-
Medical journals
- Career
Prevalence and risk factors of T-cell mediated rejection in patients after liver transplantation from deceased donors: a retrospective study over 10 years
Authors: Svetlana Adamcová-Selčanová 1; Ubomír Skladaný 1; Tomáš Koller 2
Authors‘ workplace: HEGITO (Division Hepatology, gastroenterology and liver transplantation) of Department Internal medicine II, Faculty of Medicine, Slovak Medical University, FD Roosevelt Hospital, Banská Bystrica, Slovakia 1; 5th Department Internal Medicine, Comenius University Faculty of Medicine, University Hospital Bratislava Ruzinov, Bratislava, Slovakia 2
Published in: Vnitř Lék 2020; 66(1): 59-65
Category: Original Contributions
Overview
Introduction: T-cell mediated rejection (TCMR) is still one of the most common non-surgical complications following liver transplantation (LTx).
Aims: To determine the prevalence, risk factors and outcome of TCMR after LTx from deceased donors (DDLT) in a single center.
Methods: Retrospective analysis; Study interval: May 2008-December 2017. Inclusion criteria: DDLT at this TC; exclusion criteria: patients treated with CyA or basiliximab. Recorded variables: demographics, MELD score, Child-Pugh, etiology, CIT (Cold Ischemia Time), BG (blood groups), tacrolimus (TAC) on 5th day post LTx and at discharge, length of hospital stay (LOS), survival. TCMR was defined histologically, liver biopsy was performed only in patients having an increase in liver function tests or unexplained liver dysfunction.
Results: 193 patients were included, median age was 53.6, 41.3 % were females, median MELD score 16.0; Child-Pugh score 10. TCMR was diagnosed in 21 patients (11.4 %). The comparison between groups (TCMR and no-TCMR) showed the following differences: age: 54.3 vs 42.3 years (p = 0.073); etiology of autoimmune hepatitis (AIH) 33.3 vs 6.7 %, (p = 0.001), PSC (Primary Sclerosing Cholangitis) 19.0 vs 6.7 %, (p = 0.13). We observed no significant differences among other etiologies, CIT and BG. Level of TAC on the 5th day post LTx was 5.90 [4.00-9.30] vs 4.80 [2.60-7.00] ng/ml (p = 0.097); TAC at discharge was 9.00 [6.80-11.3] vs 8.9 [7.50-10.6] ng/ml (p = NS); LOS was 35.0 vs 24.5 (p = 0.001). We observed no difference in overall survival between the groups. Multivariate analysis identified independently associated factors with TCMR: AIH (OR = 4.76, 95% CI 1.37-16.46; p = 0.014), absence of significant ascites before LTx (OR = 3.15; 95% CI 1.11-8.95, p = 0.024) and 5th day TAC level (OR = 0.85, 95% CI 0.73-0.997, p = 0.045).
Conclusion: T-cell mediated rejection diagnosed clinically and confirmed histologically occurred in 21 patients (11.4 %). Etiology of AIH, absence of ascites and lower TAC were independent risk factors for TCMR. TCMR had no impact on overall survival.
Keywords:
diagnosis – risk factors – deceased donors liver transplantation – outcome – T-cell mediated rejection
Introduction
Despite significant improvements in immunosuppressive therapy, rejection is still one of the most common non-surgical complications following liver transplantation (LTx) both in the early and later the post-transplant period. T-cell mediated rejection (TCMR) as a complication following LTx was defined in 1995 (1, 2). The prevalence of TCMR has varied between 20 %, and 40 %. More than 60 % of rejection episodes are manifested in the first 3 months after LTx, usually from the 5th to 30th post-transplant day (3, 4). Risk factors for TCMR include a low level of immunosuppression, infancy and younger age, female gender, positive lymphotoxic cross-matching, and autoimmune etiology of underlying liver disease (3–5). Rejection is mediated by antigen-presenting cells (APC), T-lymphocytes, and allogeneic MHC (Major Histocompatibility Complex) peptide complex. There are two well described pathophysiological pathways: the so-called direct activation pathway and indirect pathway (3, 6–9). In TCMR, the direct pathway prevails in the immediate post-transplant period, while the indirect one is more frequent at later stages (6–8). Clinical presentation is non-specific and often vague – patients may present with fever, fatigue, abdominal pain, icterus, progression of ascites and/or liver dysfunction. Often, the only manifestation is the increased activity of liver enzymes and serum bilirubin.
Liver biopsy (LB) remains the gold standard for diagnosing TCMR, where the diagnostic triad is:
A. inflammatory cellular infiltrate in portal fields,
B. inflammation beneath the endothelium of the portal and central veins (endothelitis), and
C. damage to the interlobular bile ducts.
Banff Classification stratifies TCMR histological findings according to Rejection Activity Index (RAI) into mild, moderate and severe (9, 10). There is still a controversy concerning indication strategy for LB, with some centers performing protocol biopsies and some performing LB only in patients with clinical evidence of graft dysfunction (4). Prevention of TCMR is based on the timely initiation of immunosuppressive treatment. Immunosuppressive agents used to treat TCMR are corticosteroid pulses (11, 12), the most common treatment of steroid-resistant TCMR is anti-lymphocyte thymoglobulin (ATG) – a polyclonal lymphocyte preparation (13–17), than antibody-based agents including anti-CD3 muromonab (OKT3), anti-CD20 antibody rituximab, basiliximab and daclizumab (18–21).
