-
Medical journals
- Career
A STUDY OF 17 PATIENTS AFFECTED WITH PLEXIFORM NEUROFIBROMAS IN UPPER AND LOWER EXTREMITIES: COMPARISON BETWEEN DIFFERENT SURGICAL TECHNIQUES
Authors: M. G. Onesti; S. Carella; G. Spinelli; A. Martano; S. Giustini; N. Scuderi
Authors‘ workplace: Department of Dermatology and Plastic Reconstructive Surgery, University of Rome “Sapienza”, Rome, Italy
Published in: ACTA CHIRURGIAE PLASTICAE, 51, 2, 2009, pp. 35-40
INTRODUCTION
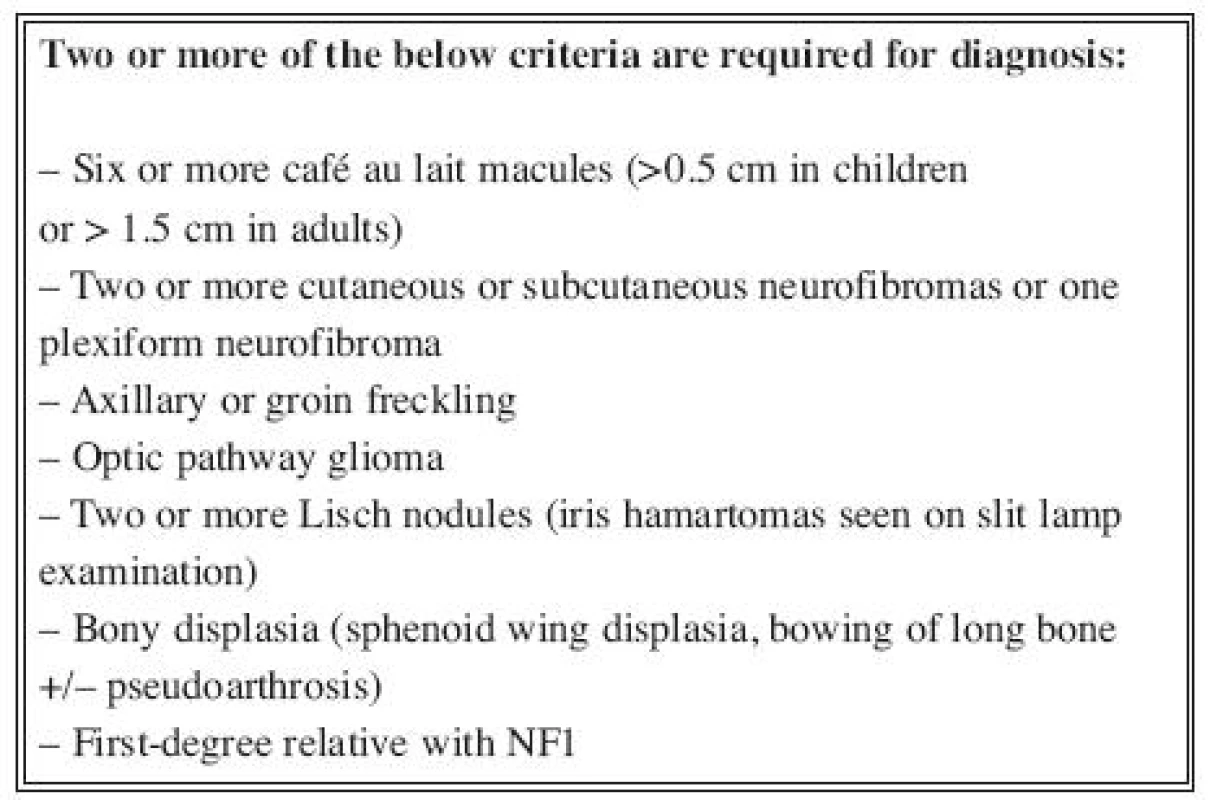
Neurofibromatosis is an autosomical dominant disorder of which there are two distinct forms (1), neurofibromatosis type 1 (NF1) (peripheral form) and neurofibromatosis type 2 (NF2) (central form). Both are characterized by skin tumours (neurofibromas) derived from peripheral nerval shealts. The NF1 gene is a large gene mapped to chromosome 17 encoding a protein named neurofibromin, which has a role in tumour suppression. Neurofibromas are the hallmark of the disease and usually appear during childhood or adolescence. Diagnosis of neurofibromatosis type 1 (incidence 1 : 2,500 newborns) (2) is based on clinical criteria. The National Institute of Health (NIH) Consensus Development Conference (3) formulated the current diagnostic criteria (Table 1) and proposed the name neurofibromatosis 1.
