-
Medical journals
- Career
Vasospasm of the Flap Pedicle - The New Experimental Model on Rat
Authors: P. Hýža 1; L. Streit 1; D. Schwarz 2; T. Kubek 1; H. E. Gilboe 3; J. Veselý 1
Authors‘ workplace: Department of Plastic and Aesthetic Surgery, St. Anne University Hospital, Brno 1; Institute of Biostatistics and Analyse, Masaryk University, Brno 2; Faculty of Medicine, Masaryk University, Brno, Czech Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 56, 1-2, 2014, pp. 3-11
Introduction
The vasospasm is a frustrating problem that microsurgeons are often confronted with. The vasospasm is a functional problem that usually resolves spontaneously. However, flap failure may follow due the vasospasm if it is prolonged (1–5). We can define vasospasm as a local and persistent spastic contraction of smooth muscles in the vessels that is caused by pathological stimulus (2,6). The vasospasm should be considered as a separate clinical entity and distinguished from systemic vasoconstriction. Systemic vasoconstriction is caused by central nervous system regulating pathways and vasoconstriction of the peripheral vessel is generalized. Vasospasm is a local vasoconstriction of the vessel and it is less influenced by central nervous system (7).
Both artery and vein may be affected by vasospasm. Four types of vascular vasospasm according to its location have been observed in a dissected flap: segmental spasm of a part of the pedicle; spasm of the whole pedicle; spasm of the small vessels inside the flap and “tissue shock of the flap” (irreversible process corresponding with no-reflow phenomenon) (3,4). Pathogenesis of vasospasm is complex and often associated with other pathological entities, such as mechanism of haemostasis, ischemia–reperfusion injury of the flap or no-reflow phenomenon (8–13). Studying pathological physiology of the vasospasm in clinical conditions is not easy and flap survival may be at risk if the vascular pedicle was traumatized. Therefore, in-vitro or in-vivo experimental models are mostly used by researchers.
The first aim of our study was to compare the effects of different types of surgical manipulations (stimuli) that can provoke vasospasm during flap dissection. The second aim was to choose the proper stimulus for creating an experimental model that would be used for subsequent testing of vasodilating drugs on vasospasm.
Material and methods
185 male Wistar rats were used. The median weight of the rats was 326 g (SD ± 64 g). The experiment was approved by the institutional and ethics committee at St. Anne University hospital in Brno. The measurements were conducted under standardized temperature (24-25°C) and light conditions. The rats were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg). Ether was used at the beginning of anaesthesia. All dissections were performed using a standardized technique by the same surgeon.
The dissection technique: The holder for laser-Doppler probe (small straight probe 407-1, Perimed AB, Jarfalla, Sweden) was placed on the skin at an exactly defined point (2 cm cranially and laterally from the genital tubercle). The groin flap based on inferior superficial epigastric artery pedicle was marked around the skin paddle. The subcutaneous tissue at the edges of the flap was first clamped with a haemostat to ensure bloodless dissection and then cut with scissors. Next, the flap was raised from lateral to medial using the scissors to sharply disect loose tissue just above the fascia of the thigh muscles. The flap pedicle was traced on the under-surface of the flap and carefully protected from touching, cutting, pulling or other injuries. The pedicle was located on the medial aspect of the flap. At this point the pedicle was sharply dissected leaving a thick layer of fat and connective tissue surrounding it (Fig. 1). Two adventitial, distally based, 0.5 cm long flaps were created on the pedicle. One of them included the inferior epigastric nerve. Only a very thin adventitial layer was left covering the remaining pedicle vessels (Fig. 2). The next steps of the flap dissection depended on which of the four different types of surgical trauma were tested: pulling on the pedicle; compression of the pedicle; blood applied on the pedicle; and dissection of the vessels.
Fig. 1. Flap dissection. The flap was dissected sharply and the pedicle was not touched by instruments neither it was pulled or traumatized in any other way to prevent induction of vasospasm 
Fig. 2. Pedicle dissection. Two adventitial flaps were created on both sides of the pedicle using sharp dissection by scissors 
Group 1 - Pulling on the pedicle: The weight was attached to the adventitial flaps described above by the atraumatic monofilament nylon suture (Premilene 6-0). The weight was gently hanged on the roller and the pedicle was pulled with different weights, providing four groups: “weight 10g, weight 15g, weight 20g and weight 25g”.
Group 2 - Compression of the pedicle: The pedicle was clamped by vascular clamps. Biemer’s vascular clamp MN2200 and MN2210 with closing pressure of 30–40g and 20–25g (Asanus GmbH, Neuhausen ob Eck, Germany) were used in “arterial clamp” and “venous clamp” groups respectively. The haemostat was clamped over the arterial clamp (MN2200) to achieve higher closing pressure in the group “arterial clamp + haemostat” (Fig. 3).