Aims
To determine the prevalence, risk factors and outcome of TCMR in patients undergoing liver transplantation from deceased donors (DDLT) in a small-volume transplant center (TC) over the last ten years.
Patient and methods
We conducted a retrospective study at the liver unit in teaching hospital in Central Slovakia. We extracted data from the hospital information center – Care Center® (NIS-CC). Study interval: May 2008 – December 2017. Inclusion criteria: DDLT at this TC; exclusion criteria – patients treated with CyA and basiliximab.
At the moment of listing, we recorded the following variables: age, sex, MELD score (Model for End-Stage Liver Disease), Child-Pugh score, etiology of liver disease, and its cirrhosis complications. Also, we recorded parameters immediately related to the liver transplantation: CIT – Cold Ischemia Time, recipient BT – blood type, tacrolimus (TAC) levels on day five following LTx and at the time of hospital discharge, length of hospital stay (LOS), and overall survival post liver transplantation.
We did not evaluate the donor-recipient matching (mismatches in HLA loci) and cross-matching.
Immunosuppression protocol in our TC includes a triple combination of intravenous methylprednisolone 500 mg in the anhepatic phase followed by a daily intravenous dose of 20 mg, tacrolimus 0.1 mg/kg/ day and mycophenolate mofetil 2 000 mg/day. We excluded patients receiving immunosuppressive induction therapy with basiliximab.
We suspected TCMR in patients having an increase in serum levels of AST (aspartate aminotransferase), ALT (alanine aminotransferase), GGT (gammaglutamyltransferase), ALP (alkaline phosphatase) and or serum bilirubin following liver transplantation or evidence of worsening liver function. To confirm the diagnosis of TCMR, patients underwent percutaneous liver biopsy under ultrasound guidance using a true-cut needle (n = 12). In cases having contraindications to percutaneous liver biopsy (platelet count lower than 50 000 × 109 /l), trans-jugular liver biopsy (n = 9) was carried out. All patients reported an early form of TCMR histologically diagnosed at an interval of up to three months after LTx. Once TCMR was histologically confirmed, we treated patients with intravenous pulses of methylprednisolone with a cumulative dose of 3 grams (1 000 mg/day/3 days). In cases with inadequate response to steroids (a mild form of TCMR), we labeled TCMR as cortico-resistant, and patients received intravenous anti-thymocyte globulin (ATG) at a dose of 2.5–5 mg/kg/day for 14 days (a moderate and severe form of TCMR).
Statistical analysis was performed using a software package MedCalc v.18, Ostende, Belgium. We present all the numerical parameters as mean and standard deviation, categorical variables as percentages. Comparison of TCMR and no-TCMR groups was performed using T-test in normally distributed variables, Mann-Whitney-test in non-normally distributed variables and chi-square test in comparing proportions. To identify factors independently associated with TCMR, we entered all parameters appearing statistically associated with TCMR (p < 0.12) into a backward multivariate logistic regression. We analyzed the overall survival between sexes and TCMR and no-TCMR groups with Kaplan-Meier survival curves. Statistical significance was defined by the probability of null-hypothesis inferior to 0.05.
The study has been carried out following the proceedings of the Helsinki declaration. All the patients have signed an informed consent before any procedure, and separately for liver transplantation. The study has a retrospective design; data were anonymized in the database and patients did not undergo any intervention apart from the usual procedures prior and post liver transplantation. All authors declare having no conflicts of interest. Dataset for this observational study is available on request from authors.
Results
During the reporting period, 196 consecutive DDLT were performed including 3 re-transplantations. The final analysis included 184 patients. Summary statistics and study group characteristics are displayed in table 1. Mean age was 53.6 (43.4–59.4), 41.3 % were females, with average MELD score 16.0 (13.5–18.8) points, mean Child-Pugh score 10.0 (8–11) points.