The major disease features involve the nervous system, the skin, and bone, and the resulting complications are numerous (4–7, 8–11).
NF1 can be classified into histological categories using growing patterns, described as nodular, plexiform and sand-glass. Growth of nodular form occurs around the nerve. Growth of plexiform neurofibromas can occur circumferentially around a nerve, longitudinally along a nerve, or both. It may involve the whole nerve network of a limb. Sand-glass form develops near the spinal roots through the conjugation courts.
Plexiform neurofibromas (PN) is one of the most common and severe types of neurofibroma that occur in neurofibromatosis type 1 (12). It is also called plexiform neuroma and classified as a benign peripheral nerve sheath tumour that surrounds multiple nervous fascicles. It is a nonmetastatic, highly vascularzed, locally invasive tumour that has slow growth. PN is one of the significant complications of NF1, which may occur during childhood and rarely develops after adolescence. PN can originate malignant peripheral nerve sheath tumour (MPNST), which occurs in 2–5% of patients (2)with plexiform neurofibroma. MPNST, previously described as neurofibrosarcomas or malignant schwannomas, are the main cause of death and the most common neoplasia in this group.
Clinically they may be visible from the surface of the body or may be internal with no evident superficial extension (13). Involvement of the skin gives rise to a thickening of the dermis, irregular hyperpigmentation and/or increased vascular markings, and palpable cord-like masses. Cutaneous plexiform neurofibroma may arise from superficial peripheral nerves, in which case there is no deep involvement, or can represent the superficial extension of deeper, more massive, plexiform tumour.
These tumours affect long portions of nerves, infiltrate the nerve and surrounding tissue thus causing significant pain, deformity, and functional problems of the involved part of the body (14). The vast majority of reports relate to plexiform neurofibromas occurring in the head and neck region (15, 16), because this area is richly innervated; while there are not many reports on the involvement of the lower extremity, a couple of reports demonstrate involvement of the feet (17, 18) while only one displays leg involvement in an adult case (19). Symptomatic plexiform neurofibromas are usually evaluated by imaging studies, such as magnetic resonance imaging (MRI) or CT scanning. In general, MRI is the modality of choice. High resolution MRI of peripheral nerves using phased-array radiofrequency coils (neurography) has been applied to imaging of plexiform neurofibromas and may prove useful in planning for surgery, following tumour growth, or identification of regions with malignant change.
Treatment of plexiform neurofibromas is currently surgical. Complete resection is often difficult due to the extensive growth of the tumour, and invasion of surrounding tissues and regrowth after surgery is common, but subtotal and total resection without functional destruction is often possible for superficial PN. Choice of optimal time for surgery is therefore challenging. The purpose of the present study was to review the surgical treatment of plexiform neurofibromas, located in the upper and lower extremities.
MATERIALS AND METHODS
Twenty-nine surgically excised specimens of neurofibromas in the upper and lower extremities from the files of the Department of Plastic and Reconstructive Surgery of University “Sapienza” in Rome from 2000 to 2007 were reviewed retrospectively. The inclusion criteria were:
- Plexiform neurofibromas located in the upper and lower extremities confirmed by biopsy and classified according to the World Health Organization (20).
- Medical records with essential information.
- The type of surgical procedure performed (subtotal or total excision, nerve sheet sparing, nerve excision).
Clinical data (anatomic distribution, sex, age, isolated or multiple neurofibromas, size and depth of lesions), therapy and follow-up information were extracted and recorded (Table 2).
