-
Medical journals
- Career
Safety of Tofacitinib in Ulcerative Colitis – Combined Results of 6 Studies
7. 12. 2023
At the annual meeting of the American Gastroenterological Association (ACG) in October 2023 in Vancouver, Canada, the results of the final safety analysis of tofacitinib in the treatment of ulcerative colitis from a clinical trial program involving 1,157 patients with exposure duration to this therapy of up to 9.2 years were presented.
Introduction
Tofacitinib is a Janus kinase (JAK) inhibitor indicated, among others, for patients with ulcerative colitis (UC). Its safety in treating this inflammatory disease was evaluated in a clinical trial program that included an 8-week phase II induction study, two identical 8-week phase III induction studies (OCTAVE Induction 1 and 2), a year-long phase III maintenance study (OCTAVE Sustain), an open-label extension maintenance study (OCTAVE Open), and a phase IIIb/IV study (RIVETING).
Analysis Methodology and Evaluated Population
Safety data of tofacitinib in patients with UC were analyzed in maintenance therapy cohorts and overall cohorts. There were 6 groups: For patients in the maintenance therapy cohort, safety was compared for treatment with tofacitinib 5 mg twice daily (1) or 10 mg twice daily (2) versus placebo (3). In the overall cohort, the safety of tofacitinib at a predominant dose of 5 mg twice daily (average daily dose < 15 mg) (4), at a predominant dose of 10 mg twice daily (average daily dose ≥ 15 mg) (5), and collectively in all patients treated with 5 or 10 mg twice daily (6) was evaluated in phase II, III studies, in the open-label extension, and in phase IIIb/IV.