Fig. 3. Compression of the pedicle by vascular clamps and vascular clamp + hemostat 
Group 3 - Blood applied on the pedicle: Presence of the blood on the pedicle was a stimulus that provoked vasospasm in this group. A branch of the pedicle was cut and bleeding from this branch covered the pedicle in the “bleeding” group. Blood was taken from the tail of the previously operated rat and applied on the pedicle in “blood” group (Fig. 4).
Fig. 4. Blood applied on the pedicle 
Group 4 - Dissection of the vessels: The artery and the vein of the pedicle were dissected from each other by opening the micro-scissors in the whole length of the pedicle in the group with “dissection of the vessels” (Fig. 5).
Fig. 5. Dissection of the vessels 
Timing of the experiment: Each step of the experiment was defined by appropriate time period using Perisoft for Windows software script. Each step was then separated by voice signal that allowed us to conduct the experiment according to an exact timing (Table 1).
1. Timing of the experiment. Time t=0 was defined as the end of the stimulus that provoked the vasospasm. From this point, the duration of the vasospasm was measured 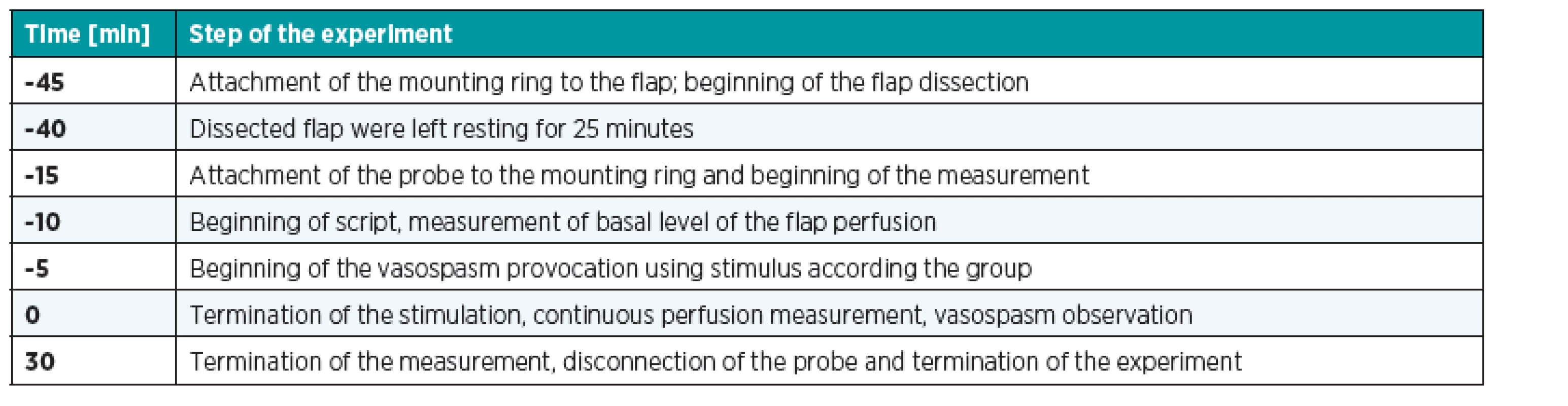
Laser Doppler perfusion measurements: A laser Doppler flow-meter (PeriFlux system 5000; Periflux 5010 LDPM unit, small straight probe 407-1 and mounting ring PH 07-4, Perimed AB, Jarfalla, Sweden) was used to measure blood perfusion through the dissected flap. The Periflux 5010 LDPM unit was attached to the probe just before measurement beginning. Sampling period was set up to τ=0.03s. Laptop with Perisoft for Windows software (PeriSoft for Windows 2.5., Perimed AB, Jarfalla, Sweden) was used as a signal recorder. All the values from the Laser Doppler flow-meter were automatically processed by PeriSoft for Windows software into ASCII for each measurement (Fig. 6).
Fig. 6. Example of a “typical” perfusion curve shape visualized in PeriSoft for Windows software. Perfusion value is minimized due to stimulus application right after a period of perfusion stabilization for at least 5 minutes. Low value of perfusion persists for certain period of time after termination of vasospasm stimulation. Blood perfusion is than restored and stabilized again. Reference time t=0 is in this case time 0:30 minutes (termination of vasospasm stimulation). We can see gradual reperfusion and consequential stabilization of perfusion after dissection of the flap on the left hand side of the green marked area – it can be understood as a justification of the resting time between flap dissection and beginning of the measurement 
Signal Processing: We categorized each measurement according to its perfusion curve shape as typical and atypical (see Fig. 6). All the measurements with atypical perfusion curve were excluded from consequential statistical processes due to impossible detection of appropriate time parameter (artefacts, errors in measurement). Savitzky-Golay polynomial filter was employed to smooth the signal that was corrupted by impulse noise. Then, two important time periods “tB” and “tC” were extracted from the signals using Matlab (MathWorks, Inc., Natick, MA, U.S.A) scripts. The time “tB” was considered to be an indicator of duration of the vasospasm and was defined as the period between t=0 and the time to reach the limit in the perfusion curve (Fig. 7; see Table 1). The time “tC” was defined as the period between t=0 and the time when the re-perfusion reached its maximal value – time to the perfusion restoration. Time “tA” represented duration of traumatisation (stimulation). This variable was arbitrary set as 0 [s] in “dissection of the vessels; blood and bleeding” groups because durations of the stimulation were negligibly short. tA lasted 5 minutes in the other groups. Time parameters tB a tC were first compared among the following stimulus categories (pulling the pedicle, compression of the pedicle and blood applied on the pedicle – Table 2). Then these groups (from relevant stimulus categories mentioned above) were compared with “dissection of the vessels” group.