2. Clinical data, therapy and follow-up information 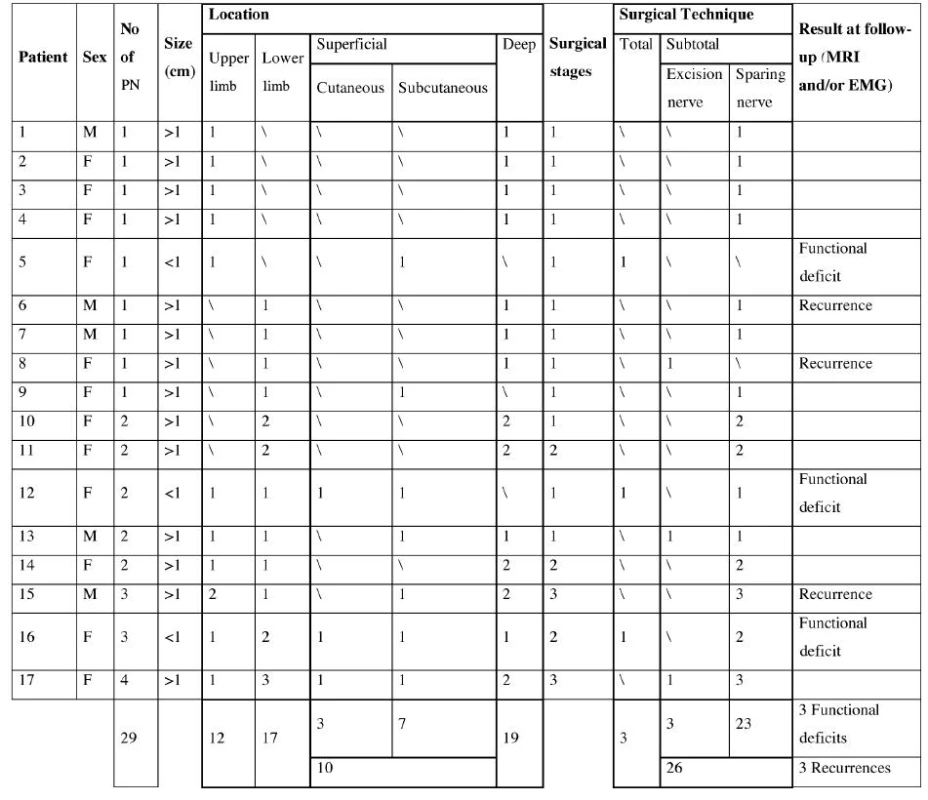
Histhopatological evaluation of the specimens was carried out in every case. Prior to surgery all patients underwent MRI scan and EMG evaluation in order to assess pre-operative nerve function, thus reducing surgical morbidity. Magnetic resonance image (MRI) was performed for the tumour regions at 1.5 Tesla with T1 - and T2 - weighted sequences including a short-tau inversion-recovery (STIR) sequence. For all patients, contiguous axial STIR (TR/TE, 6000/35; inversion time, 150 ms; echo-train length, 8) images and coronal or sagittal STIR images were obtained. The extremities were imaged with a matrix of 512–160; other anatomic locations were imaged with a 256–256 matrix. Tumour resection was performed following the graphical delineation of the affected skin and according to the MRI findings. Tumour resection included the epidermal and subcutaneous layer. Exploration of the underlying muscles was mandatory.
Follow-up made it possible to observe the presence of eventual recurrence detected with MRI; functional outcomes were evaluated with electromyography and aesthetic results obtained and recorded using a scoring scale realized in our clinic (Table 3).