1. Summary statistics and baseline characteristics of the study group of 184 patients after liver transplantation from deceased donors 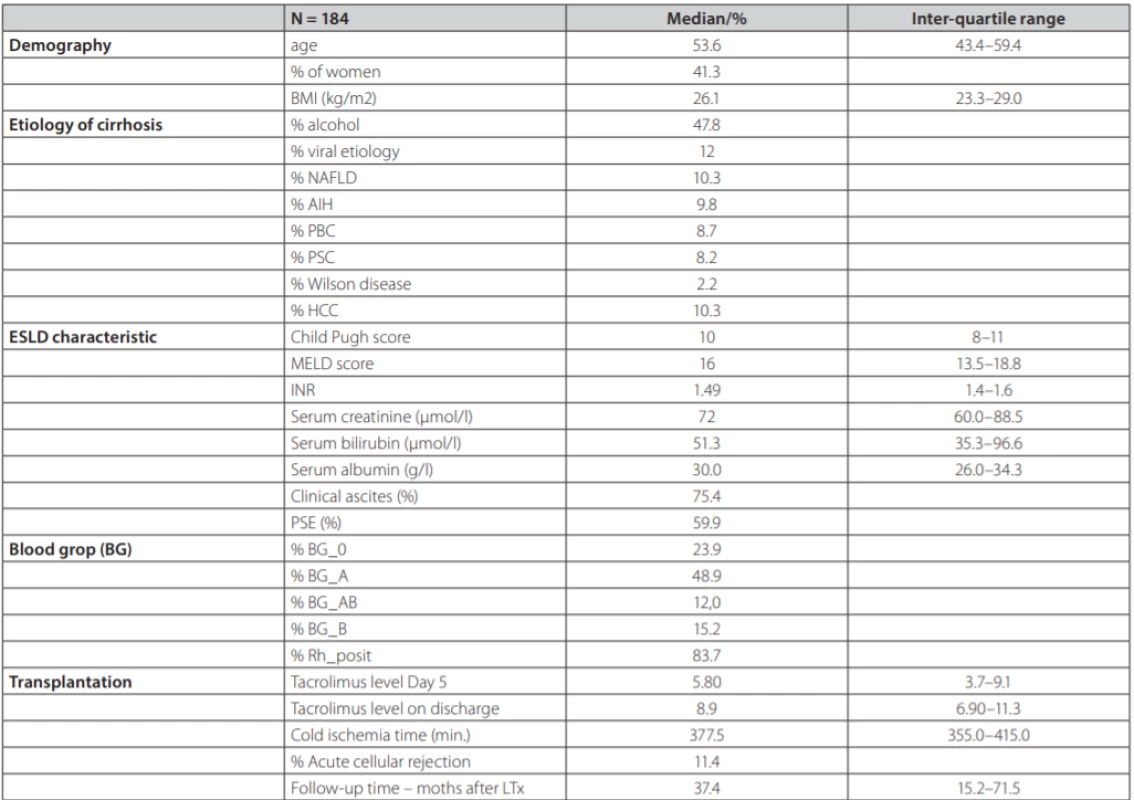
NAFLD – non alcoholic fatty liver disease, AIH – aAutoimmune hepatitis, PBC – pPrimary biliary cholangitis, PSC – primary sclerosisg cholangitis, HCC – hepatocellular carcinoma, PSE – portosystemic encephalopathy TCMR was diagnosed in 21 patients (11.4 %). Each of the patients had only one episode of TCMR. Six patients adequately responded to steroid pulse therapy, histologically, this was a mild form of TCMR. In 15 patients, the response was not adequate and they received second-line therapy with ATG, histologically, these were a mild and severe form of TCMR (table 2) displays the comparison between TCMR and no-TCMR groups. Average age in the group with TCMR vs. no-TCMR was 54.3 (46.3, 59.8) years and 42.3 (37.6, 57.7) years (p = 0.073). Etiology of liver disease was significantly different in AIH 33.3 % vs 6.7 % (p = 0.001); ALD (Alcohol-related Liver Disease) 23.8 % vs 50.9 % (p = 0.035); PSC (Primary Sclerosing Cholangitis) 19.0 % vs 6.7 % (p = 0.13); PBC (Primary Biliary Cholangitis) 14.3 % vs 8.0 % (p = NS); viral hepatitis (HBV, HCV) 4.8 % vs 12.9 % (p = NS); NAFLD (Non-Alcoholic Fatty Liver Disease) 4.8 % vs 11.0 % (p = NS); MW (Morbus Wilson) 4.8 % vs 1.8 % (p = NS) for both groups respectively. Median cold ischemia time (CIT) was 400.0 vs 377.0 min (p = NS) respectively. Representation of individual blood groups (BG) did not show any difference. Tacrolimus concentration at day 5 post LTx was 5.90 (4.00, 9.30) ng/ml vs 4.80 (2.60, 7.00) ng/ml (p = 0.097); tacrolimus concentration at discharge from the hospital was 9.00 (6.80, 11.3) ng/ml vs 8.9 (7.50, 10.6) ng/ml (p = NS) respectively. Length of hospital stay (LOS, group TCMR vs no-TCMR) was 35.0 vs 24.5 days (p = 0.001) respectively.
