results. It means that it fitted better to real geometry of the healthy breast. The highest geometrical inaccuracy appears in the area bellow the breast. The second null hypothesis was proved. It should be mentioned that comparison of a healthy and reconstructed breast from a pair might be misleading due to varying asymmetry of the female breast27. On the other hand, Yip et al. demonstrated that the breast symmetrization procedure is important and valuable for increasing satisfaction with the breast, however this is not the major outcome determinant in breast reconstruction28.
Some limitations of this study should be pointed. The anisotropy of human skin and natural pretension were not considered in our models. In fact, the incision is usually made along Langer´s lines to minimize the wound opening in the perpendicular direction. Moreover the mastectomy was done virtually, so the simulation does not take into the consideration the real geometry of the patient after the surgery. Our next clinical study is going to evaluate the calculated force by in vivo experiments and by determining of the skin press-stress around the human breast.
CONCLUSION
The knowledge of the scar after breast reconstruction is of high importance from the aesthetical point of view. Reduction of mechanical forces around the scar prevents abnormal scar formation29. Delayed wound healing is costly for the patient with breast reconstruction. The aesthetical aspect of the resulted suture is of the same importance as the breast deformation itself. In spite of this stress distribution on a resulted scar after breast reconstruction was not studied yet, according to our knowledge. It was shown, that by using numerical approach, real prediction of aesthetical results can be achieved.
Acknowledgement
This publication was written at the Technical University of Liberec as part of the project “Research of mechanical properties of selected living tissue and materials used in medicine” with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2016.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
Corresponding author:
Lukáš Čapek, M.D.
Technical University of Liberec, Department of Applied Mechanics
Studentská 2, 46 117 Liberec 1
Czech Republic
E-mail: lukas.capek@tul.cz
Sources
1. Surveillance, Epidemiology, and End Results Program [Internet]. National Cancer Institute, USA. 2011. Available from: http://seer.cancer.gov/statfacts/html/breast.html
2. Lewis JR Jr. Use of a sliding flap from the abdomen to provide cover in breast reconstructions. Plast Reconstr Surg. 1979 Oct;64(4):491–7.
3. Ryan JJ. A lower thoracic advancement flap in breast reconstruction after mastectomy. Plast Reconstr Surg. 1982 Aug;70(2):153–60.
4. Roose L, Maerteleire W, Mollemans W, Maes F, Suetens P. Biomedical Simulation. Berlin: Springer Berlin Heidelberg; 2006. Chapter, Simulation of Soft-Tissue Deformations for Breast Augmentation Planning; p. 197–205.
5. del Palomar, AP., Calvo, A., Herrero, B., Lopez, J., Doblare, JM. A finite element model to accurately predict real deformations of the breast. Med Eng Phys. 2008;30 : 1089–1097.
6. Azar FS, Metaxas DN, Schnall MD. Methods for modeling and predicting mechanical deformations of the breast under external perturbations. Med Image Anal. 2002 Mar;6(1):1–27.
7. Cardoso A, Coelho G, Zenha H, Sá V, Smirnov G, Costa H. Computer simulation of breast reduction surgery. Aesthetic Plast Surg. 2013 Feb;37(1):68–76.
8. Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16(6):547–67.
9. Holzapfel G, Ogden R. Biomechanics of soft tissue in cardiovascular systems. Springer, Udine, 2003.
10. Lim J, Hong J, Chen W, Weerasooriya T. Mechanical response of pig skin under dynamic tensile loading. J Imp Eng. 2011;38 : 130–135.
11. Capek L, Jacquet E, Dzan L, Simunek A. The analysis of forces needed for the suturing of elliptical skin wounds. Med Biol Eng Comput. 2012;50(2):193–8.
12. Butler PD, Nelson JA, Fischer JP, Chang B, Kanchwala S, Wu LC, Serletti JM. African-American women have equivalent outcomes following autologous free flap breast reconstruction despite greater preoperative risk factors. Am J Surg. 2015 Apr;209(4):589–96.
13. Naoura I, Mazouni C, Ghanimeh J, Leymarie N, Garbay JR, Karsenti G, Sarfati B, Leduey A, Kolb F, Delaloge S, Rimareix F. Factors influencing the decision to offer immediate breast reconstruction after mastectomy for ductal carcinoma in situ (DCIS): the Institute Gustave Roussy Breast Cancer Study Group experience. Breast. 2013 Oct;22(5):673–5.
14. Nelson JA, Fischer JP, Radecki MA, Pasick C, McGrath J, Serletti JM, Wu LC. Delayed autologous breast reconstruction: factors which influence patient decision making. J Plast Reconstr Aesthet Surg. 2013 Nov;66(11):1513–20.
15. Somogyi RB, Webb A, Baghdikian N, Stephenson J, Edward KL, Morrison W. Understanding the factors that influence breast reconstruction decision making in Australian women. Breast. 2015 Apr;24(2):124–30.
16. Chaudhry HR, Bukiet B, Siegel M, Findley T, Ritter AB, Guzelsu N. Optimal patterns for suturing wounds. J Biomech. 1998 Jul;31(7):653–62.