3. Scoring scale 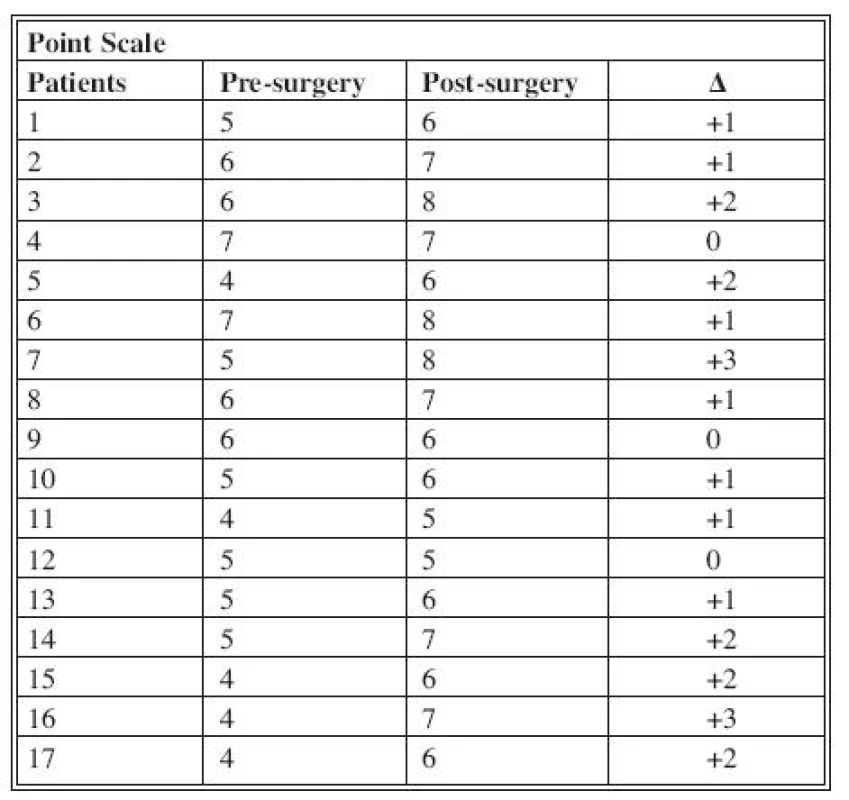
Every score is assigned on the basis of personal patient’s evaluation. RESULTS
We examined 17 patients affected with plexiform neurofibromas in lower and upper extremities, of which twelve were female and five male, evaluated between February 2000 and December 2007. They ranged in age from 12 to 53 years old. PNF were diagnosed based on the following indications: subcutaneous location on palpation, associated with thickening of the skin, local hypertrophy, and hyperpigmentation on inspection. Multiple lesions were detected in eight patients and isolated lesions in the remaining nine patients. Time of development of plexiform neurofibromas ranged from 1 to 6 years. Clinically, the neurofibromas were soft, slightly elevated, and usually did not exceed 6 cm in maximum dimension. There were 29 neurofibromas in total, 12 in the upper extremities and 17 in the lower extremities. In particular, of 29 neurofibromas 19 were deep and 10 (3 cutaneous and 7 subcutaneous) were superficial lesions. Every patient complained of movement disorders, pain and psychological stress relate to their appearance.
Among the superficial lesions 3 superficial small (<1 cm) neurofibromas were treated with total excision, 7 large (>1 cm) neurofibromas with subtotal excision. The deep lesions were treated with subtotal excision, in particular 23 with sheaths nerves sparing, while 3 with nerve excision. Follow-up time ranged from 3 to 230 months. MRI was undertaken covering the tumour regions. Tumour regrowth was monitored for 2–6 years postoperatively. One recurrence of the neurofibromas was detected with MRI 17 months after surgical treatment in three patient treated with subtotal excision; three patients treated with total excision showed minimal functional disorder documented with electromyography. We obtained satisfactory aesthetic results, and the majority of patients produced good scars without keloid or hypertrophic changes. (Fig. 1–6.)