Fig. 7. Detection of time parameters and amplitudes of flap perfusion. Duration of the vasospasm tB was calculated separately for each measurement as the time period between t=0 and the time to reach certain limit in the perfusion curve when the value of the perfusion reached certain limit. This limit represents beginning of re-perfusion and it is defined as 10<sup>th</sup> percentile of values of perfusion between vC and vD. vC was defined as the signal level when the values of perfusion were stabilized after stimulus releasing just before beginning of reperfusion; vD as the maximal value of re-perfusion. 
Results
Basic statistical parameters were calculated for both time indicators tB and tC. Medians for each group are provided in the Table 2 together with numbers of eliminated atypical measurements (see Table 2). Only 5 measurements were performed in “weight 25g” group, because there was extensive number of anatomical changes of the pedicle and atypical measurements, and this group was excluded from further statistical comparisons.
2. tB and tC median values and classification of vasospasm activity. The table shows the distribution of groups as well as their inclusion in the relevant stimulus category. Numbers of eliminated measurements and non reperfusions are mentioned 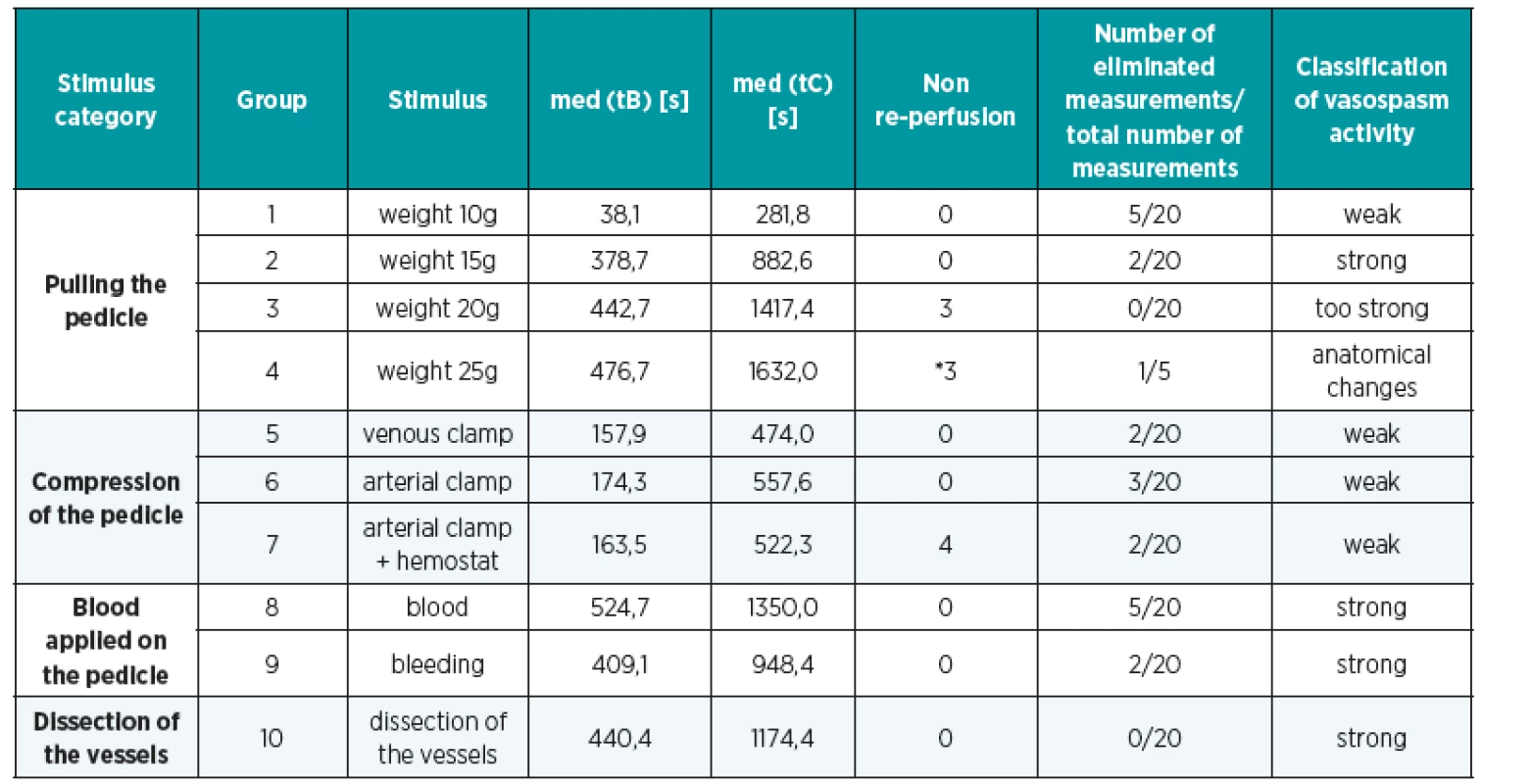
*Only 5 measurements were done in “weight 25g” group for anatomical damage of the vessels a thrombosis of the pedicle. Too high tension of the pedicle was the cause and no reperfusion was the consequence. These 5 measurements were excluded from next statistical comparison. Comparison of vasospasm duration according to the stimulus category
Non parametric ANOVA (Kruskal-Wallis, K-W) test proved significant variation between the medians of tB (P=8.25x10-6) and tC (P=1.56x10-5) in the stimulus category “pulling the pedicle”. The results of consequential Wilcoxon tests after K-W analysis revealed significant differences only between “weight 10g” group and the others (Fig. 8, Table 3).
