Meta-analysis of Efficacy and Safety of Third-Generation Antiseizure Medications in Adjunctive Treatment of Focal Seizures in Adults with Epilepsy
Epilepsy treatment is primarily symptomatic, aimed at reducing seizure frequency. However, more than 50% of patients do not achieve seizure freedom with monotherapy. Below we summarize the results of a meta-analysis comparing the efficacy and safety of third-generation antiseizure medications in the treatment of focal seizures in adults with epilepsy.
Analyzed Studies and Patient Populations
The meta-analysis included 16 randomized double-blind controlled studies published by June 2021 in the MEDLINE (PubMed), Cochrane (CENTRAL) and US National Institutes of Health Clinical Trials Registry databases. A total of 4507 adult patients, whose disease was not controlled on their current medication, were treated for focal epileptic seizures with one of the third-generation antiseizure medications (ASMs): brivaracetam (BRV; n = 803), cenobamate (CNB; n = 221), eslicarbazepine (ESL; n = 990), lacosamide (LCM; n = 1104) or perampanel (PER; n = 1389). A total of 2246 patients were included in the placebo groups. Following the titration phase, a minimum 12-week maintenance phase followed, during which patients took the medications in the following doses: BRV 50–200 mg/day, CNB 200–400 mg/day, ESL 800–1200 mg/day, LCM 200–400 mg/day, and PER 4–12 mg/day. There were no significant differences between the groups in terms of age, gender, disease duration, baseline seizure frequency, or concomitant ASMs.
The primary parameters observed were the proportion of patients with a ≥ 50% reduction in monthly seizure frequency (defined in most studies as a response to treatment) and 100% (achieving seizure freedom) and the incidence of treatment-related adverse events (TEAEs) leading to discontinuation of treatment.
Findings
Using CNB (particularly at the 400 mg/day dose) in the maintenance phase was associated with a higher rate of ≥ 50% reduction in seizure frequency (see Fig. 1) compared to BRV (odds ratio [OR] 2.02; 95% confidence interval [CI] 1.11–3.66), ESL (OR 1.93; 95% CI 1.07–3.48), LCM (OR 1.86; 95% CI 1.04–3.32), and PER (OR 2.07; 95% CI 1.16–3.70).
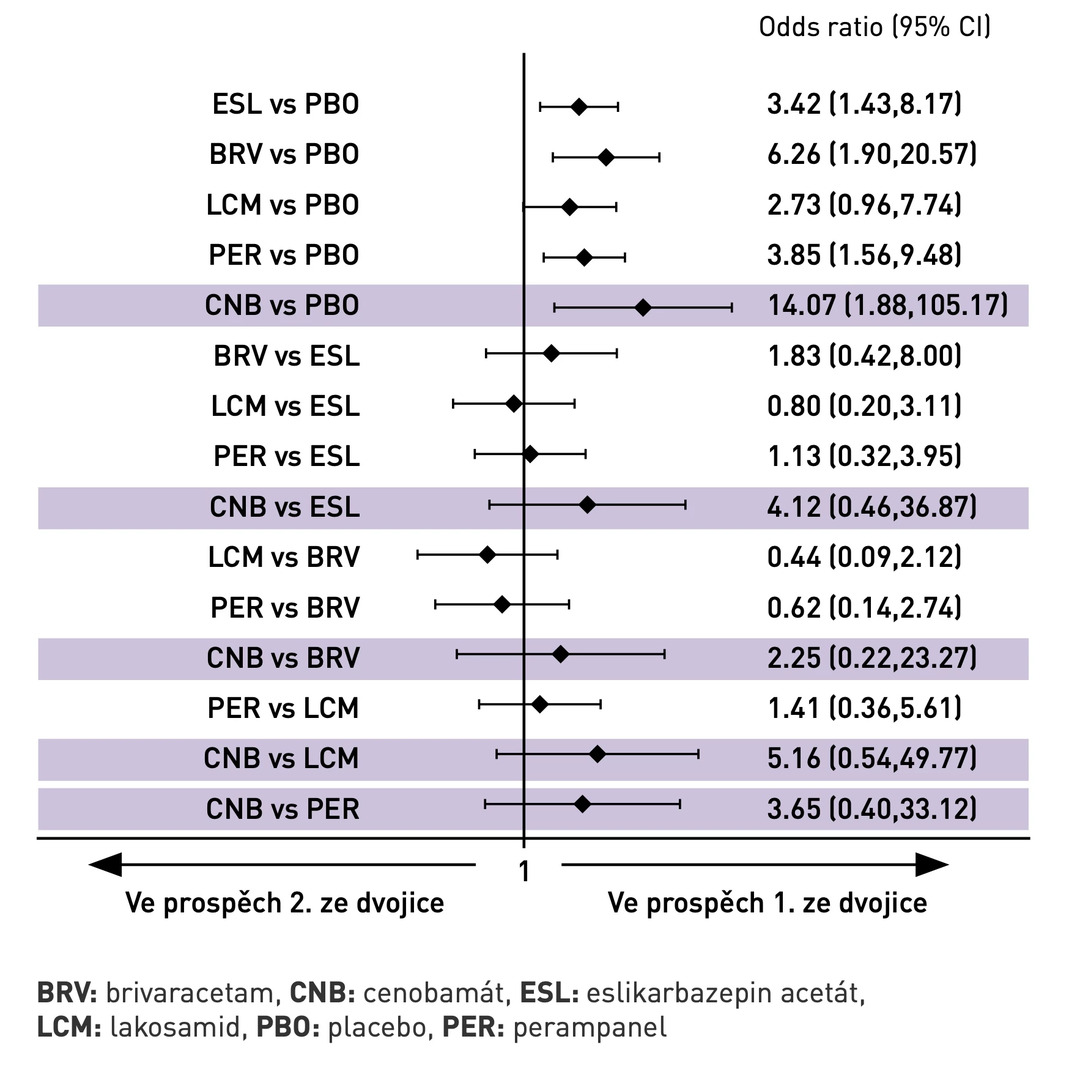
In terms of achieving seizure freedom, the network meta-analysis showed statistically significantly higher efficacy for BRV, CNB, ESL, and PER compared to placebo (see Fig. 2). When analyzed by daily dose, no statistically significant differences were observed among the individual antiseizure medications.