The overall cohort included patients with a median follow-up duration of 1.7 years (average 2.8 years, range 1 day to 9.2 years). Tofacitinib was taken for more than 2 years by 47.7% of patients, and 81.1% of all enrolled patients were taking the 10 mg dose twice daily. Parameters such as average age, overall average Mayo score, duration of UC, and the proportion of patients with previous TNF-α inhibitor treatment failure, previous use of immunosuppressive therapy, and corticosteroid use at study entry were similar across groups.
Findings
Incidence of Adverse Events
The incidence of adverse events, the most common individual adverse events, serious adverse events, and the proportion of patients who discontinued treatment due to adverse events are summarized in the following table. The most common adverse events during tofacitinib treatment were worsening UC, nasopharyngitis, and arthralgia.
Table: Incidence of Adverse Events in Tofacitinib Studies for the Treatment of Ulcerative Colitis by Evaluated Therapy
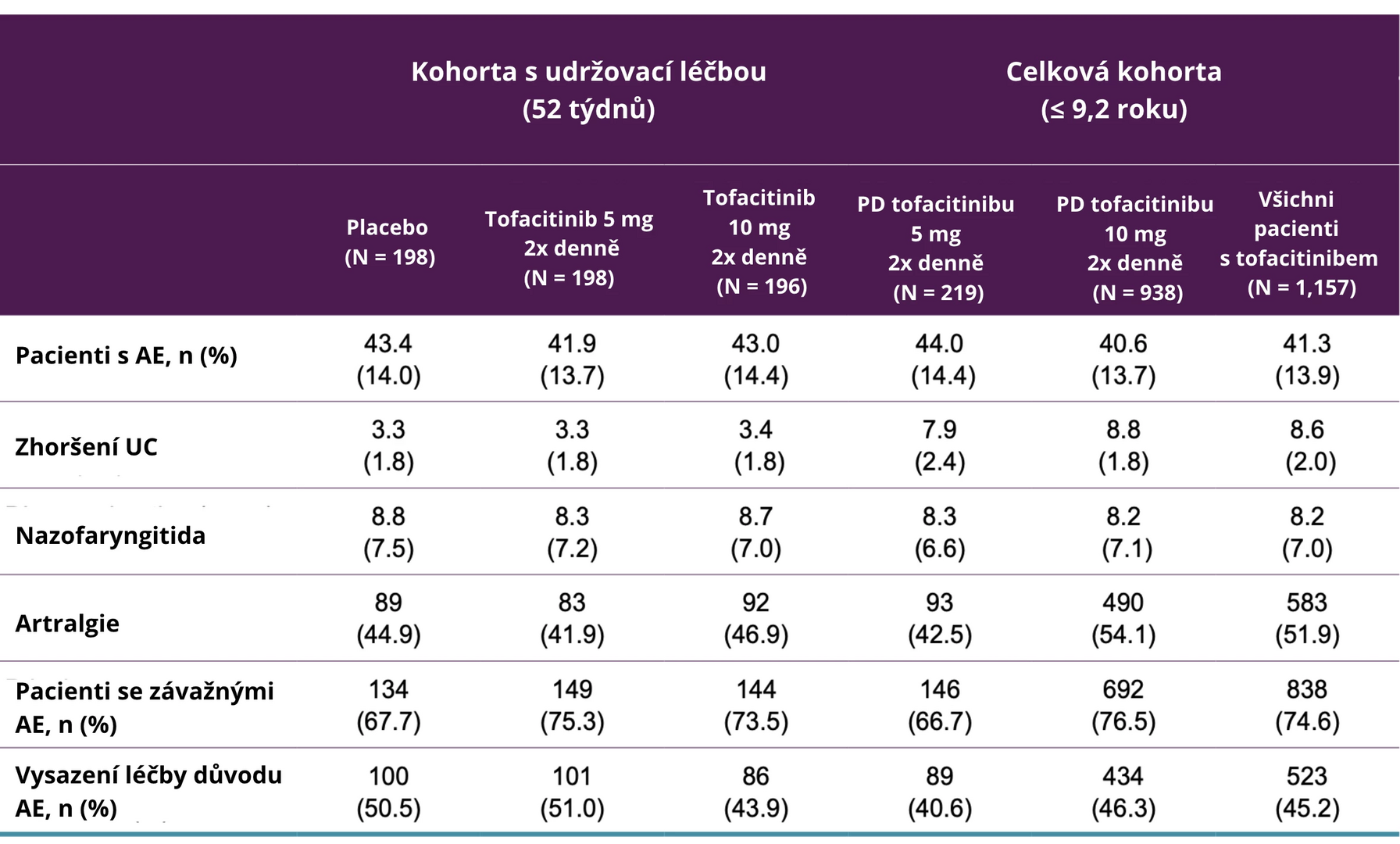
Note: AE – adverse event; PD – predominant dose; UC – ulcerative colitis.
Incidence of Adverse Events of Special Interest
The incidence of adverse events of special interest (infections, malignancies, gastrointestinal perforation, major adverse cardiovascular events, and thromboembolism) in the maintenance therapy cohort and the overall cohort is illustrated in the figure below. In the overall cohort, the incidence of adverse events of special interest, except for serious infections and all herpes zoster infections, generally remained < 1 case per 100 patient-years of exposure. The incidence of these adverse events remained stable during continued treatment for up to 9.2 years.