3. Results of Wilcoxon (rank-sum) tests for tB in „pulling the pedicle” category. P-value and P-value after Bonferroni corrections (in bracket) are mentioned in the table 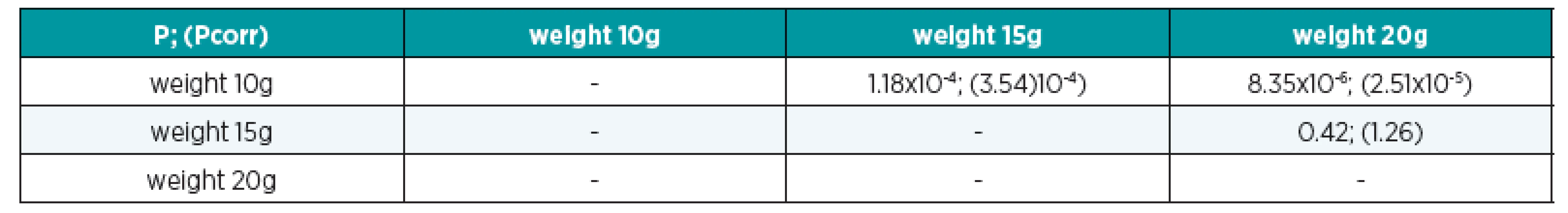
Fig. 8. BW diagram for tB values for “pulling the pedicle” stimulus category. Red line points at median, blue box edges represent quartiles, the score marks identifies ratio of the median, whiskers show 1.5x inter-quartile range, red crosses are outliers 
Non parametric ANOVA test did not manage to prove significant variation between the medians in the stimulus categories “compression of the pedicle” (tB (P=0.268) and tC (P=0.078)) and “blood applied on the pedicle” (tB (P=0.320) and tC (P=0.220) ). The subsequent Wilcoxon tests were therefore not done (Fig. 9, Fig. 10).
Fig. 9. BW diagram for tB values for “compression of the pedicle” stimulus category 
Fig. 10. BW diagram for tB values for “blood applied on the pedicle” stimulus category 
The comparison of the stimulus categories with the reference group “dissection of the vessels”
This group was chosen as a reference group because the stimulus corresponds best with the clinical situation during flap dissection. Unfortunately this stimulus is not precisely defined and easily repeatable.
Non parametric ANOVA test proved significant variation between the medians tB (P=8.25x10-6) and tC (P=1.56x10-5) in stimulus category “pulling the pedicle” and the reference group. The results of consequential Wilcoxon tests are mentioned (Fig. 11; Table 4).