17. Danielson DA, Natarajan S. Tension field theory and the stress in stretched skin. J Biomech. 1975 Mar;8(2):135–42.
18. Kirby SD, Wang B, To CW, Lampe HB. Nonlinear, three-dimensional finite-element model of skin biomechanics. J Otolaryngol. 1998 Jun;27(3):153–60.
19. Larrabee WF Jr, Galt JA. A finite element model of skin deformation. III. The finite element model. Laryngoscope. 1986 Apr;96(4):413–9.
20. Lott-Crumpler DA, Chaudhry HR. Optimal patterns for suturing wounds of complex shapes to foster healing. J Biomech. 2001 Jan;34(1):51–8.
21. Retel V, Vescovo P, Jacquet E, Trivaudey F, Varchon D, Burtheret A. Nonlinear model of skin mechanical behaviour analysis with finite element method. Skin Res Technol. 2001 Aug;7(3):152–8.
22. Yoshida H, Tsutsumi S, Mizunuma M, Yanai A. Three-dimensional finite element analysis of skin suture. Part 1: spindle model and S-shaped modified model. Med Eng Phys. 2000 Sep;22(7):481–5.
23. Yoshida H, Tsutsumi S, Mizunuma M, Yanai A. A surgical simulation system of skin sutures using a three-dimensional finite element method. Clin Biomech (Bristol, Avon). 2001 Aug;16(7):621–6.
24. Flynn C. Finite element models of wound closure. J Tissue Viability. 2010 Nov;19(4):137–49.
25. Nelson JA, Chung CU, Fischer JP, Kanchwala SK, Serletti JM, Wu LC. Wound healing complications after autologous breast reconstruction: a model to predict risk. J Plast Reconstr Aesthet Surg. 2015 Apr;68(4):531–9.
26. Werfully S, Areibi G, Toner M, Bergquist J, Walker J, Renvert S, Claffey N. Tensile strength, histological and immunohistochemical observations of periodontal wound healing in the dog. J Periodontal Res. 2002 Oct;37(5):366–74.
27. Liu YJ. Aesthetics of the Female Breast: Correlation of Pluralistic Evaluations with Volume and Surface Area. (2009). Yale Medicine Thesis Digital Library. Paper 172. Available on: http://elischolar.library.yale.edu/ymtdl/172
28. Yip JM, Watson DI, Tiggemann M, Hsia S, Smallman AE, Dean NR. Determinants of breast reconstruction outcome: How important is volume symmetry? J Plast Reconstr Aesthet Surg. 2015 May;68(5):679–85.
29. Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011 Sep;19 Suppl 1:s2–9.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2016 Issue 1-
All articles in this issue
- NUMERICAL EVALUATION OF SCAR AFTER BREAST RECONSTRUCTION WITH ABDOMINAL ADVANCEMENT FLAP
- TRANSPLANTATION OF VASCULARIZED COMPOSITE ALLOGRAFTS. REVIEW OF CURRENT KNOWLEDGE
- TRACHEAL ALLOTRANSPLANTATION AND REGENERATION
- PULMONARY EMBOLISM AFTER ABDOMINOPLASTY – ARE WE REALLY ABLE TO AVOID ALL COMPLICATIONS? CASE REPORTS AND LITERATURE REVIEW
- USE OF OSTEOTOMY IN POST-TRAUMATIC DEFORMITY OF FRONTAL SINUS ANTERIOR WALL. CASE REPORT
- IS NON-TRAUMATIC NAIL DYSTRPOPHY ONLY DUE TO CHRONIC ONYCHOMYCOSIS? THE ONYCHOMATRICOMA. CASE REPORT
- SALUTATIO ET LAUDATIO AD ANNIVERSARIUM PROFESSORIS WILLIAM GUNN
- EVALUATION OF COMPLICATIONS AFTER ENDOSCOPY ASSISTED OPEN REDUCTION AND INTERNAL FIXATION OF UNILATERAL CONDYLAR FRACTURES OF THE MANDIBLE. RETROSPECTIVE ANALYSIS 2010–2015
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- IS NON-TRAUMATIC NAIL DYSTRPOPHY ONLY DUE TO CHRONIC ONYCHOMYCOSIS? THE ONYCHOMATRICOMA. CASE REPORT
- PULMONARY EMBOLISM AFTER ABDOMINOPLASTY – ARE WE REALLY ABLE TO AVOID ALL COMPLICATIONS? CASE REPORTS AND LITERATURE REVIEW
- TRANSPLANTATION OF VASCULARIZED COMPOSITE ALLOGRAFTS. REVIEW OF CURRENT KNOWLEDGE
- EVALUATION OF COMPLICATIONS AFTER ENDOSCOPY ASSISTED OPEN REDUCTION AND INTERNAL FIXATION OF UNILATERAL CONDYLAR FRACTURES OF THE MANDIBLE. RETROSPECTIVE ANALYSIS 2010–2015
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career