Use of any ASM was associated with a higher proportion of patients experiencing at least one adverse event during treatment compared to placebo. No statistically significant differences were observed among the ASMs in terms of adverse events leading to therapy discontinuation. BRV (OR 0.61; 95% CI 0.44–0.86) and LCM (OR 0.60; 95% CI 0.40–0.88) were associated with a lower incidence of reported adverse events compared to ESL. Patients treated with PER were at a higher risk of experiencing at least one adverse event (OR 1.42; 95% CI 1.02–1.96) compared to BRV.
Fig. 1 Probability of achieving ≥ 50% reduction in seizure frequency (response to treatment)
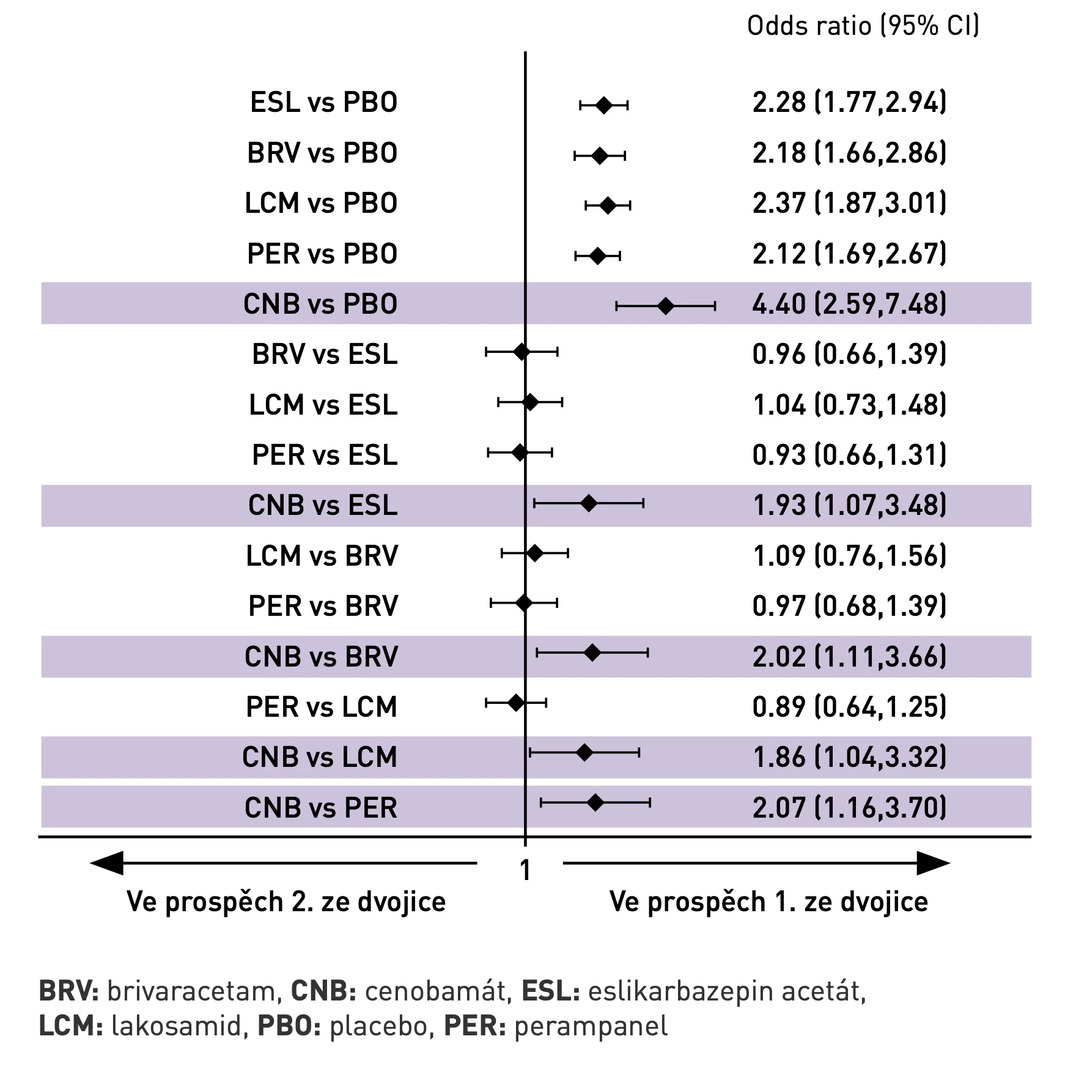
Fig. 2 Probability of achieving seizure freedom
The meta-analysis also revealed that the average disease duration was 22.8 years in the cenobamate group taking the 200 mg dose and 24.4 years in the group taking the 400 mg dose. In both groups, approximately 1/4 of the patients were concurrently taking ≥ 3 concomitant antiseizure medications.
Conclusion
Patients using cenobamate as an adjunctive treatment for focal epileptic seizures have the highest likelihood of achieving a therapeutic response and seizure freedom (compared to other third-generation antiseizure medications). However, real-world practice data will further complement and elucidate the actual therapeutic potential and clinical relevance of this recently approved ASM.
(mafi)
Source: Lattanzi S., Trinka E., Zaccara G. et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs 2022; 82 (2): 199–218, doi: 10.1007/s40265-021-01661-4.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.