4. The results of Wilcoxon (rank-sum) tests for “pulling the pedicle” stimulus category and the reference group for time parameter tB 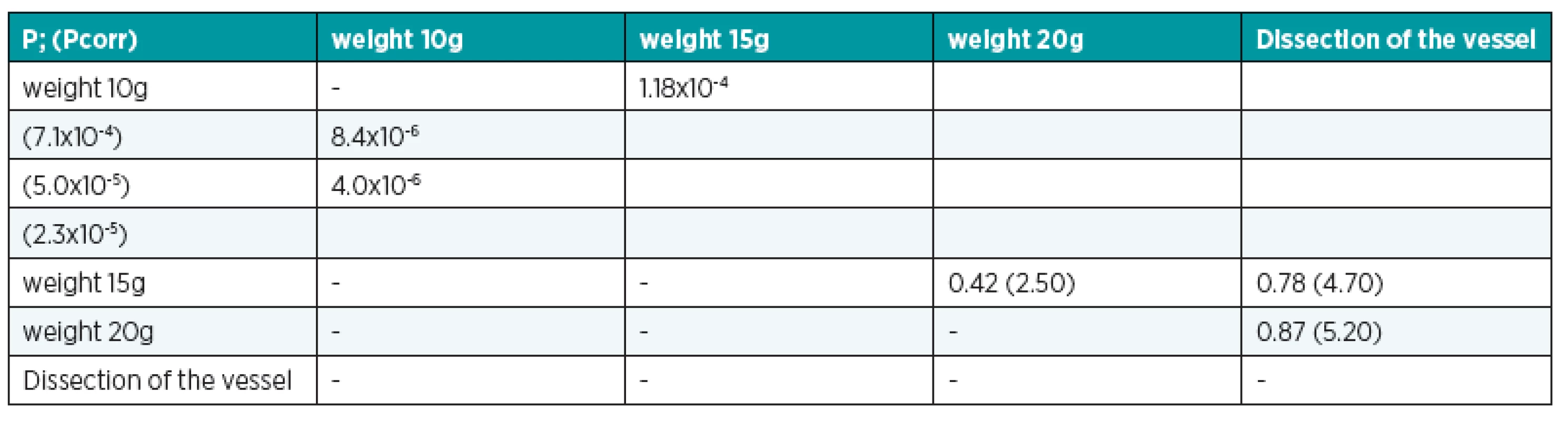
Fig. 11. BW diagram for tB values for “pulling the pedicle” stimulus category and the reference group 
Non parametric ANOVA test proved significant variation between the medians tB (P=6.4x10-7) and tC (P=8.7x10-5) in stimulus category “compression of the pedicle” and the reference group (Fig. 12; Table 5).
5. The results of Wilcoxon (rank-sum) tests for “compression of the pedicle” stimulus category and the reference group for time parameter tB 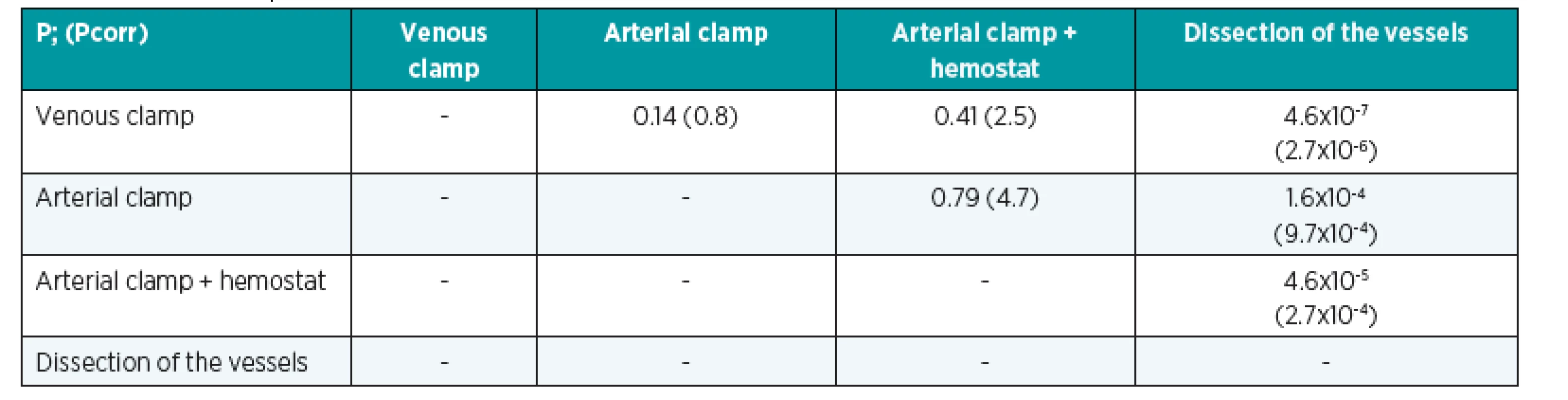
Fig. 12. BW diagram for tB values for “compression of the pedicle” stimulus category and the reference group 
Non parametric ANOVA test did prove significant variation between the medians of tB (P=0.457) and tC (P=0.291) in stimulus category “blood applied on the pedicle” and the reference group.
The comparison of the selected groups between appropriate stimulus categories
The groups that had been evaluated by the previous analysis as the most suitable for development an experimental model, “weight 15g; blood and bleeding, were tested using non parametric ANOVA test. The significant difference was not proved between the groups (tB (P=0.360) and for tC (P=0.420)). We may consider these three groups as analogical with regards to the duration of the vasospasm.
The average signal amplitudes and categorization of the stimuli
The average signal amplitudes were created for each group by calculating the mean of perfusion unit values every 0.03s. We displayed all the extracted average signal amplitudes in one single picture (Fig. 13). This extraction allowed us to categorize two different units of stimuli: the “weak” stimuli that provoke short lasting or no vasospasm, and the “strong” stimuli that provoke long lasting vasospasm. When we applied this categorization in Table 2, we could find that all medians of time parameter tB were always longer than 300s (5 minutes) for “strong” stimuli. Conversely, all medians of time parameter tB were always shorter than 180s (3 minutes) for “weak” stimuli. The “strong” stimuli included dissection of the vessels, pulling the pedicle using 15g, 20g and 25g weights and presence of the blood around the pedicle. The weak stimuli included compression of the pedicle using vascular clamps and pulling the pedicle using 10g weight.
