-
Medical journals
- Career
Perioperative oesophagogastroduodenoscopy in the prevention and therapy of anastomotic complications – a review
Authors: Stašek M. 1; Aujeský R. 1; Vrba R. 1; Chudáček J. 1; Klos D. 1; Khan Ahmad H. 1; Holm S. 2; Neoral Č. 1
Authors‘ workplace: I. chirurgická klinika LF UP a FN Olomouc 1; Oddělení plastické a maxilofaciální chirurgie, Univerzitní nemocnice Uppsala, Švédsko 2
Published in: Gastroent Hepatol 2020; 74(3): 217-227
Category: Digestive Endoscopy: Review Article
doi: https://doi.org/10.14735/amgh2020217Overview
The current development of endoscopic and minimally invasive methods show new techniques to treat early anastomotic complications in the upper gastrointestinal tract. The aim of this paper is to summarise current knowledge about endoscopic methods and their relation to surgical treatment in terms of their timing of use, alternative procedures and previous study results. The main methods discussed are endoscopic vacuum therapy (EVAC) and the use of stents and clips, particularly the over-the-scope variant (OTSC). Intraoperative endoscopy is an important option, due to the navigation of surgery and combined surgical and endoscopic therapy. Included in this paper is an evaluation of the benefits of individual methods employed in the complications of oesophagogastric and oesophagojejunal anastomoses as well as in bariatric surgery (sleeve gastrectomy, gastric bypass). An evaluation of the benefits of individual methods employed in the complications of oesophagogastric and oesophagojejunal anastomoses as well as in bariatric surgery (sleeve gastrectomy, gastric bypass) is included in a comprehensive review.
Keywords:
gastroscopy – negative-pressure wound therapy – self-expandable metallic stent – anastomotic leakage – clips – bariatric surgical procedures
Introduction
The actual trend of using minimal invasiveness and target interventions in upper gastrointestinal surgery aims to reduce the incidence of complications and length of hospitalisation and postoperative recovery periods. Essentially, we must consider the issues of safety, oncological radicality and, last but not least, the possibility of an active, targeted and minimally invasive approach to the prevention and therapy of complications. The modalities of therapeutic endoscopic procedures in the treatment of pathologies in the upper digestive tract have recently implemented new technological and tactical developments.
Most comprehensive publications are focused on the general treatment of defects without further dividing to perforations or anastomotic complications, and timing of their occurrence [1,2]. The aim of this paper is to summarise the current possibilities of intraoperative and early postoperative surgical complications while evaluating possible intraoperative and perioperative endoscopic interventions.
The safety of intraoperative endoscopy in stapling anastomoses
The most important task for endoscopy and anastomosis quality evaluation is safety. Intraluminal pressure measurement in the common stapler and primarily reinforced anastomoses in animal models determine the tolerance of insufflation pressure to 27–44 mm Hg, in some staplers even up to 80 mm Hg [3,4].
Clinical studies using intraoperative endoscopy confirm the safety of endoscopy and leakage testing. Rare studies in bariatric patients manometrically measuring the level of intraluminal pressure ratios confirm a pressure of 25,6 mm H2O (12–60 mm Hg) [5]. The results support the conclusion that testing the leakage with an orogastric tube or intraoperative endoscopy does not cause iatrogenic damage in a properly performed stapler suture. Endoscopic intraoperative diagnosis has been studied, among other techniques, on a large population of bariatric patients and can be considered as safe for clinical use [6].
Intraoperative testing of anastomoses
Intraoperative anastomosis assessment techniques have a major purpose in diagnosing air leakage, anastomosis viability, bleeding, and patency maintenance. The primary reason for testing is to reduce postoperative complications that may be solved by the additive surgical technique, though endoscopic methods may be beneficial. These can be used in the primary procedure itself or in early post-operative care or during follow-up surgery.