2. Comparison of selected parameters between groups according to T cell mediated rejection occurring among 184 patients after liver transplantation (LTx) from deceased donor 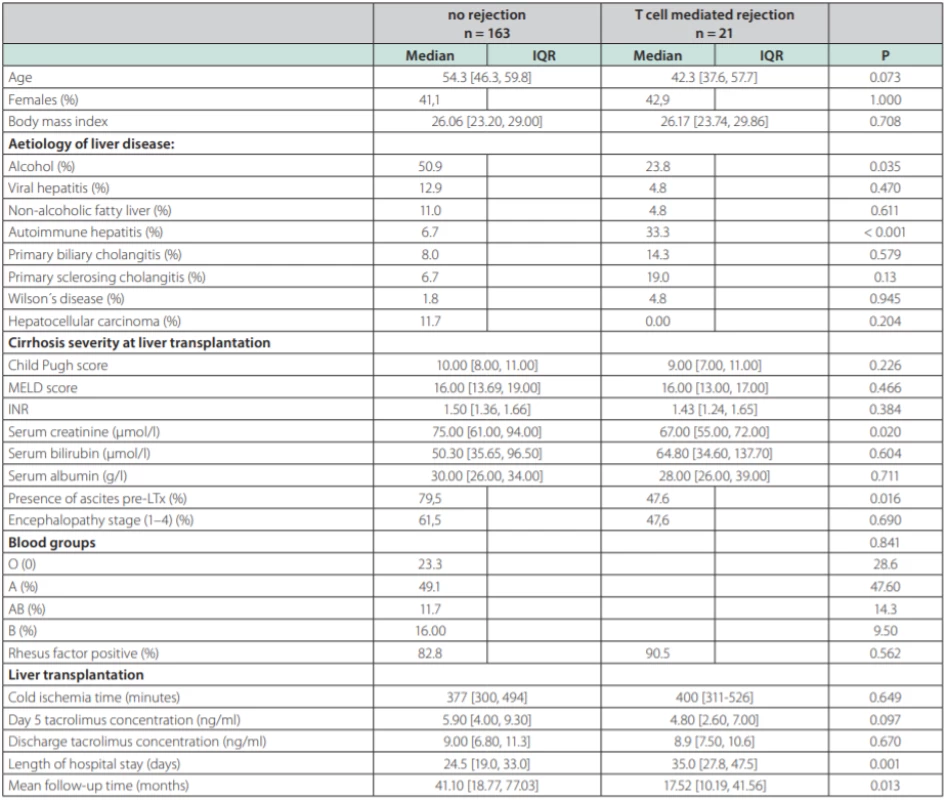
In univariate analysis, recipient age, alcoholic and autoimmune etiology, pre-transplant serum creatinine, ascites and 5th day tacrolimus concentration were identified as factors being associated with the TCMR. Multivariate logistic model displayed in table 3 reveals three independent risk factors of TCMR: autoimmune liver disease (OR = 4.76, 95% CI 1.37-16.46; p = 0.014); absence of clinically significant ascites prior to LTx (OR = 3.15; 95% CI 1.11-8.95, p = 0.024) and 5th day tacrolimus concentration (OR = 0.85, 95% CI 0.73-0.997, p = 0.045).
3. Multivariate logistic regression in search for independent predictors of T-cell mediated rejection in 184 patients undergoing liver transplantation from deceased donors 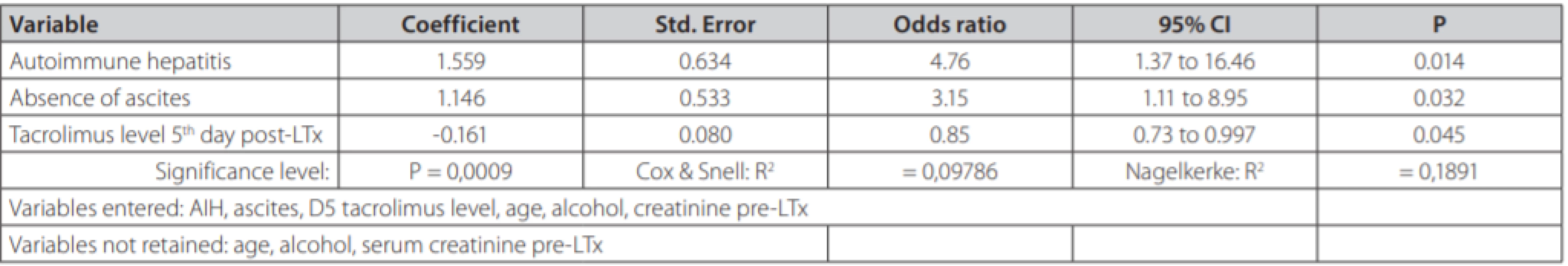
Analysis of overall survival post LTx and the survival outcome of TCMR are displayed in figures 1 and 2. Kaplan-Meier survival curves reveal no difference in overall survival between genders (fig. 1) and according to TCMR status (fig. 2).