Fig. 13. The average signal amplitudes 
We used the method of elimination to choose the appropriate stimulus for creation of the experimental model that would be used for subsequent testing of vasodilating drug on vasospasm. We choose the appropriate one from the “strong” stimuli, as we believe that the spasm duration of 5 minutes is long enough to study the effectiveness of drugs. We observed too many cases of non-reperfusion or anatomical damages of the vessels in the group of weight 20g and 25g (see Table 2). Presence of blood around the flap would not allow us to maintain an exact concentration of the drug. Between the two remaining groups, we considered a higher risk of subjective error in “dissection of the vessel” group comparing with “weight 15g” group. The stimulus pulling the pedicle by 15g weight was chosen as the most appropriate for development of the experimental model.
Discussion
Pathogenesis of vasospasm is a complex process often associated with several pathological mechanisms (haemostasis, ischemia–reperfusion injury of the flap and no-reflow phenomenon).
Three possible causes of vasospasm are cited:
- Direct smooth muscles damage, which is associated in clinical practice with flap dissection and manipulations of flap pedicle. Wingard proved that smooth muscle distension causes myosin light chain phosphorylation and muscle contraction known as myogenic response (14).
- Perivasal blood presence leading to vasospasm of adjacent vessels was documented by neurosurgeons during subarachnoid bleeding (15).
- Endothelial damage disrupting the physiological equilibrium between spasmogenic (endothelin-1, TXA2, free oxide radicals) and vasodilating substances (NO, PGE1, PGI2) may lead to the vasospasm formation (16).
Four possible pathophysiological processes of endothelial damage are discussed:
- Micro-traumatisation of the vascular endothelium – reduced production of NO, PGE1, PGI2 by endothelial cells and thrombocytes aggregation with increased production of serotonin and TXA2 provoke vasospasm (17,18).
- Free oxide radicals overproduction during flap reperfusion – NO – nitroxide is inactivated and production of PGE1, PGI2 is inhibited by free oxide radicals that are produced by tissue ischemia (19,20).
- Endothelin-1 production or overproduction from damaged endothelial cells (21–23). However, we did not confirm the causality of the vasospasm pathogenesis and expression of ET-1 in our previous study (24).
- Reduction of NO production by endothelial cell during reperfusion as a consequence of endothelial ischemia (22,25-26).
The basic aim of our study was to find out what kind of surgical manipulation was mostly responsible for vasospasm. Pulling the pedicle longitudinally, compression of the pedicle using a vascular clamp and presence of the blood around the pedicle were the particular stimuli used to simulate and simplify complex clinical situation during pedicle dissection. Dissection of the vessels of the pedicle was the most corresponding with the clinical situation during flap dissection. This was - on the other hand - the least precisely defined stimulus and the most influenced by variability and subjective approach of the surgeon. “Dissection of the vessels” was used for these reasons as a reference group.
The reasons for selecting these surgical stimuli for our comparison were the following:
- Preparation of the pedicle from surrounding tissue may cause a significant loss of endothelial cells although blood flow through the vessel is continuing (27). Provocation of the vasospasm was observed and proved after adventitial stripping (28).
- Tensile stress on the pedicle induced by the end of the instrument may be considerable during flap dissection.
- Endothelial damage caused by micro-vascular clamp is frequent and directly proportional to the pressure on the clamp. Closing pressure of the clamps should be lower than 30g/mm2 to minimize the risk of vascular damage (29-30).
- Process of haemostasis is ongoing and vasoconstrictors are released if a small branch of the pedicle is cut. Presence of the blood from the surrounding tissues itself may provoke vasospasm. Hemoglobin and the other vasoconstrictors contained in blood were probable spasmogens (15). We compared separately the effect of bleeding from the pedicle branch and the effect of the blood applied from exterior (31).
Use of laser Doppler device allowed us to obtain an exact continuous and objective record of the flap perfusion (32). The disadvantages include its sensitivity to the slightest movements and the fact that the laser Doppler flow-meter cannot provide absolute perfusion values. Measurements are expressed as Perfusion Units (PU). The indicators of perfusion grade were not considered to be suitable for the evaluation of vasospasm because we observed a significant dispersion of reference values in PU. Time parameters of perfusion progress were chosen for that reasons.