Important testing options for the anastomotic coherence include leak testing, fluid leakage and visual endoscopic assessment. The air leak test is usually done by instilling air via an orogastric tube with visual inspection by a surgeon, possibly under water surface. The advantages are the simplicity of procedure, reproducibility and possibility of calibration of the anastomosis in case of large tubes. On the other hand, we do not obtain direct visualization of the anastomosis including bleeding and viability and miss the possibility to perform the endoscopic treatment. Sleeve gastrectomy studies repeatedly show an insufficient correlation between a positive air leak test result and subsequent leakage. [7,8,9]. Using both blue dye and air gives comparable results.
Endoscopy is a more accurate method as it includes the possibility of visual inspection, yet, like other methods, cannot be considered completely reliable for the assessment of perforation, especially in complicated postoperative conditions (fundoplication, perforation in the diverticulum or tumour fissuration). However, the difficulty in the method of determining the leak (especially without specification of intraluminal insufflation pressure) may be a problem [6,7,10].
Endoscopic assessment of suture or anastomosis viability has been the subject of several studies, both intraoperatively and in the early postoperative period, as mentioned later. Further methods for viability assessment comprise the surgeon’s visual assessment, the use of indocyanine green and oxygen saturation measurements. A wide range of further possibilities was studied in gastrointestinal anastomoses in practical or study use (a polarographic measurement of oxygen tension, near-infrared spectrophotometry, Doppler ultrasound, infrared imaging, laser Doppler flowmetry, pH measurements and microdialysis), most of them are not in common clinical use [11].
The visual assessment is a basic, but not entirely reliable, method for verifying its viability, complicated especially in mediastinal anastomoses provided from the abdominal approach.
ICG application [11,12] is a safe method that offers adequate perfusion assessment for both open and laparoscopic procedures. In the case of gastric tube assessment during oesophagectomy, the exact length of the tubing or the technique and approach to the anastomosis may help. The assessment of anastomotic oxygenation by oximetry measurement of oxygen saturation has been verified in animal models [13] and clinically, especially in oesophagal replacements [14]. The problem may be both segmental ischemia and stapler ischemia [15]. Measuring oximetry may be useful in ischemic conditioning prior to esophagectomy [16]. Technically, a probe can be used from both the mucosa and the serosa side, and there are probes for both open and minimally invasive surgery. However, the benefits of using oximetry need to be verified by further studies.
The information on intraoperative endoscopy in preventing or treating suture line bleeding is limited.
Bleeding, particularly from stapler anastomosis, reaches an incidence of 0–2% [17,18]. Less than half of the cases require endoscopic intervention. It is possible to use endoscopic injection therapy with adrenaline or polidocanol, coagulation or clipping [19,20].
In some cases, suture reinforcement of the suture line or the re-anastomosis may be necessary. The contribution of endoscopy to the risk of recurrence of bleeding cannot be overlooked [21]. Significant bleeding occurs usually intraoperatively or within the first four hours after surgery [22]. Therefore, it is highly advisable to perform this procedure under general anaesthesia with orotracheal intubation to prevent the risk of aspiration and the possibility of synchronous or subsequent surgicaltreatment.
The calibration of the lumen is used especially for bariatric procedures, an orogastric probe of the required diameter (size 34–40 charr., in some cases up to 60 charr.), is applied [23]. In particular, endoscopy is used to control luminal patency in wedge resections (oesophagal diverticulum).
Resection or wedge resection of the stomach) and anastomosis in particularly poorly verifiable procedures (anastomosis in lower mediastinum via an abdominal approach). Endoscopy provides the more the diagnosis of technical problems involving inappropriate stapler suturing.
Technical possibilities of intraoperative and early postoperative treatment of anastomotic healing complications
Endoscopic treatment of anastomotic leaks should be considered especially for complications ranked grade III according to Clavien-Dindo classification [24]. Grade I-II usually require conservative treatment [25]. Grade IV complications (severe septic anastomotic complications with the development of multiple organ failure) refer to surgical treatment. Grade III complications require intervention, and, in particular, this is the important space for targeted and limited invasivity therapy including endoscopic techniques as leading or important additive modality.