1. Overall survival of 193 patients undergoing liver transplantation according to gender. Dotted line: females, full line: males, P = 0.80 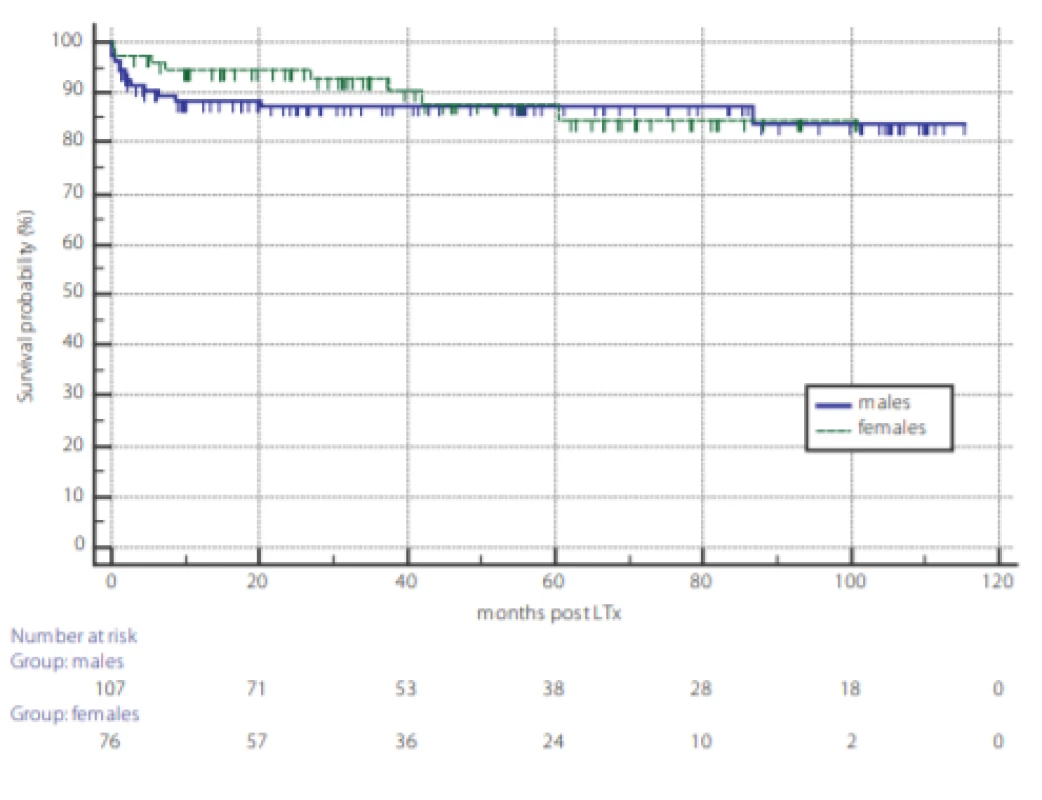
2. Survival probability after liver transplantation according to the occurrence of T cell mediated rejection (TCMR). TCMR – dotted line, no-TCMR – full line, P = 0.232 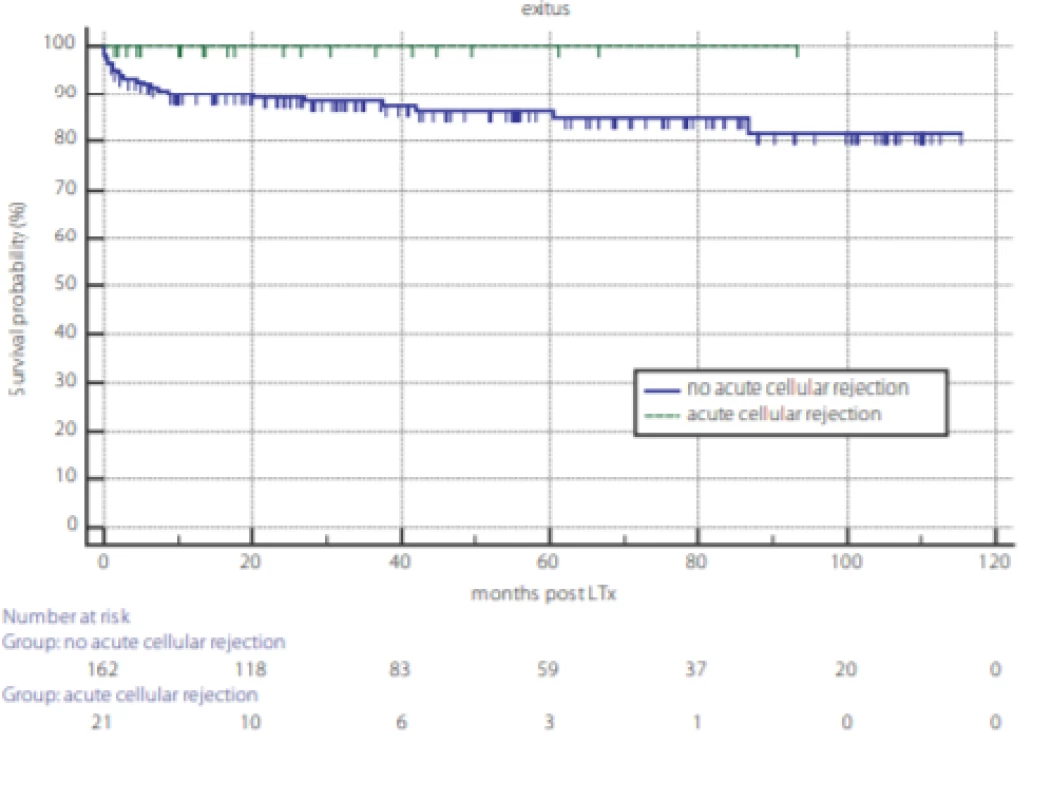
Discussion
In our study, in a single low-volume liver transplant center over past 10 years, TCMR prevalence was 11.4 %. Three independent risk factors were identified: autoimmune liver disease, absence of clinically significant ascites prior to liver transplantation and lower serum tacrolimus level on the 5th post-transplant day.
