-
Medical journals
- Career
Impact of Tofacitinib on the Quality of Life of Patients with Ulcerative Colitis
7. 12. 2023
Tofacitinib is an oral Janus kinase (JAK) inhibitor used in the treatment of certain rheumatic diseases and ulcerative colitis (UC). The work presented at this year's 18th congress of the European Crohn's and Colitis Organization (ECCO) in Copenhagen, Denmark, focused on its impact on the quality of life in terms of physical and mental functions in patients with UC.
Study Methodology
The results presented at the conference were derived from the analysis of data from the OCTAVE Sustain clinical study. Patients with UC who participated in either of the OCTAVE Induction 1 or 2 studies could participate in the OCTAVE Sustain study. In the OCTAVE Sustain study, the effect of tofacitinib at doses of 5 and 10 mg taken twice daily as maintenance therapy compared to placebo was monitored.
For drugs administered long-term, it is necessary to determine whether their efficacy also translates into an improvement in patients' quality of life. The occurrence of symptoms and dysfunctions associated with UC was assessed by patients in the OCTAVE Sustain study using the IBDQ (Inflammatory Bowel Disease Questionnaire-32), which monitors 4 areas (bowel symptoms, general symptoms, emotional and social functioning), and the SF-36 (Short Form-36 Health Survey) which deals with a total of 8 domains within the summary of physical and mental components of quality of life.
The results were analyzed after 52 weeks of treatment. The statistical method of least squares was used and the standardized effect size (ES) was calculated. ES of 0.1 was labeled as insignificant, 0.2 as small, 0.5 as medium, and 0.8 as large.
Results
The study involved 593 patients. In the 52nd week of follow-up, tofacitinib showed significantly better results than placebo. The largest difference (large ES) in the evaluation of the IBDQ questionnaire was noted for fatigue and exhaustion (see Fig. 1a) and stool urgency (see Fig. 1b), with improvements (medium ES) also observed in abdominal pain (see Fig. 1c) and sexual dysfunction (see Fig. 1d).
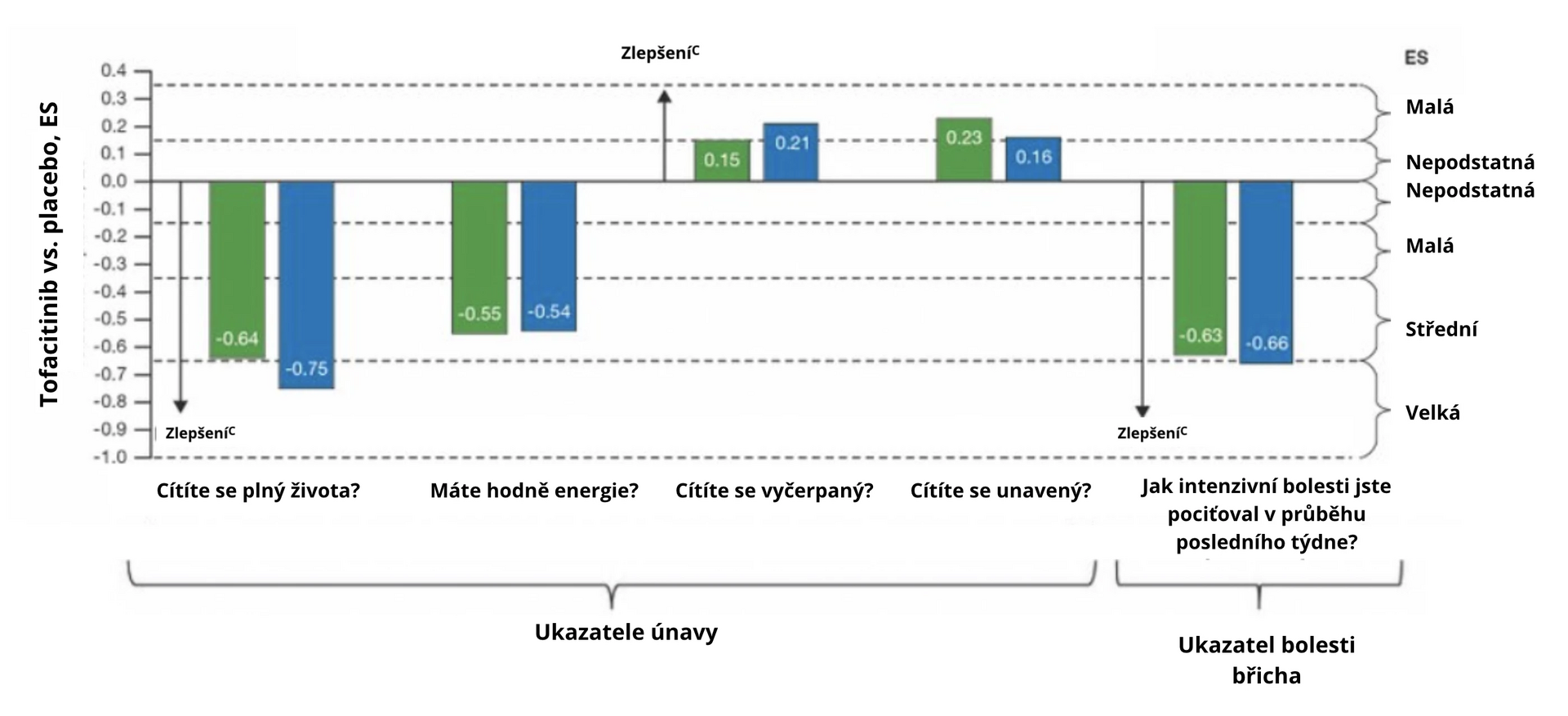
Improvement was also observed with the SF-36 questionnaire in the summary of physical and mental components, with significant differences noted in each of the 8 areas individually. Only in the assessment of fatigue was the impact not as marked (see Fig. 2a). Significant improvement was noted in the assessment of abdominal pain (see Fig. 2b).