Endoscopic diagnosis of the leak extent can be classified according to the Schuchert and Carboni classification [26,27], which divides anastomotic leaks into 4 groups (Table 1).
1. Classification of anastomotic leaks by Schuchert and Carboni. Tab. 1. Klasifikace anastomotických leaků podle Schucherta a Carboniho. 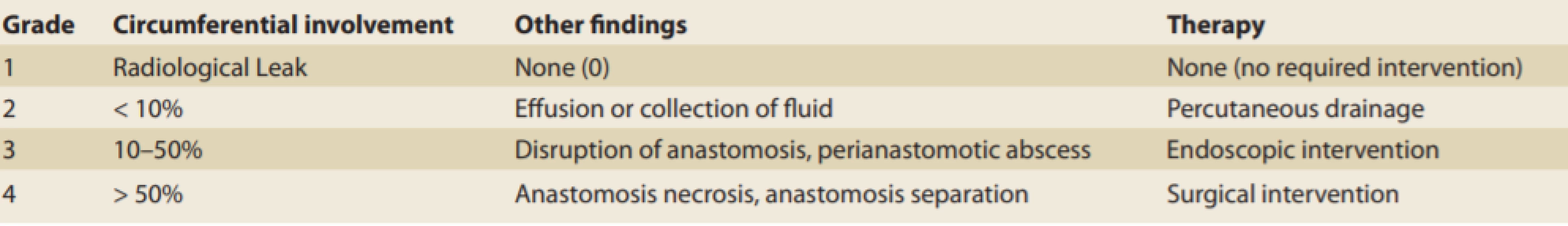
Endoscopy assisted surgery
In the prevention of foregut surgery complications, the intraoperative endoscopy offers the navigation of surgical intervention, possible targeted surgical suturing in bleeding or stapling or manual suture insufficiency. Primary or secondary suture line reinforcement could be a matter of discussion. In primary reinforcement, the decision is usually preoperative and the intraoperative endoscopy has usually marginal importance. Nevertheless, in the secondary reinforcement decision making, omental reinforcement may be beneficial in oesophagal and bariatric surgery [6,28–31]. It may decrease the incidence of leakage, although without affection of either mortality or incidence of stenoses, particularly in esophagectomies.
Simultaneous laparoscopy and endoscopy or their specific sequential order are important in the decision-making process of situations where surgery and endoscopy can be used as a therapeutic alternative. The more, endoscopic therapy of the leak can be combined with additional surgery (tracheostomy, drainage, wound revision or jejunostomy) [32].
Minimally invasive and endoscopic modalities
Internal and external drainage
Internal drainage by endoprosthesis (biliary plastic endoprosthesis, pigtail) [33,34] is useful in limited leakage with a perianastomotic collection. It uses a pressure drop mechanism with intraluminal drainage. The controlled fistula construction using external internal drainage brings another possibility, combining endoscopy with CT or fluoroscopic guidance with the introduction of pigtail through the inner fistula orifice [35]. The success rate in contained leaks with a perianastomotic abscess is high, reaching up to 100 %. Possible undesirable results are low-output external fistulas intended for further intervention, usually stent, OTSC clipping or plug. An important advantage is the possibility of an early start of liquid nutrition. An alternative is the combined laparoendoscopic introduction of T-drain [36,37]. The less commonly used methods are transoral or transnasal drainage with continuous suction.
Stents
The use of stents in upper gastrointestinal surgery [38,39] is widespread and appears to be the most common endoscopic method to treat early leakage or bleeding. The main principle involves the exclusion of the affected part of the passage. The indications include oesophagal injury, postoperative leaks and strictures. It is possible to use both fully or partially coated metallic stents or biodegradable stents.
