Myasthenia Gravis and Unmet Patient Needs – Data from Clinical Practice
Myasthenia gravis (MG) is a rare, chronic, disabling, unpredictable, and potentially life-threatening autoimmune neuromuscular disease. There is still a lack of sufficient data from real clinical practice to better meet the needs of MG patients. What is the current situation in European countries? And how are Czech patients doing?
Why collect data on MG treatment?
In evidence-based medicine, the highest value is placed on data collected in randomized controlled clinical trials (RCTs). Insights from real clinical practice supplement important information about the course of the disease and treatment outcomes in a broader patient population. Data collection in clinical practice is crucial especially for rare diseases (with a prevalence of < 0.5/1,000 inhabitants) with a limited patient population meeting the strict entry criteria of RCTs.
European Analysis
In 2020, a survey was conducted in 5 European countries (France, Italy, Germany, Spain, United Kingdom) where MG patients and their doctors filled out information about their disease.1 A total of 144 doctors (neurologists, geriatricians, and practitioners) recorded data on 778 patients.
Status at the time of diagnosis
The average age of patients at symptom onset was 47.7 years, and the average time from symptom onset to MG diagnosis was 11 months. At the time of diagnosis, the severity of the disease according to the Myasthenia Gravis Foundation of America (MGFA) criteria was classified as category ≥ II in 65.3% of patients, and they reported an average of 5 symptoms. The 5 most bothersome symptoms at the time of diagnosis included weakness and fatigability of the eye and eyelid muscles, ptosis, diplopia, general fatigue, and arm weakness.
Symptoms at the time of the survey
At the time of the survey, the average age of patients was 54 years, and 52% of them were women. Patients participated in the survey on average 5.2 years after symptom onset (range 0.04–61.3 years). Generalized disease was recorded in 71.5% of them. Participants reported the same number and type of disease symptoms on average as at the time of diagnosis. The most common symptom was ocular myasthenia (60.7%), and general fatigue was reported as the most bothersome (51.2%).
MG Therapy in Real Practice
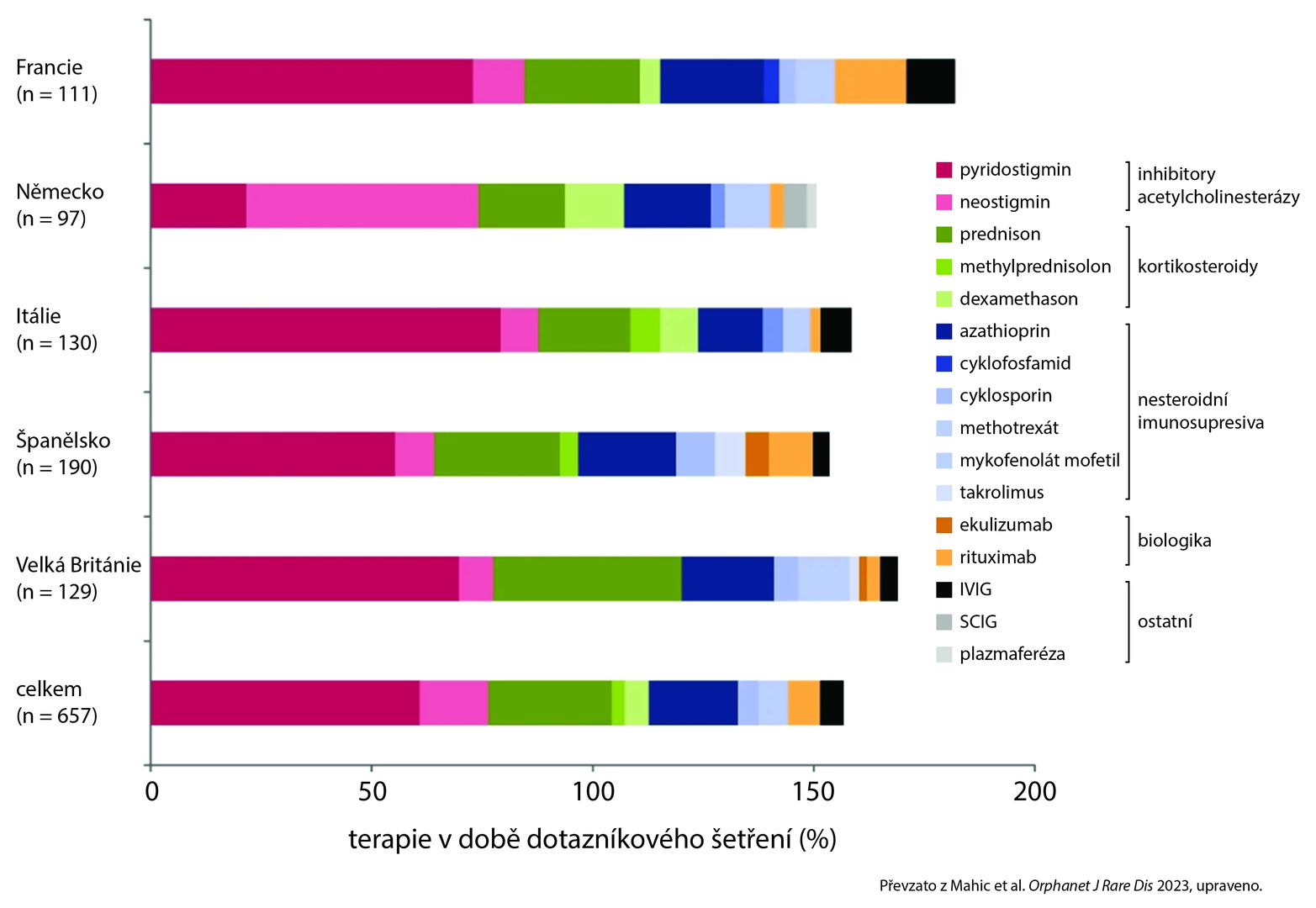