The positive effect of tofacitinib persisted throughout the monitoring period. While the difference between baseline values and week 52 was not very large overall with tofacitinib treatment, patients on placebo experienced a clear worsening in all evaluated areas of both questionnaires.
Fig. 1 ES for UC symptoms treated with tofacitinib compared to placebo at week 52 using the IBDQ questionnaire: a) fatigue, b) stool urgency, c) abdominal pain, d) sexual dysfunction
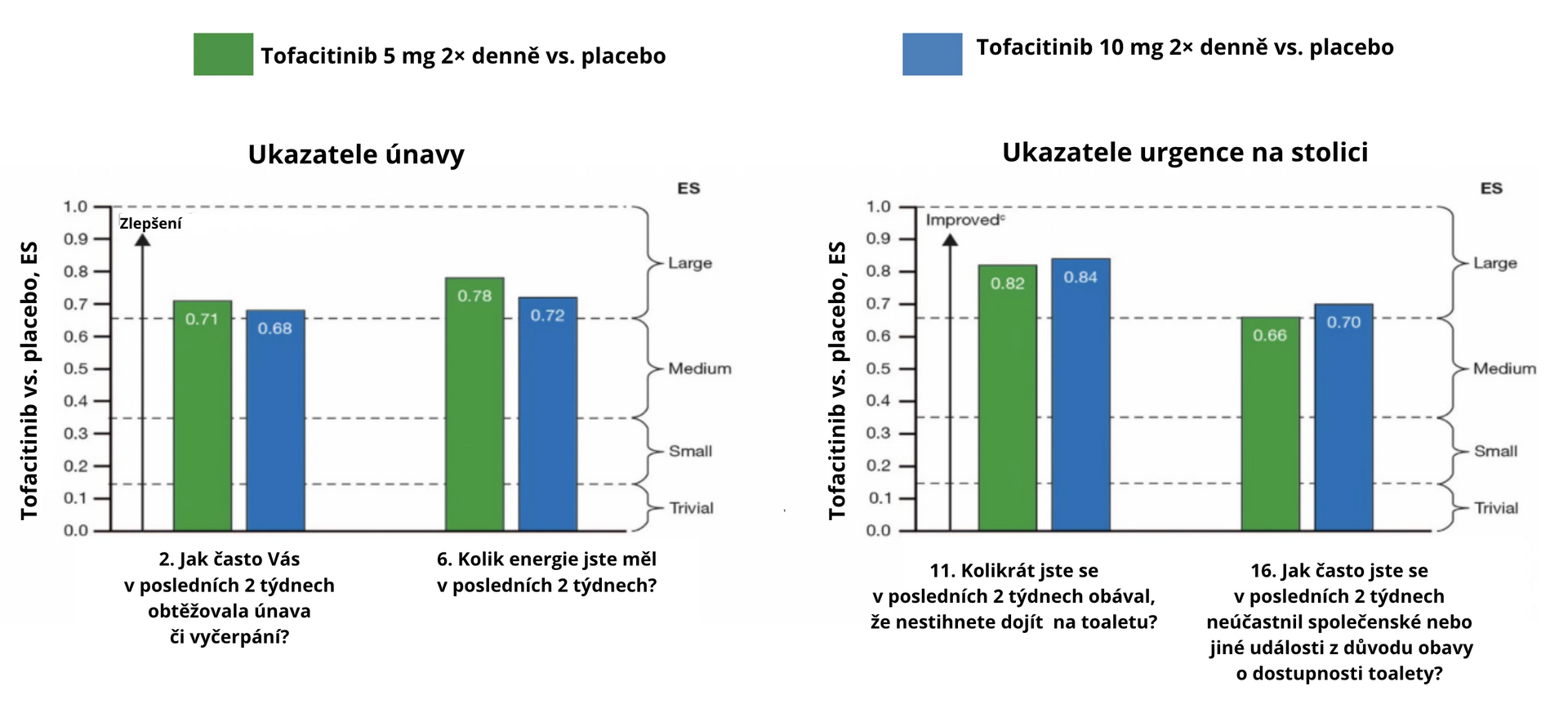
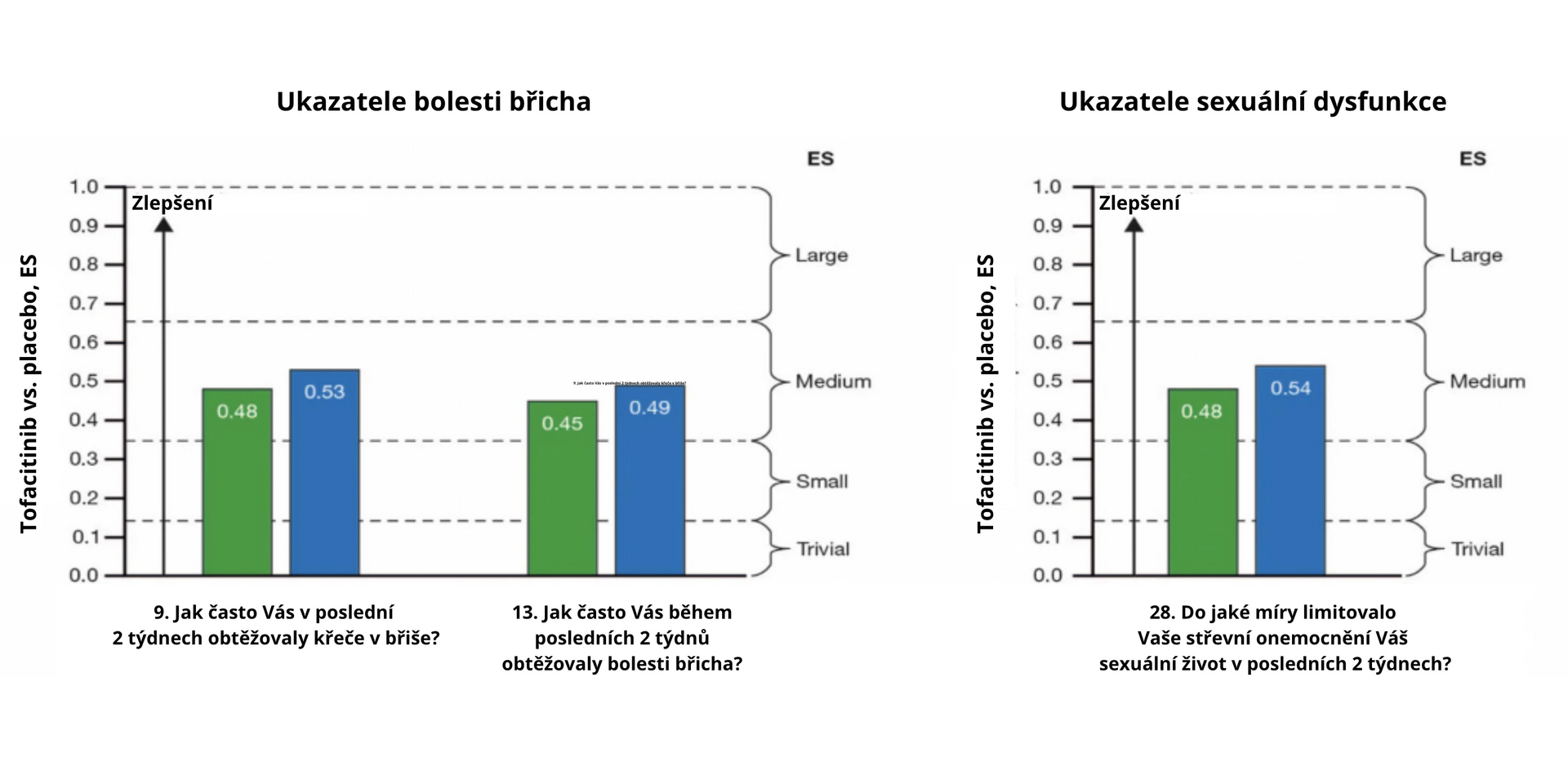
Fig. 2 ES for UC symptoms treated with tofacitinib compared to placebo at week 52 using the SF-36 questionnaire: a) fatigue, b) abdominal pain
Note:
a Standardized ES 0,1 was defined as insignificant, 0,2 as small, 0,5 as medium, and 0,8 as large. Intermediate values (0.15, 0.35, 0.65) were used to define ES intervals, i.e., 0–0,15 insignificant, > 0,15 to 0,35 small, > 0,35 to 0,65 medium, and > 0,65 large.
b Individual items on the IBDQ/SF-36 represented indicators speaking to the individual symptoms of UC.
c Increasing ES value represents improvement in quality of life compared to baseline when comparing tofacitinib with placebo.Conclusion
Compared to placebo, tofacitinib at doses of 5 or 10 mg taken twice daily showed significantly better results in the assessment of quality of life. The positive effects were consistent and lasted throughout the monitoring period.
(kali)
Source: Dubinski M. C., Gardiner S., Hur P et al. Impact of tofacitinib maintenance therapy on key ulcerative colitis patient-reported outcomes of fatigue, urgency, abdominal pain and sexual dysfunction using IBDQ and SF-36 individual items as proxies. J Crohns Colitis 2023; 17 (Suppl. 1): 339–342, doi: 10.1093/ecco-jcc/jjac190.0316.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Paediatric gastroenterology Gastroenterology and hepatology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