The most common technique of stent introduction is radiologic guidance with the advantage of instantaneous assessment of contrast passage and confirmation of leakage contrast and stent deployment. The endoscopy offers the assessment of leakage, may help to choose the most appropriate therapy including combination therapy, selection of a particular type of stent including double stenting method and adjusting the stent position and dilatation [40–44]. Stenting may require targeted drainage or surgical revision (necrectomy, drainage, omentoplasty, etc.)
Overall, a successful stent insertion rate of 40–80 % can be expected with a stent placement of at least 2–6 weeks. The main risk factors of failure are proximal cervical anastomosis, oesophagocardial junction injury, oesophagal injury of more than 6 cm, and anastomotic leak from the distal gastric conduit after oesophagectomy [44]. A dislocation occurs in up to 40% of cases. An important problem, however, may also be an ingrown stent and the inability to extract, overgrowth with the granulation tissue (Fig. 1), followed by stricture, or even the risk of developing fistulation to adjacent organs (aorta, trachea, bronchi).
1. Granulation tissue overgrowth with minor persistent leakage around the stent. Obr. 1. Přerůstání stentu granulační tkání s malým persistentním leakem kolem stentu. 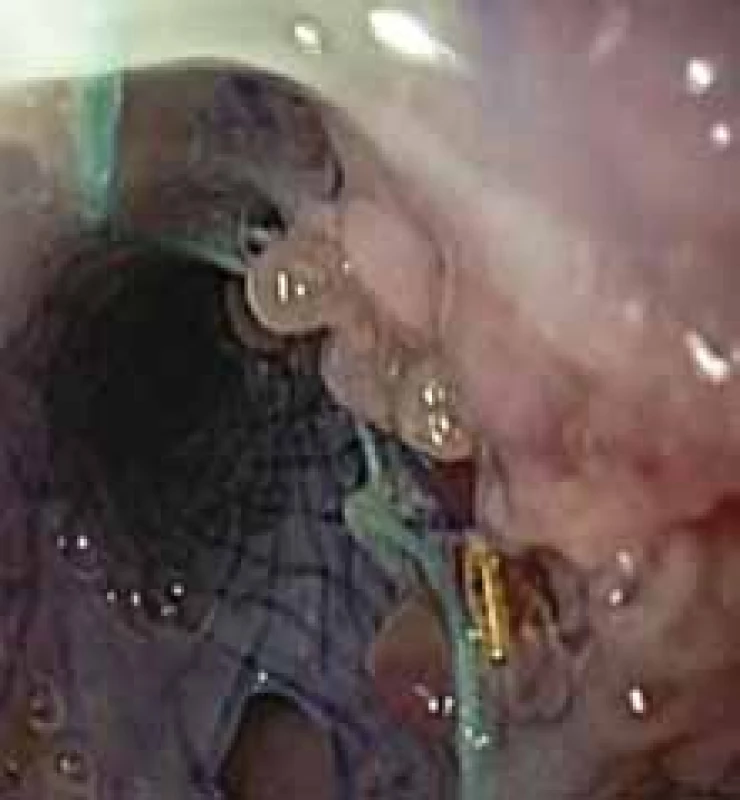
Clipping
Endoscopic haemoclips (TTSC – though-the-scope clips) (Fig. 2) are usually used in general for endoscopic clipping of bleeding in anastomotic complication therapy and were reported rarely for the anastomotic leak closure [45]. OTSC (Ovesco Endoscopy AG, Tübingen, Germany) (Fig. 3) [46-48] offers the possibility of solving minor leaks, including those intraoperatively detected, in poorly accessible locations of surgical treatment. The accessibility, increasing experience and technical acceptability of this method make it an option in both perioperative and early postoperatively diagnosed leaks. Last but not least, other methods (EVAC, stent, etc.) can serve as the final methods used after successful application. However, their use is difficult in the area of the cervical anastomosis.
