-
Medical journals
- Career
Facilitated Immunoglobulin Substitution Therapy in Paediatric Patients with Immunodeficiency – Real-World Data
16. 10. 2023
Patients with humoral immunodeficiency are indicated for immunoglobulin substitution therapy. The study presented below summarizes real-world data concerning the latest substitution method (i.e., facilitated subcutaneous administration) in paediatric patients.
Study Methodology and Evaluated Population
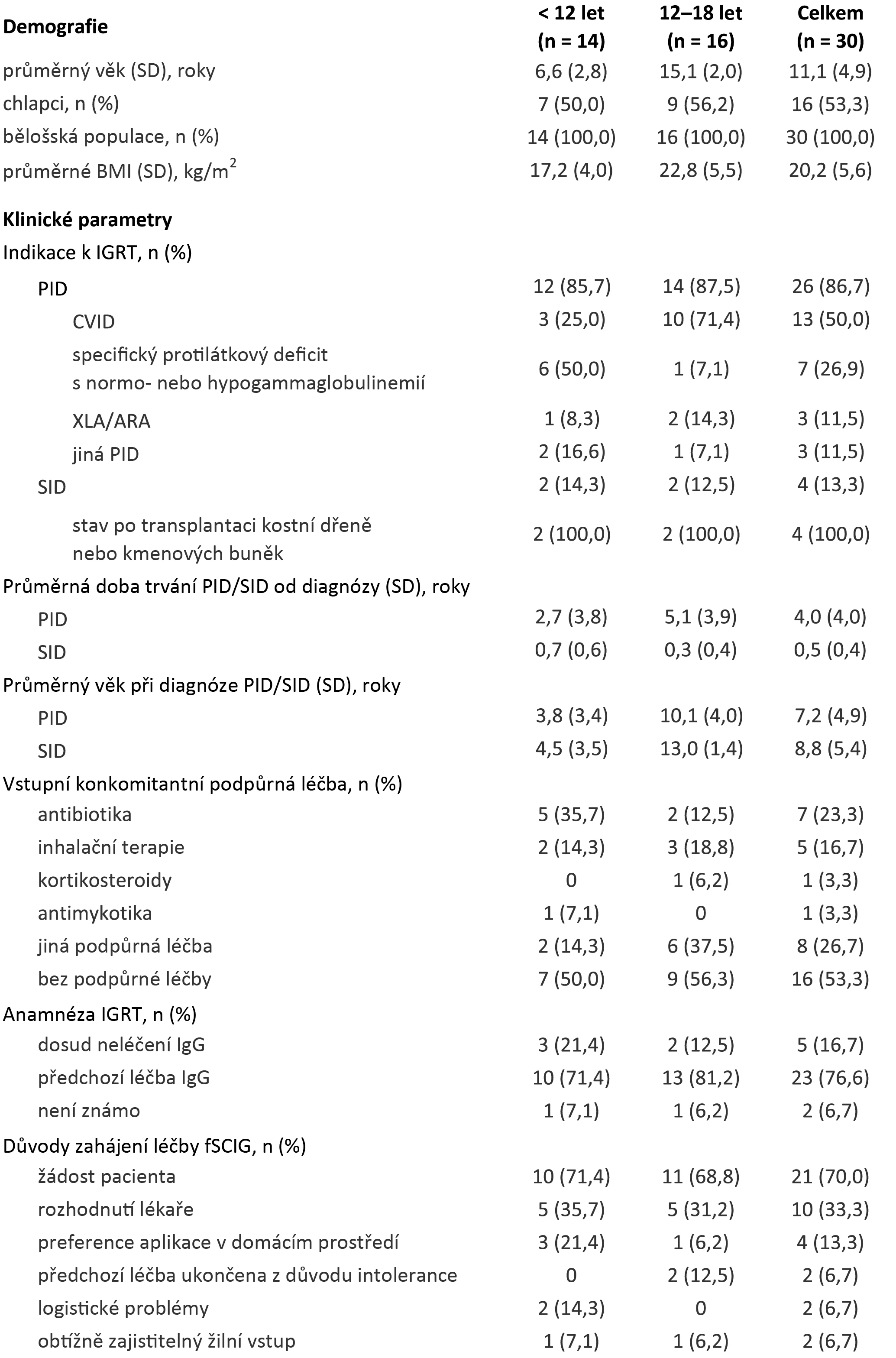
A total of 30 patients (53.3% boys) under the age of 18 with immunodeficiency, both primary (PID; n = 26; most commonly with common variable immunodeficiency) and secondary (SID; n = 4; post-bone marrow or stem cell transplant), who underwent facilitated subcutaneous immunoglobulin therapy (fSCIG) for ≥ 6 months were included in a German multicentre retrospective study1 between May 2019 and October 2020. Fourteen (46.7%) of them were younger than 12 years, and the remaining 16 (53.3%) were aged 12–18 years.
Before starting fSCIG therapy, most of them (n = 23) used another substitution immunoglobulin product – a total of 12 patients with PID were treated with ≥ 1 subcutaneous product and another 12 (9 with PID and 3 with SID) with ≥ 1 intravenous product. In patients with PID, fSCIG therapy was mostly initiated at their request, while in those with SID, it was usually on a doctor's recommendation (see table).
Table. Baseline Demographic and Clinical Characteristics of Patients
The technique of infusion administration, tolerance, and safety of the treatment were evaluated.
Results
The first infusion was administered in a healthcare facility for most patients (90%), and within 6 months, all treatments were conducted at home. Most adolescents (12/16) managed the application themselves, while children (12/14) were usually infused by a caregiver.
After 6 months, the target dose was achieved in all patients. In adolescents (not in children under 12 years), an increase in infusion volume and rate was observed during the treatment. The average infusion duration was 104 minutes, usually administered into the same site every 3–4 weeks (96.7%). No technical issues with administration were reported, nor was it necessary to interrupt or slow down the infusion rate. After 6 months, infusions were administered on a precisely set day (67.9%) or within ± 1–3 days (32.1%) in most patients.
No pre-medication was required for any patient. Following the first administration, within 72 hours, 4 patients experienced mild local inflammatory reactions at the infusion site. Over the 6 months of use, local adverse reactions were reported in 6 and systemic reactions (fever, vomiting, and cough) in 2 patients; none of the adverse reactions were serious.
IgG levels after 6 months, for all patients where data were available (n = 12), were evaluated as satisfactory.
During the first 6 months of therapy, participants underwent an average of 2 training applications in healthcare and 1 at home. The application method, tolerance, and effectiveness of treatment were rated as good or very good by 96.4%, 82.1%, and 89.3% of treating physicians after 6 months.
Conclusion
This study supports facilitated subcutaneous immunoglobulin therapy in paediatric patients with immunodeficiency, demonstrating good tolerance and safety. Unlike conventional subcutaneous therapy, it allows for longer intervals (3–4 weeks) between individual infusions, enabling administration at home.
(mafi)
Source: Baumann U., Fasshauer M., Pausch C. et al. Facilitated subcutaneous immunoglobulin use in pediatric patients with primary or secondary immunodeficiency diseases. Immunotherapy 2022; 14 (2): 135–143, doi: 10.2217/imt-2021-0167.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Allergology and clinical immunology Paediatric ENT Paediatric pneumology Haematology ENT (Otorhinolaryngology) Paediatrics Pneumology and ftiseology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

