-
Medical journals
- Career
Brodalumab in the Treatment of Psoriasis Symptoms on the Scalp and Nails
24. 6. 2020
A post-hoc analysis of 3 clinical studies (AMAGINE-1/-2/-3) evaluates the effect of treatment with brodalumab or ustekinumab (compared to placebo) in patients with mild to severe psoriasis on the scalp and in the nail areas.
Psoriasis on the Scalp and Nail Areas
Up to 80% of psoriasis patients have scalp involvement. Lesions of psoriasis on the scalp lead to complications such as itching, scarring, hair loss, and significantly diminish the quality of life. Topical application in the hair-covered area is challenging, whereas systemic treatment (methotrexate or biologics) is generally indicated for more severe cases.
Nail involvement affects approximately half of psoriasis patients. Nail psoriasis is painful and also significantly lowers the quality of life. The treatment effect of nail psoriasis is delayed compared to other affected areas, resulting in reduced patient adherence to treatment. Both topical and biologic preparations are used; however, standardized treatment guidelines are lacking.
Analyzed Studies
The AMAGINE-1 study evaluated the reduction of scalp involvement using the PSSI (Psoriasis Scalp Severity Index) score in patients treated with brodalumab (210 mg) or placebo every 2 weeks for 12 weeks. Studies AMAGINE-2/-3 evaluated the reduction of nail involvement using the NAPSI (Nail Psoriasis Severity Index) score in patients treated with brodalumab every 2 weeks or continuously with ustekinumab for 52 weeks.
The Effect of Brodalumab on Scalp Psoriasis
After 12 weeks of treatment, a significantly larger number of patients treated with brodalumab achieved PSSI 75 (89% brodalumab vs. 9.5% placebo) and PSSI 100 (63.5% brodalumab vs. 3.2% placebo) compared to the start of the study. Patients treated with brodalumab had a lower average PSSI score over the 12 weeks compared to the placebo group, with significant improvement in PSSI evident as early as 2 weeks of treatment.

Average improvement from baseline PSSI over twelve weeks.
N1: number of patients with a valid measurement at the given week, PSSI: psoriasis scalp severity index, Q2W: every two weeks
*p<.001 vs. placeboComparison of Brodalumab and Ustekinumab in Nail Psoriasis Treatment
Over 52 weeks, more patients treated with brodalumab achieved complete clearance of nail psoriasis compared to ustekinumab − at week 52, NAPSI 0 was achieved in 63.8% of patients treated with brodalumab compared to 39.1% treated with ustekinumab. Patients on brodalumab had a lower average NAPSI score and a higher average improvement percentage from baseline NAPSI compared to those on ustekinumab after one year of treatment.
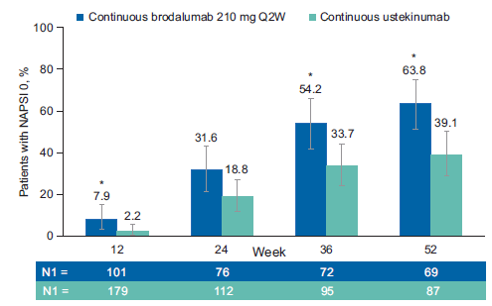
Percentage of patients with NAPSI 0 at weeks 12, 24, and 52 continuing with brodalumab 210 mg every two weeks (Q2W) or ustekinumab.
N1: number of patients with a valid measurement at the given week, NAPSI: nail psoriasis severity index, Q2: every two weeks. *p<.0.5 vs. ustekinumab.Conclusion
Brodalumab has proven effective in significantly reducing or resolving psoriasis symptoms on the scalp after 12 weeks of treatment, and similarly exhibited high efficacy in the treatment of nail psoriasis after 52 weeks.
(eko)
Source: Elewski B., Rich P., Lain E. et al. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatol Treat 2020 Apr 21, doi: 10.1080/09546634.2020.1749546 [Epub ahead of print].
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Dermatology & STDs Paediatric dermatology & STDs
Popular this week- Oncology Care Coordinators Will Shorten Patients’ Journey Through the System
- How Could the Gut Microbiota Be Linked to a Higher Risk of Abdominal Aortic Aneurysm Progression?
- AI Will Help Personalize the Treatment of Atrial Fibrillation
- Czech Gastroenterology Keeps Up with the Times. For 80 Years
- Why Endometriosis Requires the Principles of Precision Medicine
Recommended for you- The Wound Healing Process Step by Step and What Can Complicate It
- Another Positive Effect of Diosmin − Influence on Oxidative Stress
- Dupilumab – The First Biologic Umbrellaing Type 2 Inflammation Treatment: How Does It Work and Who Is It For?
- Diosmin – Still an Important Modality in the Treatment of Venous Insufficiency
- Efficacy and Speed of Pain Relief in Rheumatoid Arthritis When Adding Baricitinib to Methotrexate
- How to Choose the Ideal Venotonic or Vasoprotective – Which Properties Are Key?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