Figure: Incidence of Adverse Events of Special Interest per 100 Patient-Years of Tofacitinib Exposure
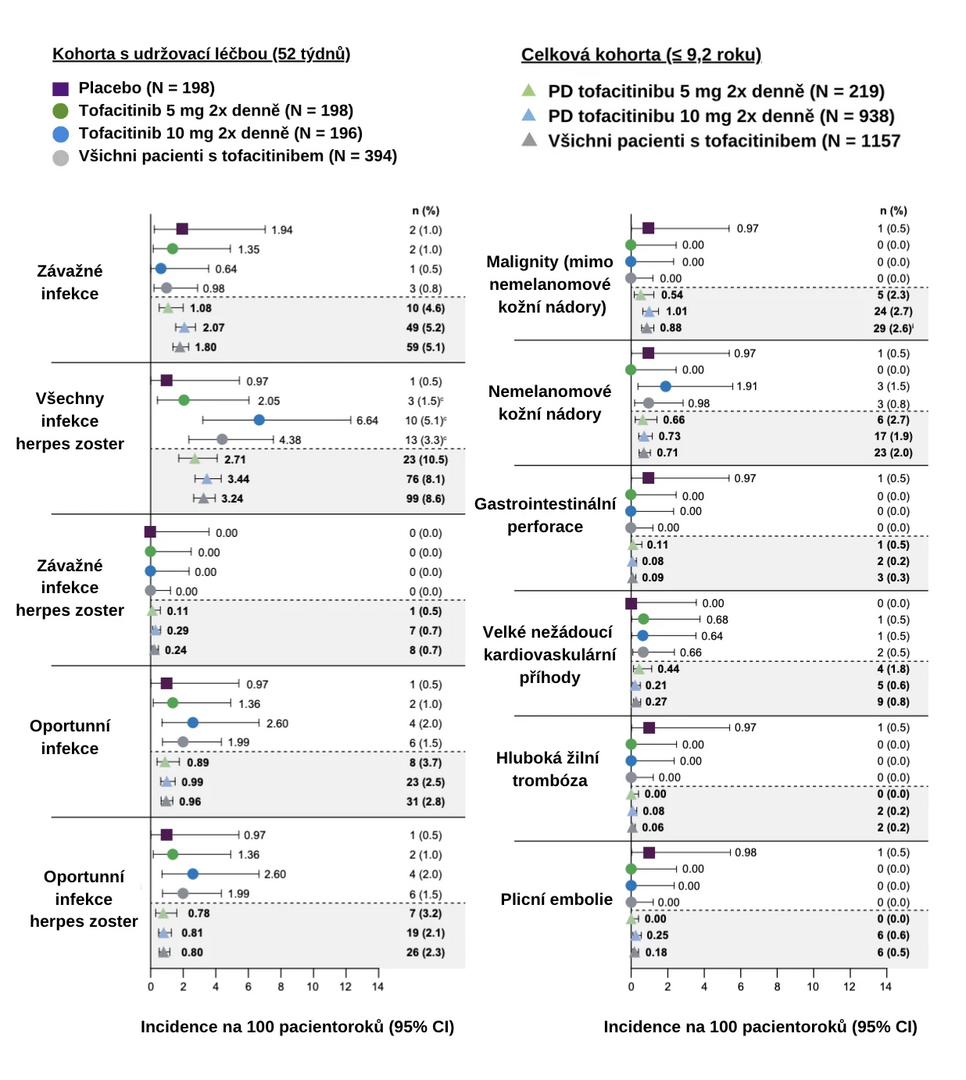
Note: CI – confidence interval; PD – predominant dose.
Fatal Adverse Events
During tofacitinib treatment, there were 8 deaths, each with a different cause: acute myeloid leukemia (after 347 days of tofacitinib treatment), aortic dissection (after 31 days), cardiac arrest due to lung cancer (after 1,725 days), pneumonia and respiratory failure due to COVID-19 infection (after 2,342 days), liver angiosarcoma (after 187 days), malignant melanoma (after 1,359 days), metastatic colorectal cancer (after 2,124 days), and pulmonary embolism due to cholangiocarcinoma (after 378 days).
Limitations
When interpreting these results, it is important to note that the overall cohort includes patients with dose changes of tofacitinib, thus lacking dose-dependent safety analysis. Additionally, overall exposure to tofacitinib was 3 times greater at the dose of 10 mg twice daily compared to 5 mg twice daily.
Conclusion
The safety profile of tofacitinib in the treatment of patients with UC aligns with findings obtained for other indications of this drug and data collected from real-world clinical practice.
(zza)
Source: Panés J., D’Haens G. R., Sands B. E. et al. P3632 Tofacitinib for the treatment of ulcerative colitis: up to 9.2 years of safety data from the global clinical program. ACG 2023 Annual Scientific Meeting, Vancouver, 2023 Oct 20–25.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Paediatric gastroenterology Gastroenterology and hepatology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

