-
Medical journals
- Career
Adjuvant Treatment of Early Invasive HER2+ Breast Cancer − Results of the Katherine Study
8. 4. 2021
Women with breast cancer found to have residual invasive disease during surgery after neoadjuvant chemotherapy and HER2-targeted therapy have a significantly worse prognosis. For these patients, the Czech Oncology Society ČLS JEP recommends adjuvant therapy with trastuzumab emtansine based on data from the Katherine clinical study. However, this treatment has not yet been covered by public health insurance, with reimbursement starting from March 1, 2021.
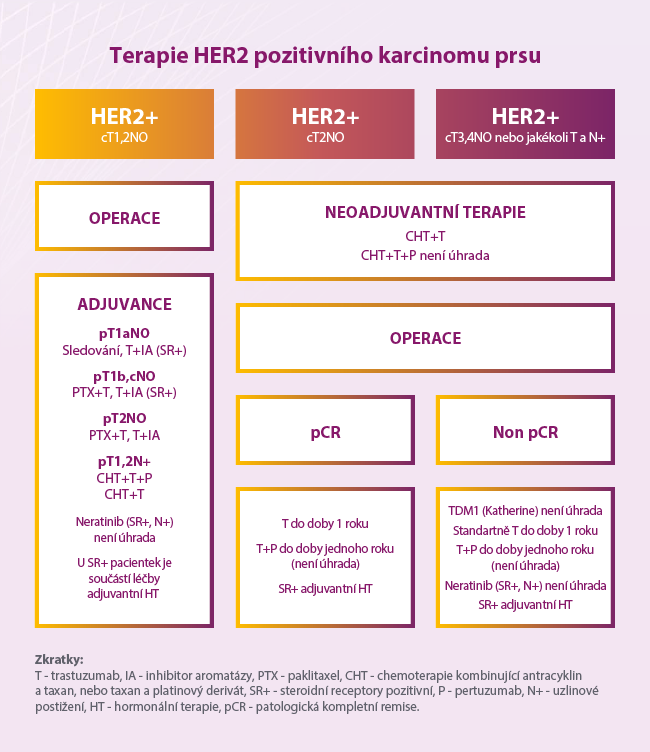
Source: Linkos.cz - Blue BookTrastuzumab emtansine in the treatment of breast cancer
Trastuzumab emtansine (T-DM1) is a conjugate of the microtubule inhibitor DM1 with trastuzumab, a humanized IgG1 antibody targeting the HER2 receptor. Binding of DM1 to trastuzumab allows for selective action of this cytostatic agent directly in HER2-expressing tumor cells.
In two previous phase III clinical trials that included patients with advanced HER2-positive breast cancer pretreated with trastuzumab and chemotherapy, T-DM1 showed better efficacy than the combination of capecitabine with lapatinib or treatment according to the physician's choice. The Katherine clinical study aimed to evaluate the benefit of T-DM1 treatment compared to trastuzumab monotherapy in patients with early breast cancer with residual invasive disease after neoadjuvant treatment.
Study methodology
The study included 1486 patients with HER2-positive early breast cancer who underwent neoadjuvant treatment with trastuzumab and a taxane (possibly combined with an anthracycline) and were found to have residual invasive disease in breast tissue or axillary nodes during subsequent surgery. Patients were randomized 1 : 1 to 14 cycles of adjuvant T-DM1 or trastuzumab. The primary goal of the study was invasive disease-free survival.
Results
At the time of analysis, invasive disease or death occurred in 12.2% of patients treated with T-DM1 and 22.2% of patients in the trastuzumab arm. The estimated 3-year invasive disease-free survival rate was 88.3% for patients treated with T-DM1 compared to 77.0% in the trastuzumab monotherapy group. Invasive disease-free survival was significantly higher with T-DM1 treatment (hazard ratio [HR] for invasive disease or death 0.5; 95% confidence interval [CI] 0.39−0.64; p < 0.001).
Distant metastases occurred as the first recurrence of invasive disease in 10.5% of patients treated with T-DM1 and 15.9% of those on trastuzumab monotherapy. The overall safety profile was consistent with previous observations, and no new or unexpected toxicities were reported. More adverse events occurred in the T-DM1 group compared to trastuzumab.
Conclusion
In patients with HER2-positive early breast cancer who had residual invasive disease after neoadjuvant treatment, subsequent adjuvant treatment with T-DM1 reduced the risk of tumor recurrence or death by 50% compared to trastuzumab monotherapy.
(este)
Sources:
1. von Minckwitz G., Huang C. S., Mano M. S. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380 (7): 617−628, doi: 10.1056/NEJMoa1814017.
2. Malignant Neoplasm of Breast (C50). In: Blue Book of the Czech Oncology Society, 27th update. Masaryk Memorial Cancer Institute, Brno, March 1, 2021. Available at: www.linkos.cz/lekar-a-multidisciplinarni-tym/diagnostika-a-lecba/modra-kniha-cos/aktualni-vydani-modre-knihy/27-12-zhoubny-novotvar-prsu-c50
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Clinical oncology Paediatric clinical oncology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