The prevalence of TCMR following liver transplantation varies greatly among studies (3, 4) and is mostly dependent on the diagnostic strategy that has been used to define TCMR. Newer studies evaluating tacrolimus-based regimen tend to report lower incidence of TCMR compared with older studies using cyclosporin (22). Combination regimen with tacrolimus and mycophenolate has further decreased the risk. Studies with protocol biopsies have also reported a slightly higher prevalence of TCMR compared with studies when liver biopsies were performed only when clinical or laboratory evidence of graft dysfunction was suspected (23). Our study reports TCMR prevalence in the lower end of the reported intervals. The observation might be explained by lower prevalence of autoimmune liver disease and by our diagnostic strategy based on clinical and biochemical evidence. Per-protocol biopsies at pre-specified intervals after liver transplantation were not performed in our center (2, 3, 5, 23). Liver histology was used only for TCMR confirmation thereby underestimating the true prevalence of histological TCMR. However, possible under-diagnosis of TCMR did not appear to have any adverse impact on patients´ prognosis.
As for TCMR risk factors, patients with AIH had a 4.76-times higher likelihood of TCMR than other etiologies. We also tested the hypothesis, that both autoimmune hepatitis and primary sclerosing cholangitis carry a higher risk of TCMR. However, odds ratio for both diseases in the same multivariate model would be much lower (1.16) compared with odds ratio for solo autoimmune hepatitis (table 3). The absence of clinically significant ascites (stage 2 and 3) prior to LTx, increased the risk 3.15 times. Tacrolimus concentration in TCMR group was lower compared with non-TCMR group, but the difference did not reach statistical significance probably due to different numbers in both groups. However, in multivariate analysis after correction for age, alcohol etiology, autoimmune etiology, creatinine and ascites we found an independent association between lower tacrolimus concentration on day 5 and the higher risk of TCMR. This finding appears consistent with previous reports and also appears plausible. In the end, it is the serum concentration of this immunosupressive drug as the single modifiable risk factor of TCMR. We did not confirm significant association between TCMR and Child-Pugh, MELD scores, the blood type or the length of cold ischemia time. However, the presence of TCMR, with a need for its adequate treatment, led to a significantly prolonged hospital stay compared with patients without TCMR. In the literature, autoimmune liver disease has been frequently reported as ACR risk factor (4, 23, 24). The impact of liver function prior to LTx on the risk of TCMR has been evaluated in 133 patients by Gomez-Manero et al [25]. The study reports that patients with a Child-Pugh score of A and those without ascites had higher risk of TCMR following LTx. In addition, a multicenter study has also identified a trend for lower pre-transplant MELD score among patients developing port-transplant TCMR (26). In our study, MELD score did not predict TCMR, but it was lower than in the mentioned reports. Different selection criteria and liver allocation protocols between centers might also explain observed differences. More severe liver disease in patients with ascites likely leads to more profound immune system paralysis, eventually being associated with lower probability of rejection post LTx. Ascites is often associated with bacterial translocation leading to constant interaction of translocated bacteria with antigen presenting cells and toll-like receptors. Our study brings evidence, that the absence of ascites prior to LTx is a single most important clinical predictor for TCMR. However, the number of candidates for LTx without ascites in real-life is probably low. Low serum levels of immunosuppressive medication in the early post-transplant period have been described as a risk factor for TCMR (27–29). To date, the impact of TCMR on patient and graft survival after LTx has been evaluated in a single report by (23), revealing that TCMR had no impact on patients´ survival.