2. Through the scope clipping in minor tracheoesophageal fistula. Obr. 2. Klipování malé tracheoezofageální píštěle hemoklipy. 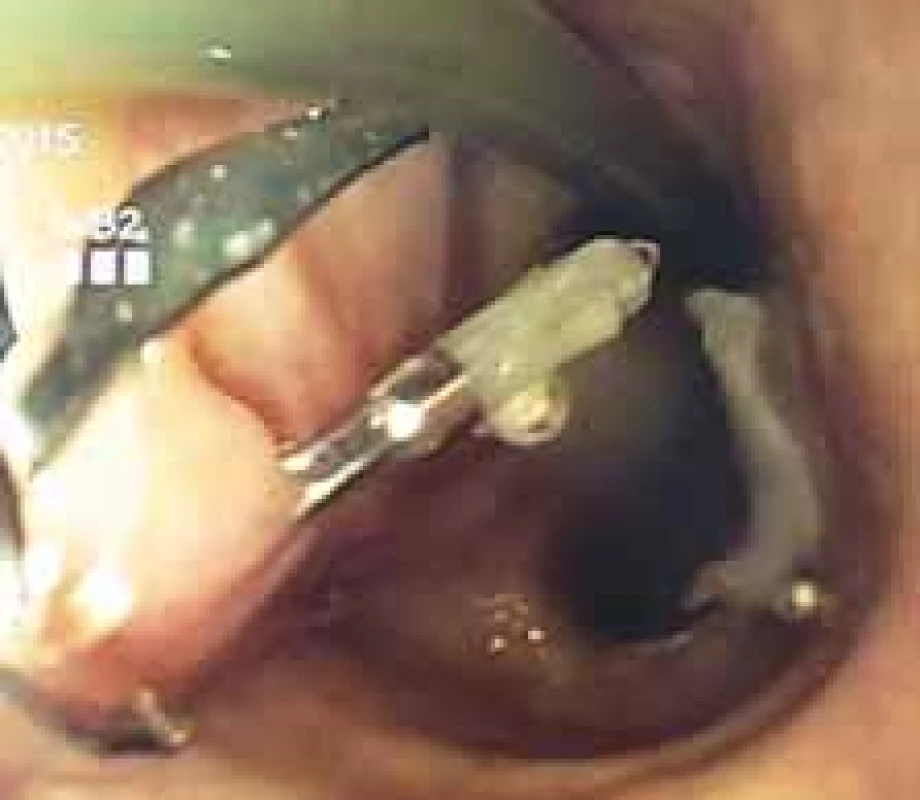
3. Persistent oesophagobronchial fistula after OTSC application following wedge resection of the oesophagus. Obr. 3. Perzistující ezofagobronchiální píštěl po klínovité resekci jícnu, stav po aplikaci OTSC klipu. 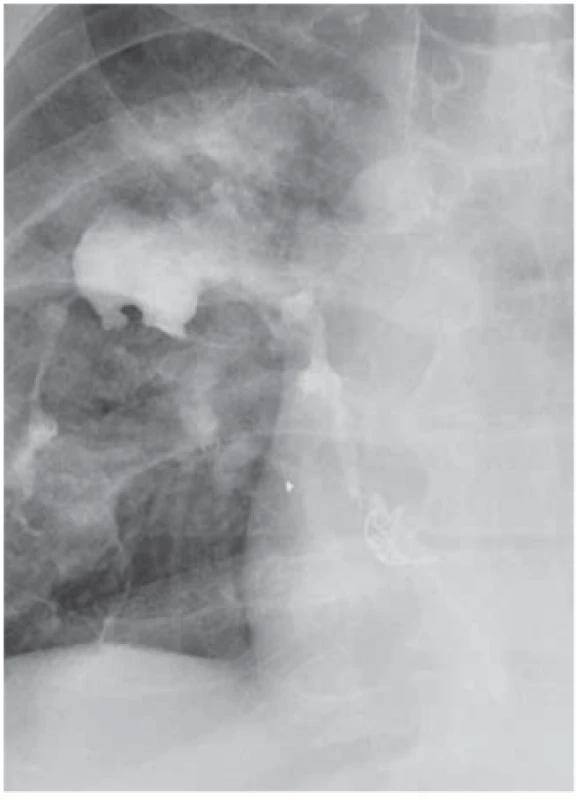
Technically, clips are matched to the endoscope extension with a release through the working channel, comparable to the application of ligation rings. The additive instrumentation is an anchor for tissue retraction or grasper with two lateral branches that approach the edges of the perforation or leakage.
