-
Medical journals
- Career
Prediction of Hypocalcemia Risk in Patients Treated with Denosumab and Active Form of Vitamin D
8. 6. 2023
According to new work by Japanese authors, it appears that the risk factors for the development of hypocalcemia in patients treated with denosumab and calcitriol (the active form of vitamin D) are different from those when natural vitamin D is added to denosumab.
Forms of Vitamin D and Its Metabolism
Vitamin D is a fat-soluble steroid that occurs in nature in 2 forms. Plants and fungi produce plant vitamin D2 (ergocalciferol) from ergosterol. Animal vitamin D3 (cholecalciferol) is formed in the skin from 7-dehydrocholesterol by a two-step non-enzymatic conversion using UVB radiation. For most processes mediated by vitamin D, it requires initial metabolism to 25-hydroxyvitamin D3 (calcidiol), which occurs predominantly in the liver. Calcidiol is the main circulating metabolite of vitamin D. Further hydroxylation to 1α,25-dihydroxyvitamin D3 (calcitriol) occurs primarily in the proximal tubules of the kidneys. Calcitriol is the active form of vitamin D3.1, 2
Image 1. Fig. Vitamin D metabolism 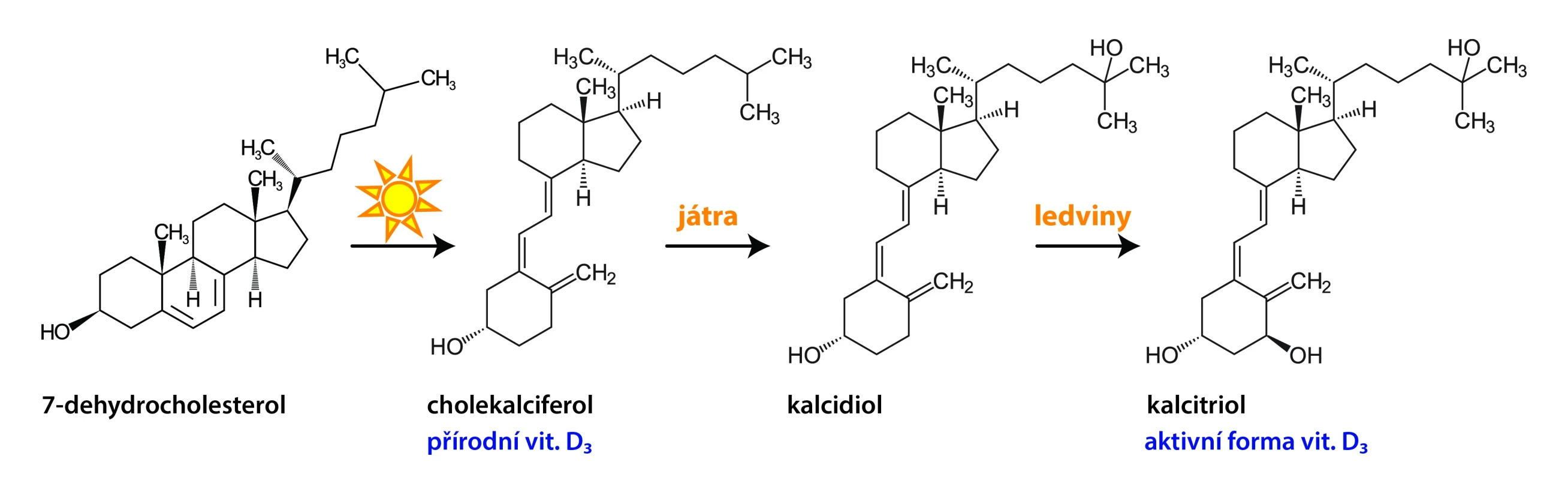
Hypocalcemia and Denosumab Treatment
Denosumab is used in the prevention of bone events in patients with advanced malignancies; however, its administration is associated with the risk of hypocalcemia. In the prevention of denosumab-induced hypocalcemia, vitamin D is added to the treatment. Based on practical experience, patients with renal insufficiency often receive the active form of vitamin D instead of the natural form due to their reduced ability to activate vitamin D.3 The development of grade ≥ 2 hypocalcemia was observed in retrospective studies even with vitamin D supplementation.
Methodology and Study Progress
In their previous work, Japanese authors created a model to predict the risk of hypocalcemia in patients treated with denosumab and cholecalciferol.4 The model takes into account gender, serum calcium levels, albumin, and alkaline phosphatase, presence of osteoporosis, breast or stomach cancer, combination of administered proton pump inhibitors, and pretreatment with zoledronic acid.
Now, their goal was to evaluate the type of vitamin D administered to patients with bone metastases treated with denosumab and the risk factors for the development of grade ≥ 2 hypocalcemia in patients with the active form of vitamin D. They conducted a retrospective observational study,3 utilizing data from June 2013 to May 2020 from a database covering 25% of Japanese hospitals providing acute care. Patients were divided according to the type of vitamin D they were taking. Then the investigators identified factors associated with the development of hypocalcemia in patients with calcitriol.
Findings
Out of 33,442 patients treated with denosumab, 22,347 were also taking cholecalciferol and 3,560 were taking calcitriol. Patients in the calcitriol group had significantly worse renal function (median estimated glomerular filtration rate /eGFR/ 74.0 vs. 69.7 ml/min/1.73 m2), but 23.6% of them had sufficient kidney function (eGFR ≥ 90 ml/min/1.73 m2).
Among patients taking calcitriol, 166 without hypocalcemia and 17 with hypocalcemia were analyzed, as they met the study inclusion criteria (data on calcium levels before denosumab treatment and up to 28 days after the first dose of denosumab, albumin determination, vitamin D use ≥ 28 days). Significant factors predicting the development of grade ≥ 2 hypocalcemia during denosumab and calcitriol treatment included high serum aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea (BUN − blood urea nitrogen), and low hemoglobin levels.
Conclusion
The authors concluded that some patients treated with denosumab who do not have impaired kidney function still use calcitriol. For the prediction of hypocalcemia risk in patients treated with denosumab and calcitriol, a different protocol, which includes serum AST, ALP, hemoglobin, and urea levels, may be required compared to those who receive cholecalciferol.
(zza)
Sources:
1. Bikle D., Christakos S. New aspects of vitamin D metabolism and action − addressing the skin as source and target. Nat Rev Endocrinol 2020; 16 (4): 234–252, doi: 10.1038/s41574-019-0312-5.
2. Dusso A. S., Bauerle K. T., Bernal-Mizrachi C. Non-classical vitamin D actions for renal protection. Front Med (Lausanne) 2021; 8 : 790513, doi: 10.3389/fmed.2021.790513.
3. Ikegami K., Saito M., Imai S. et al. Investigation of prescription status and exploration of risk factors related to denosumab-induced hypocalcemia in combination therapy with 1α,25-dihydroxy-vitamin D3. Biol Pharm Bull 2023; 46 (1): 95−101, doi: 10.1248/bpb.b22-00649.
4. Ikegami K., Hashiguchi M., Kizaki H. et al. Development of risk prediction model for grade 2 or higher hypocalcemia in patients with bone metastasis treated with denosumab plus cholecalciferol (vitamin D3)/calcium supplement. J Clin Pharmacol 2022; 62 (9): 1151–1159, doi: 10.1002/jcph.2057.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Clinical oncology Pneumology and ftiseology Radiotherapy
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