It has been previously shown, that agreement among clinicians from multiple liver transplant centers on the clinical criteria for selecting patients for liver biopsy is very poor (26). These data probably reflect differences among protocols which are being followed in liver transplant centers. In the management of liver transplant recipients, there is apparently a clinical challenge. On one side, the diagnosis of TCMR requires liver biopsy with its´ non-negligible drawbacks. On the other, currently we are unable to diagnose TCMR without liver histology. Although undiagnosed TCMR in our study did not lead to any significant adverse outcome, there is evidence that some patients with undiagnosed TCMR without biochemical graft dysfunction develop clinically overt TCMR over time. In a meta-analysis of 15 studies including 1 566 patients undergoing per-protocol biopsies, 331 of patients had histological evidence of TCMR with no biochemical graft dysfunction, and 36 of them eventually developed clinically significant TCMR. In the study, 7 patients had steroid-refractory TCMR and 9 patients subsequently developed chronic rejection (30, 31). However, the risk of progressive graft dysfunction in undiagnosed TCMR was very low, and authors did not recommend protocol liver biopsies in all patients. Recently, Rodriguez-Peralvarez et al in their multicenter study have proposed a risk score for non-invasive prediction of histologically diagnosed TCMR (26). This model has not been used in our transplant center while we are currently awaiting results of validation studies. Meanwhile, even the issue of per-protocol liver biopsies in high-risk patients (lower age, autoimmune etiology of the primary disease and potentially patients with less severe liver disease) remains open. Our study brings some more evidence in favor of performing LB in selected patients only, with the selection strategy open to results of additional studies. Alternative strategy would be a pre-emptive use of induction immunosuppressive regimen in patients at risk. For example, basiliximab given in combination with a tacrolimus-based immunosuppressive regimen, has been associated with lower incidence of TCMR, excellent short-term rejection-free graft and higher overall survival after LTx (32–34). In addition, anti-IL-2 induced regimen could prevent subsequent treatment with higher doses of calcineurin inhibitors with potential nephrotoxicity (35). Further studies validating the efficacy and safety of both strategies are warranted.
Our study has several strengths. We report TCMR prevalence in consecutive cases from a single small-volume liver transplantation center for the period of 10 years. The prevalence of TCMR from the Middle or Eastern European transplant centers has not been reported yet. Limitation of our study is a single center experience with relatively low number of cases. However, single center experience provides results for a homogenous group of patients with unified selection criteria for liver transplantation candidates, single immunosuppressive protocol and one protocol for diagnosing TCMR and selecting patients for liver biopsy. Obvious limitation of our study is the lack of pre-scheduled per-protocol liver biopsies in all transplanted patients, but this strategy is not widely recommended.
Conclusions
T-cell mediated rejection diagnosed clinically and confirmed histologically occurred in 21 patients (11.4 %). Etiology of AIH, absence of ascites and lower TAC were independent risk factors for TCMR. TCMR had no impact on overall survival.
KORESPONDENČNÍ ADRESA AUTORA:
MUDr. Svetlana Adamcová Selčanová, Ph.D.,
II. interná klinika SZU, FNsP F. D. Roosevelta,
Nám. L. Svobodu 1,
Banská Bystrica,
Slovakia
Cit. zkr: Vnitř Lék 2020; 66(E-1): e4–e10
Článek přijat redakcí: 24. 1. 2019
Článek přijat k publikaci: 24. 4. 2019
Sources
1. Terminology for hepatic allograft rejection. International Working Party. Hepatology 1995; 22 : 648–654.
2. Fisher L, Henley K, Lucey M Acute cellular rejection after liver transplantation: Variability, morbidity and mortality. Liver Transpl Surg 1995; 1 : 10–15.
3. Neil DA, Hübscher SG. Current views on rejection pathology in liver transplantation. Transpl Int 2010; 23 : 971–983.
4. Sticova E, Honsova E Diagnostika rejekce v transplantovaných játrech. Cesk Patol 2015; 51 : 166–168.
5. Deschenes M, Belle SH, Krom RA, et al. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation 1998; 15 : 302–310.
6. Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens 2007; 69 : 545–556.
7. Stefanova I, Dorfman JR, Tsukamoto M, et al. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev 2003; 191 : 97–106.
8. Afzali B, Lombardi G, Lechler RI Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant 2008; 13 : 438–444.
9. Demetris AJ, Bellamy C, Hübscher SG, et al. 2016 comprehensive update of the Banff Working Group on Liver Allograft Pathology: Introduction of antibody-mediated rejection. Am J Transplant 2016; 16 : 2816–2835.
10. Banff Working Group on Liver Allograft Pathology. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 1997; 25 : 658–663.
11. Goddard S, Adams DH Methylprednisolone therapy for acute rejection: too much of a good thing? Liver Transpl 2002; 8 : 535.
12. Volpin R, Angeli P, Galioto A, et al. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl 2002; 8 : 527.
13. Wiesner RH, Menon KV. Late hepatic allograft dysfunction. Liver Transpl 2001; 7 : 60.
14. Eason JD, Nair S, Cohen AJ, et al. Steroid-free liver transplantation using rabbit anti-thymocyte globulin and early tacrolimus monotherapy. Transplantation 2003; 75 : 1396–1399.
15. Lee JG, Lee J, Lee JJ, et al. Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation. Medicine (Baltimore) 2016; 95 : 3711.
16. Aydogan C, Sevmis S, Aktas S, et al. Steroidresistant acute rejections after liver transplant. Exp Clin Transplant 2010; 8 : 172–177.