When evaluating OTSC clip results in anastomotic complications, a success rate of 68% (81 out of 120 patients) is reported [25]. The best results can be expected for freshly detected dehiscence or perforations without significant inflammation or fibrous changes and adequate removal of foreign matter (Fig. 4). CO2 insufflation is recommended due to better absorption in the pneumoperitoneum, which may require additive surgery. The advantage is in possible combination with other methods (endoscopic vacuum-assisted closure, tissue sealant or stenting). However, there is still insufficient evidence to specify the indications for early dehiscence.
4. Conic shape of the fistula in fibrous tissue. Risk factor for clipping failure. Obr. 4. Píštěl konického tvaru ve vazivovém terénu. Rizikový faktor pro selhání klipování. 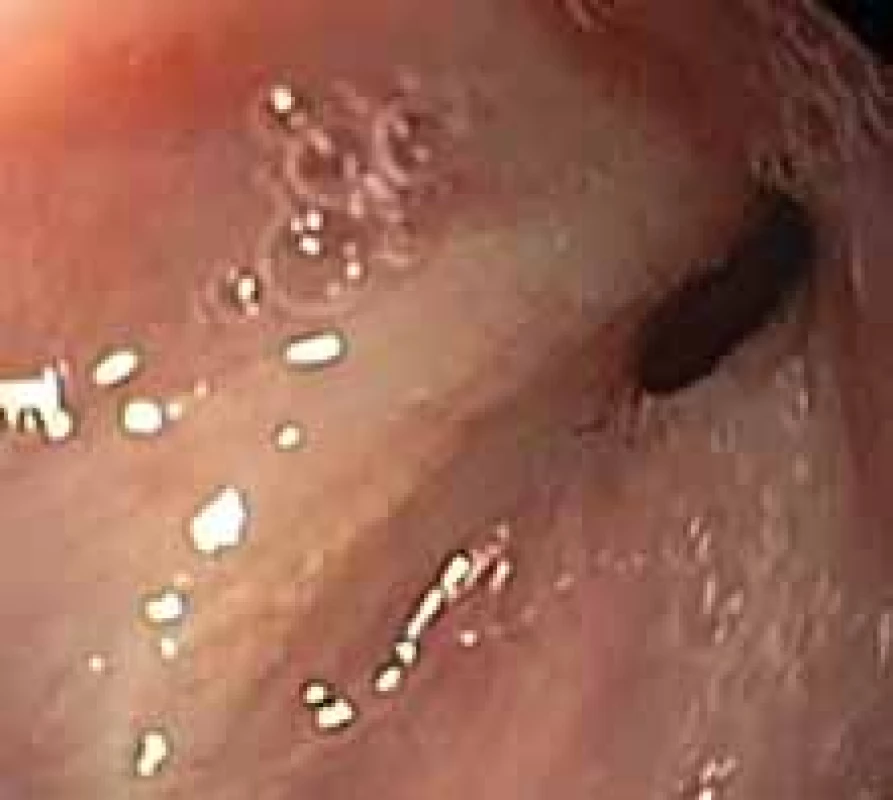
Endoscopic vacuum therapy
Endoscopic vacuum-assisted closure (EVAC) is a therapeutic method used in the upper digestive tract and dates back 12 years ago [49,50]. Technically, it is an intracavitary or intraluminal application of a polyurethane sponge that is coupled to a vacuum generator by a transnasal probe to provide active aspiration, granulation progression, reduction of infectious agents and gradual reduction of fistula volume. Technically, we can insert the sponge usually through an overtube using forceps for extraluminal application or using the intraluminal application with a commercially available set (Endo-sponge system, B.Braun, Melsungen, Germany) (Fig. 5A-C,6). The endoscopy enables the lavage and necrectomy, removal of foreign bodies (stitches, food remnants, clamps) and a targeted cultivation yield. The suction of 80–125 mm Hg is usually applied.