Only 4% of patients were not on any MG treatment. The most commonly prescribed medicines across all countries were acetylcholinesterase inhibitors, followed by corticosteroids and non-steroidal immunosuppressive therapy (see fig.). Despite long-term treatment, 62% of patients experienced moderate to severe disease symptoms. In the last 12 months, 23% of patients reported a need for hospitalization.
Fig. The ten most commonly prescribed modalities in MG treatment in the respective country at the time of the survey. The total number exceeds 100%, as concomitant treatment with multiple modalities was possible for a given patient.
And what is the situation in the Czech Republic?
Since 2015, the national registry MyReg has been collecting data on MG patients in the Czech Republic. According to European prevalence, it is estimated that there should be 1,500–2,000 MG patients in the Czech Republic. The registry currently contains data on more than 2,000 patients from 15 centers, and the number of records in the registry is still growing. According to the latest summary analysis of data as of October 2019 (n = 932; 53% men), the average age at disease onset was 62 years for men and 50 years for women. The most common initial symptoms were ocular symptoms (79%), followed by bulbar (46%), limb (32%), and respiratory symptoms (10%). Generalized MG was present in 76% of patients.
The most commonly prescribed treatment, in line with European data, was pyridostigmine followed by prednisone and azathioprine.
Conclusion
Despite available treatment, the disease burden for MG patients remains high. Even if MG is diagnosed timely and correctly, many patients still experience symptoms and their disease worsens. According to recent data from European real clinical practice, only a quarter of these patients observe improvement as a result of therapy. Therefore, it is necessary to introduce other treatment options into practice.
(este)
Sources:
1. Mahic M., Bozorg A., DeCourcy J. et al. Physician- and patient-reported perspectives on myasthenia gravis in Europe: a real-world survey. Orphanet J Rare Dis 2023; 18 (1): 169, doi: 10.1186/s13023-023-02727-0.
2. Status of the MyReg registry. Myasthenia Gravis Registry. Institute of Biostatistics and Analyses, 26. 9. 2023. Available at: https://myreg.registry.cz/index.php?pg=stav-registru
3. Botiková D., Horáková M, Voháňka S. The Czech National registry of Myasthenia Gravis MyReg. Treat NMD Congress, Leiden, 10. 12. 2019. Available at: https://myreg.registry.cz/res/file/myreg/vystupy/daniela-botikova%CC%81_day-1.pdf
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