17. Lu XJ, Chen YH, Ma Y, et al. Strategies in clinical diagnosis and treatment of steroid-resistant acute rejection after orthotopic liver transplantation. Zhonghua Gan Zang Bing Za Zhi 2016; 24 : 297–301.
18. Bonnefoy-Berard N, Revillard JP Mechanisms of immunosuppression induced by antithymocyte globulins and OKT3. J Heart Lung Transplant 1996; 15 : 435–442.
19. Usuda M, Fujimori K, Koyamada N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation 2005; 79 : 12–16.
20. Koch M, Niemeyer G, Patel I, et al. Pharmacokinetics, pharmacodynamics, and immunodynamics of daclizumab in a two-dose regimen in liver transplantation. Transplantation 2002; 73 : 1640.
21. Fernandes ML, Lee YM, Sutedja D, et al. Treatment of steroid-resistant acute liver transplant rejection with basiliximab. Transplant Proc 2005; 37 : 2179.
22. Haddad EM, McAlister VC, Renouf E, et al. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev 2006; 4: CD005161.
23. Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 1998; 28 : 638–645.
24. Neuberger J, Adams DH. What is the significance of acute liver allograft rejection? J Hepatol 1998; 29 : 143.
25. Gomez-Manero N, Herrero JI, Quiroga J, et al. Prognostic model for early acute rejection after liver transplantation. Liver Transpl 2001; 7 : 246–254.
26. Rodríguez-Perálvarez M, García-Caparrós C, Tsochatzis E, et al. Lack of agreement for defining ‘clinical suspicion of rejection’ in liver transplantation: a model to select candidates for liver biopsy. Transpl Int 2015; 28 : 455–464.
27. Sánchez-Fueyo A, Strom TB Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011; 140 : 51–64.
28. Germani G, Rodriguez-Castro K, Russo FP, el al. Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol 2015; 21 : 1061–1068.
29. Bathgate AJ, Hynd P, Sommerville D, et al. The prediction of acute cellular rejection in orthotopic liver transplantation. Liver Transpl Surg 1999; 5 : 475.
30. Bartlett AS, Ramadas R, Furness S, et al. The natural history of acute histologic rejection without biochemical graft dysfunction in orthotopic liver transplantation: a systematic review. Liver Transpl 2002; 8 : 1147.
31. Choudhary NS, Saigal S, Saraf N, et al. Acute and chronic rejection after liver transplantation: what a clinician needs to know. Journal of Clinical and Experimental Hepatology 2017; 7 : 358–366.
32. Ramirez CB, Marino IR The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther 2007; 7 : 137–148.
33. Ramirez CB, Doria C, di Francesco F, et al. Anti-IL2 induction in liver transplantation with 93% rejection free patient and graft survival at 18 months. J Surg Res 2007; 138 : 198.
34. Gruttadauria S, Vasta F, Mandalà L, et al. Basiliximab in a triple-drug regimen with tacrolimu and steroids in liver transplantation. Transplant Proc 2005; 37 : 2611.
35. Raimondo ML, Burroughs AK. Single-agent immunosuppression after liver transplantation: what is possible? Drugs 2002; 62 : 1587–1597.
Labels
Diabetology Endocrinology Internal medicine
Article was published inInternal Medicine

2020 Issue 1-
All articles in this issue
- Ve spojení a jednotě je síla
- Hlavní téma: Metabolický syndrom
- Treatment of arterial hypertension in metabolic syndrome
- Atherogenic dyslipidemia typical for metabolic syndrome
- Type 2 diabetes in praxis – balancing between resistance and secretion
- Hepatotoxicity induced by bodybuilding supplements
- Chronic stress, mental discomfort, and depression increase the rates of infectious, autoimmune as well as malignant diseases
- Sarcopenic obesity – current view
- Autoimmune hepatitis
- 20 years of nephrologist‘s journey into the depths of phosphorus toxicity
- Monitoring and tailoring the P2Y12 ADP receptor blocker therapy
- Acute myocardial infarction in a male patient with metabolic syndrome and obstructive sleep apnea syndrome
- Difficulties in the Diagnosis of Cardiac Amyloidosis and Treatment Options: Case Report
- Mandibular pain and deformation as a presentation of fibrous dysplasia of the mandible
- What is the clinical use of total cholesterol results measurement?
- A few notes from reading of the 2019 dyslipidemia guidelines
- Založení profesního spolku SAI – sdružení ambulantních internistů, z. s.
- Odešel velký člověk a lékař prof. MUDr. Vítězslav Kolek, DrSc., FCCP
- Prevalence and risk factors of T-cell mediated rejection in patients after liver transplantation from deceased donors: a retrospective study over 10 years
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Sarcopenic obesity – current view
- Chronic stress, mental discomfort, and depression increase the rates of infectious, autoimmune as well as malignant diseases
- Odešel velký člověk a lékař prof. MUDr. Vítězslav Kolek, DrSc., FCCP
- Autoimmune hepatitis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