Fig. 1. Neurofibroma in upper extremity (size: > 1 cm) 
Fig. 2. Neurofibroma in lower extremity (size: < 1 cm) 
Fig. 3. Functionality of upper extremity (extension) pre treatment 
Fig. 4. Functionality of upper extremity (extension) post treatment 
Fig. 5. Functionality of upper extremity (flexion) pre treatment 
Fig. 6. Functionality of upper extremity (flexion) post treatment 
DISCUSSION
Symptomatic plexiform neurofibromas are usually evaluated by imaging studies such as magnetic resonance imaging (MRI) or CT scanning. In general, MRI is the modality of choice. High resolution MRI of peripheral nerves using phased-array radiofrequency coils (neurography) has been applied to imaging of plexiform neurofibromas and may prove useful in planning for surgery, following tumour growth, or identification of regions with malignant change. STIR is a fluid-sensitive, fat-suppressing sequence that was chosen for the imaging protocol because of the very bright signal characteristics of neurofibromas relative to surrounding muscle, fat and bone. Contrast-enhanced imaging was not performed for two main reasons: first, Iannicelli et al. (21) showed that plexiform neurofibromas are consistently bright in T2 signal intensity and therefore very conspicuous on STIR and other T2-weighted sequences. In addition, they found that plexiform neurofibromas show variable enhancement after IV gadolinium administration on T1-weighted sequences; therefore their conspicuity on contrast-enhanced images is inconsistent. Since IV contrast material provides no gain in conspicuity of plexiform neurofibromas, it was decided that there was no justification for the added risk or expense of gadolinium-enhanced imaging in this group of voluntary subjects.
Friedrich et al. reported that PN can be distinguished into three growth categories using magnetic resonance imaging (MRI): superficial, displacing and invasive. Superficial PN arise from subcutaneous or cutaneous nerves and may remain within the upper layer of the skin – usually not involving major nerves (22). Subtotal and total resection without functional destruction is often possible for superficial NF (23). In contrast, invasive PN infiltrate multiple tissue planes and are thus much more difficult or impossible to resect. Treatment of large plexiform neurofibromas still remains a challenge for surgeons. The surgical management of plexiform neurofibromas has been discussed in the literature, but reports focused primarily on the trunk (24, 25), head and neck (26–27), and other areas. Lee L.Q. Pu et al. (28) reported that after complete resection of the tumour a subsequent free tissue transfer is possible for soft-tissue reconstruction.
This type of tumour is problematic not only because of its appearance but also because of its vascular vulnerability, which causes bleeding tendency in the tumour. In addition, 5% of diffuse plexiform neurofibromas show a malignant change, but this is difficult to detect in the early stage. Therefore it is recommended that a diffuse plexiform neurofibroma should be removed whenever possible (29, 30). However, these extremely vulnerable tumour vessels do not respond very well to vasoconstricting drugs. This makes it difficult to arrest bleeding intra-operatively, and even a biopsy is fraught with danger. Junji Tanaka et al. (31) reported that pre-operative embolization therapy employs the standard technique of trans-arterial embolization. As the primary purpose of embolization is to reduce intra-operative bleeding, it would be sufficient if blood flow to the tumour is decreased to some extent. This method should be useful for enhancing the safety of the operation and expanding the therapeutic applications for diffuse plexiform neurofibroma. Laser CO2 can be considered a useful technique too; it makes it possible to perform the incision of the lesion to assure adequate hemostasis.
There is little published data about non-surgical treatment of plexiform neurofibromas. Cytoreductive treatments such as radiation or chemotherapy have not been thoroughly investigated. It is unclear whether slow growing plexiform neurofibromas would respond to such treatments, and there is concern that such treatments might result in secondary malignancies. Radiation and chemotherapy have been used for treatment of optic gliomas and malignant peripheral nerve sheath tumours. It is unfortunate that there has been no study of the incidental effects of such treatments on neurofibromas that may have been present on such patients. A number of converging advances in molecular oncology research, as well as research on NF1 itself, offer hope that novel therapies may soon be available for plexiform neurofibromas. These include the prospect for treatments that target the Ras signalling pathway, in which the NF1 gene product plays a role, as well as unrelated treatments involving inducers of differentiation or inhibitors of angiogenesis (12).
CONCLUSIONS
In accordance with the literature, surgical intervention is presently the only treatment option for PN; in particular, early surgical intervention makes it possible to prevent the progression of PN affecting upper and lower extremities. Surgical treatments can be total and subtotal. The advantage of total excision is that it avoids eventual recurrence of the tumour, while the disadvantage is the functional deficit. Therefore this surgical technique is indicated for small and superficial neurofibromas where functional impairment would be minimal because of their small size and superficial location. In contrast, subtotal excision has the advantage of reducing functional deficit to a minimal but the disadvantage of possible recurrence. Therefore we would underline the importance of achieving the right compromise between a possible functional deficit due to total resection and a possible recurrence of PN caused by the subtotal excision.
