-
Medical journals
- Career
Reduction of Risk of Hospitalizations for Heart Failure in Type 2 Diabetics
2. 12. 2021
The EMPA-REG OUTCOME study already in 2015 highlighted one of the benefits of the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, which is the reduction of the risk of hospitalization for heart failure in patients with type 2 diabetes mellitus (T2DM) with high cardiovascular (CV) risk. Compared to placebo, empagliflozin reduced this risk by a relative 35%.
Study Progress, Population and Target Parameters
EMPA-REG OUTCOME was an international randomized placebo-controlled trial that included 7020 patients with type 2 diabetes (with HbA1c 53–75 mmol/mol without antidiabetic treatment or with HbA1c 53–86 mmol/mol on stable antidiabetic treatment) and known CV disease. As part of the study, patients were given either empagliflozin at a dose of 10 or 25 mg once daily or placebo in addition to their existing treatment.
The primary monitored parameter was composite and included death from CV causes, non-fatal myocardial infarction (MI), and non-fatal stroke. The median follow-up duration was 3.1 years.
Results
Primary Parameter
The incidence of the primary monitored parameter was significantly lower with empagliflozin treatment (10.5%) than with placebo (12.1%): hazard ratio (HR) 0.86; 95% confidence interval (CI) 0.74–0.99; p < 0.001 for non-inferiority and p = 0.04 for superiority of empagliflozin. In addition, treatment with empagliflozin was associated with a significant reduction in CV mortality (by 38%), overall mortality (by 32%), and hospitalizations for heart failure (by 35%).
Rate of Hospitalization
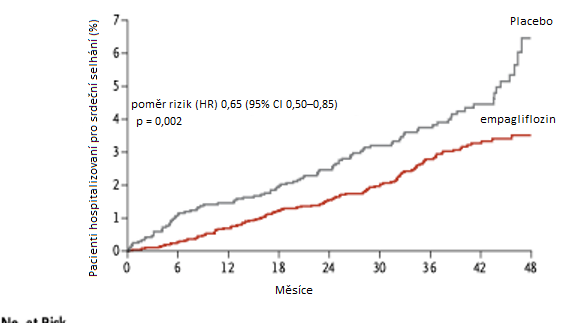
During the study, 4.1% of patients in the placebo group were hospitalized for heart failure compared to 2.7% of patients with empagliflozin. This corresponds to 14.5 hospitalizations for heart failure per 1000 patient-years with placebo and 9.4 hospitalizations for heart failure per 1000 patient-years with empagliflozin (HR 0.65; 95% CI 0.50–0.85; p = 0.002). The divergence in the curves illustrating hospitalization for heart failure occurred within the first months of treatment. A post-hoc analysis of this study also showed that the benefit of empagliflozin was similar in patients with and without a history of heart failure at study entry.
Fig. Hospitalizations for heart failure in the EMPA-REG OUTCOME study with empagliflozin treatment and placebo in patients with T2DM and high CV risk
Conclusion
The presence of type 2 diabetes represents a risk for the development of heart failure. Empagliflozin is the first antidiabetic medication that has been shown, in addition to reducing CV risk compared to placebo, to significantly decrease the incidence of hospitalizations for heart failure in patients with T2DM and known CV disease.
(zza)
Source: Zinman B., Wanner C., Lachin J. M. et al.; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373 (22): 2117–2128, doi: 10.1056/NEJMoa1504720.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Angiology Internal medicine Cardiology
Latest courses
Authors: MUDr. Sylvie Štrégl Hrušková, prof. MUDr. Michal Vrablík, Ph.D., prof. MUDr. Vojtěch Melenovský, CSc., MUDr. Marie Lazárová
Go to courses
Popular this week Whole articleLogin#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