Longitudinal tension on the pedicle applied by pulling the adventitial flap with a weight hanging on a thread on the block demonstrated weight-dependent duration of the vasospasm. Duration of the vasospasm was significantly shorter only in “weight 10g” group in the stimulus category with pulling the pedicle. The 20g and 25g weight were too heavy for the purpose of creating experimental model because anatomical damage of the vessel wall, macroscopic thrombosis and no-reperfusion phenomenon occurred several times in these groups. We cancelled further experiments in “weight 25g” group after 5 measurements for high number of these incidents. 15g weight caused vasospasm lasting for a significant period c mparable to those mentioned above. No significant differences were found between the reference group “dissection of the pedicle” and pulling the pedicle with weights above 15g. Thus, pulling the pedicle using 15g weight provoked the same intensity of the vasospasm as if it was provoked by dissection of the vessels of the pedicle. Moreover, the stimulus was uncomplicated; exactly defined; easily repeatable; it provoked a reliably long vasospasm. The longitudinal tension applied on the pedicle was chosen as the most suitable stimulus for further investigation of the drugs for relieving vasospasm. The conclusion can also be made that the tension exerted on the pedicle should be avoided during flap dissection, because it consistently provokes vasospasm of pedicle vessels.
Clamping of the vessels produced only short-lasting vasospasm. Moreover, when the strength of microvascular clamps was forced using a haemostat, the stimulus was too strong and too many cases of anatomical damage and thrombosis of the pedicle vessels occurred in this group. In cases when thrombosis did not develop, the duration of vasospasm was on average not longer than in the groups using microvascular clamps alone. Gentle holding the vessel with atraumatic forceps may be recommended for manipulation of the small vessels during flap dissection, carefully avoiding excessive to damage the vessel and develop thrombosis.
The presence of blood on the pedicle, either from the tail of another rat or from a branch of the pedicle, caused long lasting vasospasm. There was no significant difference between these groups. This stimulus was, however, not considered as suitable for further investigation of vasospasm, because blood would have had to be removed before the tested drugs could have been applied on the vessels. Nevertheless, the conclusion can be made that bleeding should be avoided during flap dissection to avoid vasospasm.
Conclusion
Effects of different type of surgical manipulation that can provoke vasospasm have been compared. Pressure produced by the vascular clamps and tensile stress caused by using 10g weight provoked short vasospasm (“weak” stimuli). “Strong” stimuli had the ability to provoke significantly longer vasospasm: dissection of the vessel of the pedicle, blood applied on the pedicle and pulling the pedicle by using 15g, 20g and 25g weight. A new experimental model for studying the effects of different vasodilating drugs on vasospasm was constructed. Pulling the pedicle using 15g weight was the most appropriate for this purpose. The fact that certain stimuli cause vasospasm longer than the others may help microsurgeons to review different methods of surgical manipulation, even though these findings cannot be precisely applicable in human medicine.
Acknowledgement
This study was supported by a grant from Internal Grant Agency of the Ministry of Health, Czech Republic, IGA - NR 8368-5. Authors have neither conflict of interest nor funding support for publishing this paper.
Address for correspondence:
Libor Streit, M.D.
Department of Plastic and Aesthetic Surgery
Berkova 34
612 00, Brno
Czech Republic
E-mail: streit@fnusa.cz
Sources
1. Acland RD. Factors that influence success in microvascular surgery. In: Serafin D, Buncke HJ. eds. Microsurgical Composite Tissue Transplantation. St. Louis, Mo: CV Mosby Co; 1979, 76–82.
2. Puckett CL., Winters RW., Geter RK., Goebel D. Studies of pathologic vasoconstriction (vasospasm) in microvascular surgery. J. Hand. Surg., 10, 1985, 343–349.
3. Vesely J., Samohyl J., Barinka L., Nemec A. Tissue shock in free flaps in the experiment on the rat. Significance, classification and effect. Handchir. Mikrochir. Plast. Chir., 19, 1987, 269–272.
4. Vesely J., Samohyl J., Barinka L., Nemec A., Smrcka V. Spastic complications in free flap transfers. Rozhl. Chir, 69, 1990, 682–688.
5. Weinzweig N., Gonzalez M. Free tissue failure is not an all-or-none phenomenon. Plast. Reconstr. Surg., 96, 1995, 648–660.
6. Seaber A. Experimental vasospasm. Microsurgery, 8, 1987, 234–241.
7. Wadstrom J., Gerdin B. Nervous influence on traumatic vasospasm in the rabbit ear artery. Microsurgery, 12, 1991, 89–95.
8. Saetzler RK., Lehr HA., Barker JH., Kamler M., Galla TJ., Messmer K. Visualization of nutritive perfusion following tourniquet ischemia in arterial pattern skin flaps: effect of vasoactive medication. Plast. Reconstr. Surg., 94, 1994, 652–660.
9. Hjortdal VE., Sinclair T., Kerrigan CL., Solymoss S. Venous ischemia in skin flaps: microcirculatory intravascular thrombosis. Plast. Reconstr. Surg., 93, 1994, 366–374.
10. Hjortdal VE., Sinclair T., Kerrigan CL., Solymoss S. Arterial ischemia in skin flaps: microcirculatory intravascular thrombosis. Plast. Reconstr. Surg., 93, 1994, 375–385.
