-
Medical journals
- Career
Cenobamate − A Case Study from Slovak Real Practice
23. 12. 2022
Cenobamate is indicated for adjunctive therapy of focal seizures with or without secondary generalization in adult patients who have not achieved sufficient results with previous treatment with at least 2 antiepileptics. This new anti-seizure medication with a dual mechanism of action thus brings another therapeutic option for patients refractory to treatment. At the November 34th Slovak-Czech Epileptology Congress in Bratislava, during a symposium titled “Is there hope for a quality life with epilepsy?” doc. MUDr. Eva Feketeová, PhD., from the Neurological Clinic LF UPJŠ and UNLP in Košice shared her first experiences with cenobamate in clinical practice.
Pharmacoresistant Epilepsy and Treatment Options
Epilepsy is considered pharmacoresistant in the event of failure of 2 therapeutic trials, meaning seizures that significantly impact quality of life persist despite an adequate maximally tolerated dose of the chosen treatment. Further steps in such cases are challenging. Resection epilepsy surgery is effective and often leads to seizure compensation, but it is not indicated in all cases, or the patients themselves may refuse it.
A promising molecule for managing epilepsy that does not respond adequately to existing treatment is cenobamate, due to its good efficacy and favorable safety profile without severe adverse effects. In Slovakia, it has been available through regular distribution networks since August 1, 2022, and with that come the first clinical experiences with this substance.
Characteristics of Cenobamate
According to SPC recommendations, cenobamate is suitable for adult patients with focal epilepsy after the failure of at least 2 previous therapeutic attempts to compensate for epilepsy. It has a dual mechanism of action − it acts on presynaptic membranes by influencing voltage-gated Na+ channels and also has an inhibitory effect through allosteric modulation of GABAA receptor function on the postsynaptic membrane.
The spectrum of adverse effects (AEs) of cenobamate most commonly includes somnolence, dizziness, fatigue, and headache, AEs typical for other anti-seizure medications as well. From a therapeutic limitation perspective, dose adjustments to 200 or 300 mg/day are recommended for patients with liver and renal disorders, respectively. Patients with hemodialysis, end-stage renal disease, and severe liver dysfunction should not use cenobamate.
Regarding drug interactions, cenobamate is not highly risky. It is metabolized through glucuronidation, decreases exposure to carbamazepine, lamotrigine, and substrates of cytochrome P450 CYP3A4/5 and CYP2B6, and increases exposure to phenobarbital, phenytoin, and substrates of cytochrome P450 CYP2C19. Doses of cenobamate up to 500 mg/day do not cause QT interval prolongation.
Findings from Clinical Research
The phase II study C017 published in 2020 evaluated the treatment response rate and change in seizure frequency after a 12-week maintenance phase of cenobamate treatment. The results showed reaching ≥ 50% response rate in almost 2/3 of patients taking a daily dose of 400 mg.
The double-blind phase of the study also focused on the median change in seizure frequency after 18 weeks of adjunctive cenobamate therapy in graded doses (100 mg, 200 mg, 400 mg) compared to placebo administration. Seizure frequency reduction was 55% in patient groups receiving 200 or 400 mg of cenobamate (p < 0.001 vs. placebo).
Cenobamate has a high ability to affect secondarily generalized focal seizures, i.e., those progressing to bilateral tonic-clonic seizures, which are associated with high risk of injury and patient stigmatization.
A Case Study from Practice and Its Results
Docent Feketeová further described experiences with the first 12 patients in her clinic prescribed cenobamate. The average age of these patients was 40 years, with focal seizure epilepsy averaging 26 years. Resection epilepsy surgery was not indicated for them, or the patients strictly refused it. Palliative procedures such as vagus nerve stimulator implantation were performed or planned for half of them, and one patient underwent callosotomy. Patients were taking an average of 3 antiepileptics before cenobamate introduction, with a quarter receiving 2 antiepileptics. Before adding cenobamate, all patients were on sodium transmission influencing preparations, with 75% using 2 such preparations. Patients also received SV2A protein influencing drugs, AMPA receptor drugs, or drugs with multiple mechanisms, with one patient taking a substance affecting the GABAA receptor.
Fig. Composition of Patients' Concomitant Medication
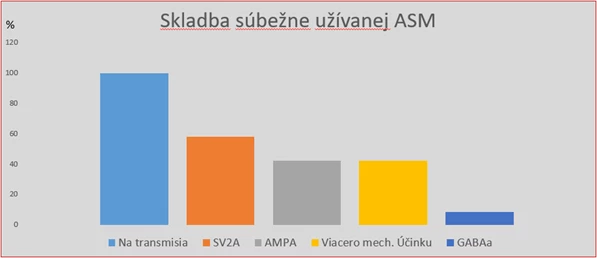
Treatment with cenobamate started according to the recommended protocol with a daily dose of 12.5 mg, gradually increasing to the target dose of 200 mg daily by the 11th week of therapy. This dose was achieved at the time of preparing the lecture by one patient; for others, titration was slower as they noticed effects even at lower doses. Based on clinical response, it will be possible to further increase the daily dose up to 400 mg. Patients were monitored for 2−3 months.
Initial results are very limited due to the observation period, but seizure frequency reduction was observed from a dose of 50 mg. This occurred in all patients, including those who had their other antiepileptic medications reduced. Three patients at 100 mg dose were seizure-free for 2 or 3 months. Adverse effects were relatively mild; one patient experienced a rash after the initial dose. Patients had no issues with morning fatigue or daytime drowsiness, none had DRESS syndrome (drug reaction with eosinophilia and systemic symptoms), suicidal tendencies, or any other serious adverse effects.
Conclusion
Cenobamate is a highly effective anti-seizure medication with a rapid onset of effect, a simple dosing regimen, and a high probability of treatment adherence. Combination with antiepileptics with different mechanisms is advisable. Cenobamate is well tolerated by patients, and one in five has a chance for complete seizure freedom. Based on current observations, significant effect in suppressing seizures is to be expected.
(lexi)
Sources:
1. Feketeová E. Cenobamate − from Theory to Practice. 34th Slovak-Czech Epileptology Congress, Bratislava, November 16, 2022. Available at:
www.kongres.tv/epilepsie2v/existuje-nádej-na-kvalitný-život-s-epilepsiou
2. Krauss G. L., Klein P., Brandt C. et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicenter, double-blind, randomized, placebo-controlled, dose-response trial. Lancet Neurol 2020; 19 (1): 38−48, doi: 10.1016/S1474-4422(19)30399-0.
3. SPC Ontozry. Available at: www.ema.europa.eu/en/documents/product-information/ontozry-epar-product-information_cs.pdf
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Neurology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