Address for correspondence:
Maria G. Onesti, M.D.
Via Augusto Valenziani 12
00187 Rome
Italy
E-mail: mariagiuseppina.onesti@uniroma1.it
Sources
1. Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol., 6, 2007, p. 340-351.
2. Darrigo LG. Jr., Geller M., Filho AB., Azulay DR. Prevalence of plexiform neurofibroma in children and adolescents with type I neurofibromatosis. J. Pediatr. (Rio J), 83, 2007, p. 571-573.
3. National Institutes of Health Consensus Development Conference Statement: Neurofibromatosis. Arch. Neurol., 45, 1988, p. 575-578.
4. Daston MM., Scrable H., Nordlund M. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells and oligodendrocytes. Neuron, 8, 1992, p. 415-428.
5. Evans DG., Baser ME., McGaughran J. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet., 39, 2002, p. 311-314.
6. Ferner RE., Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumours in neurofibromatosis 1. Canc. Res., 62, 2002, p. 1573-1577.
7. Ferner RE., Huson SM., Thomas N. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet., 44, 2006, p. 81-88.
8. Listernick R., Louis DN., Packer RJ. Optic pathway gliomas in children with neurofibromatosis type 1: consensus statement from the NF1 optic pathway glioma study. J. Paediatr., 125, 1994, p. 63-66.
9. Lubs ML., Bauer MS., Formas ME. Lisch nodules in neurofibromatosis 1. N. Engl. J. Med., 324, 1991, p. 1264-1266.
10. North KN., Riccardi V., Samango-Sprouse C. Cognitive function and academic performance in neurofibromatosis. 1: Consensus statement from the NF1 Cognitive Disorders Task Force. Neurology, 48, 1997, p. 1121-1127.
11. Tonsgard JH., Kwak SM., Short MP. CT imaging in adults with neurofibromatosis 1: frequent asymptomatic plexiform lesions. Neurology, 50, 1998, p. 1755-1760.
12. Korf BR. Plexiform neurofibromas. Am. J. Med. Genet., 89, 1999, p. 31-37.
13. Agaram NP., Prakash S., Antonescu CR. Deep-seated plexiform schwannoma: A pathologic study of 16 cases and comparative analysis with the superficial variety. Am. J. Surg. Pathol., 29, 2005, p. 1042-1048.
14. Shetty GM., Murari AS., Song HR., Lee SH., Yang JH. Neurofibromatous sensory neuropathy of the thigh in a 7-year-old boy. Arch. Orthop. Trauma Surg., 128, 2008, p. 1093-1097.
15. Salazar F., Machado A., Murthy S., Boulis NM. Thoracoscopically guided transaxillary resection of adjoining intercostal plexiform neurofibromas: review of mosaicism in neurofibromatosis: technical note. Neurosurgery, 57 (4 Suppl.), 2005, E407; discussion E407.
16. Wise JB., Cryer JE., Belasco JB., Jacobs I., Elden L. Management of head and neck plexiform neurofibromas in pediatric patients with neurofibromatosis type 1. Arch. Otolaryngol. Head Neck Surg., 131, 2005, p. 712-718.
17. Blitz NM., Hutchinson B., Grabowski MV. Pedal plexiform neurofibroma: review of the literature and case report. J. Foot Ankle Surg., 41, 2002, p. 117-124.
18. Kraus DH. Management issues in massive pediatric facial plexiform neurofibroma with neurofibromatosis type 1. Head Neck, 24, 2002, p. 207-211.
19. Nagel A., Greenebaum E., Singson RD., Rosenwasser MP. McCann PD. Foot drop in a long-distance runner. An unusual presentation of neurofibromatosis. Orthop. Rev., 23, 1994, p. 526-530.
20. Weiss SW. et al. Neural tumours. In: Weiss SW. et al. Histological typing of soft tissue tumours. Springer: Berlin Heidelberg New York, 1994, p. 38-39.