11. Khalil AA., Avis FA., Hall JC. Reperfusion injury. Plast. Reconstr. Surg., 117, 2006, 1024–1033.
12. May JW. Jr., Chait LA., O’Brien BM., Hurley JV. The no-reflow phenomenon in experimental free flaps. Plast. Reconstr. Surg., 61, 1978, 256–267.
13. Feng LJ., Berger BE., Lysz TW., Shaw WW. Vasoactive prostaglandins in the impending no-reflow state: Evidence for a primary disturbance in microvascular tone. Plast. Reconstr. Surg., 81, 1988, 755–767.
14. Wingard CJ., Browne AK., Murphy RA. Dependence of force on length at constant cross-bridge phosphorylation in the swine carotid media. J. Physiol., 488, 1995, 729–739.
15. Macdonald RL. Pathophysiology and molecular genetics of vasospasm. Acta Neurochir. Suppl., 77, 2001, 7–11.
16. Rubanyi GM. Endothelium-derived relaxing and contracting factors. J. Cell Biochem., 46, 1991, 27–36.
17. Shepherd JT., Katusic ZS., Vedernikov Y., Vanhoutte PM. Mechanism of coronary vasospasm: Role of endotelium. J. Mo. Cell Cardiol., 23, 1991, 125–131.
18. Pearson PJ., Lin PJ., Schaff HV. Global myocardial ischemia and reperfusion impair endotelium-dependent relaxations to aggregating platelets in the canine coronary artery: A possible cause of vasospasm after cardiopulmonary by-pass. J. Thorac. Cardiovasc. Surg., 103, 1992, 1147–1157.
19. Nelson CW., Wei EP., Povlishock JT., Kontos HA., Moskowitz MA. Oxygen radicals in cerebral ischemia. Am. J. Physiol., 263, 1992, 1356–1362.
20. Seccombe JF., Pearson PJ., Schaff HV. Oxygen radical-mediated vascular injury selectively inhibits receptor-dependent release of nitric oxide from canine coronary arteries. J. Thorac. Cardiovasc. Surg., 107, 1994, 505–509.
21. Miyauchi T., Yanagisawa M., Tomizawa,T. et al. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet, 2, 1989, 53–54.
22. Tsao PS., Aoki N., Lefer DJ., Johnson G., Lefer AM. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation, 82, 1990, 1402–1412.
23. Ray SG., McMurray JJ., Morton JJ., Dargie HJ. Circulating endothelin in acute ischemic syndromes. B. Heart J., 67, 1992, 383–386.
24. Hyza P., Streit L., Gopfert E., Schwarz D., Masarik M., Jurajda M. et. al. Gene expression of the Endothelin-1 in vasospastic flap pedicle – an experimental study on a porcine model. Acta Vet. Brno, 79, 2010, 453–457.
25. Kawata H., Aoki M., Mater JE. Jr. Nitroglycerin improves functional recovery of neonatal lamb hearts after 2 hours of cold ischemia. Circulation, 88, 1993, 366–371.
26. Sternbergh WC., Makhoul RG., Adelman B. Nitric oxide-mediated, endothelium-dependent vasodilation is selectively attenuated in the postischemic extremity. Surgery, 114, 1993, 960–967.
27. Margic K. Early changes in dissected small vessels: Experimental study on rat arteries and veins. Plast. Reconstr. Surg., 75, 1985, 375–383.
28. Ueda K., Harii K. Comparative study of topical use of vasodilating solutions. Scand. J. Plast. Reconstr. Surg. Hand Surg., 37, 2003, 201–207.
29. Harashina T., Fujino T., Watanabe S. The intimal healing of microvascular anastomoses. Plast. Reconstr. Surg., 58, 1976, 608–613.
30. Harashina T. The site of reapplication of microvascular clamps. Plast. Reconstr. Surg., 58, 1976, 719–720.
31. Hyza P., Vesely J., Schwarz D., Vasku A., Choudry U., Streit L., et. al. The effect of blood around a flap pedicle on flap perfusion in an experimental rodent model. Acta Chir. Plast., 51, 2009, 21–25.
32. Place MJ., Witt P., Hendricks D. Cutaneous blood-flow patterns in free flaps determined by laser Doppler flowmetry. J. Reconstr. Microsurg., 12, 1996, 355–358.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2014 Issue 1-2-
All articles in this issue
- Electrical burns in our workplace
- The history and safety of breast implants
- Reconstruction of eyelids with washio flap in anophthalmia
- The Use of Medicinal Leeches in Fingertip Replantation without Venous Anastomosis – Case Report of a 4-year-old Patient
- Contents Acta Chir. Plast. Vol. 56, 2014
- Index Acta Chir. Plast. Vol. 56, 2014
- Haemophilia - unexpected complication of rhinoplasty
- Vasospasm of the Flap Pedicle - The New Experimental Model on Rat
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Haemophilia - unexpected complication of rhinoplasty
- Reconstruction of eyelids with washio flap in anophthalmia
- The history and safety of breast implants
- The Use of Medicinal Leeches in Fingertip Replantation without Venous Anastomosis – Case Report of a 4-year-old Patient
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