21. Iannicelli E., Rossi G., Almberger M., Drudi FM., Giustini S., Calvieri S., David V. Integrated imaging in peripheral nerve lesions in type 1 neurofibromatosis. Radiol. Med. (Torino), 103, 2002, p. 332-343.
22. Friedrich RE., Korf B., Funsterer C., Mautner VF. Growth type of plexiform neurofibromas in NF1 determined on magnetic resonance images. Anticancer Res., 23, 2003, p. 949-952.
23. Friedrich RE., Schmelzle R., Hartmann M., Mautner VF. Subtotal and total resection of superficial plexiform neurofibromas of face and neck. J. Craniomaxillofac. Surg., 33, 2005, p. 55-60.
24. Nahabedian MY., Rozen SM., Namnoum JD., Vander Kolk CA. Giant plexiform neurofibroma of the back. Ann. Plast. Surg., 45, 2000, p. 442-445.
25. Salazar R., Robotti EB., Chin DHL., Grossman JAI. Giant neurofibromatosis of the chest wall: two patient reports. Ann. Plast. Surg., 41, 1998, p. 211-221.
26. Park BY., Hong JP., Lee WJ. Netting operation to control neurofibroma of the face. Plast. Reconstr. Surg., 109, 2002, p. 1228-1236.
27. Neville HL., Seymour-Dempsey K., Slopis J., Gill BS., Moore BD., Lally KP., Andrassy RJ. The role of surgery in children with neurofibromatosis. J. Pediatr. Surg., 36, 2001, p. 25-29.
28. Pu LLQ., Vasconez HC. Large recurrent plexiform neurofibroma of the foot and ankle. Microsurgery, 24, 2004, p. 67-71.
29. Jackson IT., Carbonnel A., Potparic Z., Shaw K. Orbitotemporal neurofibromatosis: classification and treatment. Plast. Reconstr. Surg., 92, 1993, p. 1-11.
30. Friedrich RE., Schmelzle R., Hartmann M., Fünsterer C., Mautner VF. Resection of small plexiform neurofibromas in neurofibromatosis type 1 children. World. J. Surg. Oncol., 3, 2005, p. 6.
31. Tanaka J., Kuramochi A., Nishi N., Yuasa M., Heshiki A. Preoperative transarterial embolization enhances the surgical management of diffuse plexiform neurofibroma: A case report. Cardiovasc. Intervent. Radiol., 28, 2005, p. 686-688.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2009 Issue 2-
All articles in this issue
- A STUDY OF 17 PATIENTS AFFECTED WITH PLEXIFORM NEUROFIBROMAS IN UPPER AND LOWER EXTREMITIES: COMPARISON BETWEEN DIFFERENT SURGICAL TECHNIQUES
- RECONSTRUCTION OF DEFECT AFTER RADICAL VULVECTOMY BY THE USE OF FOUR-FLAP LOCAL TRANSFER – A CASE REPORT
- LACERATION AND DEGLOVING INJURY OF A CHILD'S FOOT – A CASE REPORT
- NEW METHOD OF FIXATION IN ABOVE-WRIST REPLANTATION IN PATIENT WITH TRAUMATIC TOTAL CARPAL LOSS – A CASE REPORT
- NASAL PROSTHESIS SUPPORTED WITH SELF-TAPPING IMPLANTS WITH BIOACTIVE SURFACE – A CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- LACERATION AND DEGLOVING INJURY OF A CHILD'S FOOT – A CASE REPORT
- A STUDY OF 17 PATIENTS AFFECTED WITH PLEXIFORM NEUROFIBROMAS IN UPPER AND LOWER EXTREMITIES: COMPARISON BETWEEN DIFFERENT SURGICAL TECHNIQUES
- RECONSTRUCTION OF DEFECT AFTER RADICAL VULVECTOMY BY THE USE OF FOUR-FLAP LOCAL TRANSFER – A CASE REPORT
- NASAL PROSTHESIS SUPPORTED WITH SELF-TAPPING IMPLANTS WITH BIOACTIVE SURFACE – A CASE REPORT
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career