5. A. Commercially available set for endoscopic vacuum assisted closure therapy. B. Sponge. C. Overtube and applicator.
Obr. 5. A) Komerčně dostupný set pro endoskopickou vakuovou terapii. B) Houbička. C) Převlečná hadice a aplikátor.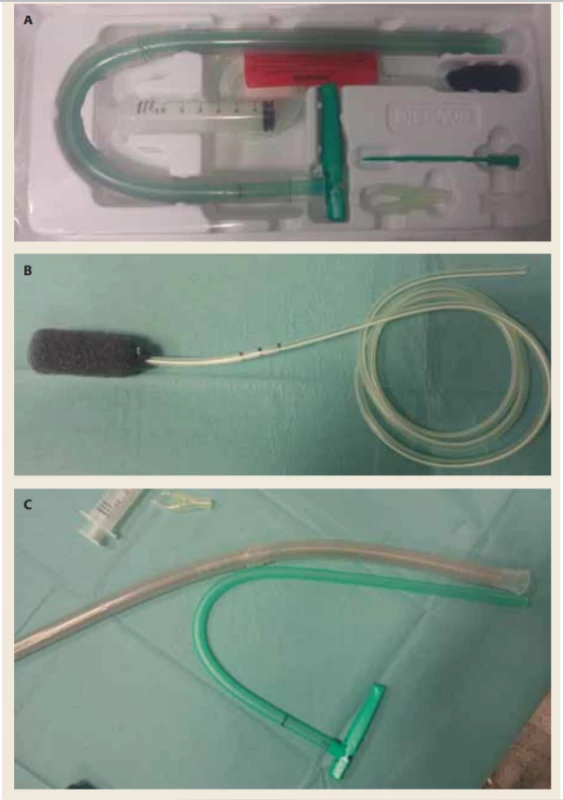
6. Intraluminal application of endoscopic vacuum assisted closure using overtube.
Obr. 6. Intraluminální aplikace endoskopické vakuové terapie s použitím převlečné hadice.
The main disadvantage of the procedure is the necessity of repeated exchanges of the sponges every 3–5 days, the performance is usually carried out in general anaesthesia with orotracheal intubation unless a tracheostomy is performed due to respiratory failure. It can be mandatory to exclude the indication of other additional interventions (CT navigated drainage collections, etc.), or surgical interventions (establishment of tracheostomy, jejunostomy, treatment of morning or cavity complications in terms of thoracic empyema or intra-abdominal collections). The success of the EVAC method has been reported at 84–100% for the upper gastrointestinal leakage and is applicable throughout the upper gastrointestinal tract, from the oesophagus to the rare indications in the duodenum. As a rule, therapy can be usually stopped at a leak size of 1 × 2 cm, of course, when granulation tissue is reached. Endoscopic controls are recommended at intervals of 1–3 weeks until complete healing is reached. Late complications, especially stenosis, are relatively uncommon.
Thus, it can be concluded that EVAC can be considered as a potential first-choice method for the treatment of acute anastomotic dehiscence. A comparison with stents has been evaluated so far in two retrospective studies (Schniewind, Brangewitz) [51,52] with significantly better results for EVAC (albeit in the second study with comparable final mortality). The alternative of sponge application is stent-over-sponge, still without wide evidence [53].
Further possibilities for endoscopic therapy of anastomotic leaks
Tissue sealants have the main importance in combined therapy with other endoscopic methods, the therapy usually requires large amounts of glue [54]. Plug may be beneficial in chronic leaks and complex fistulas. The matter used as a plug is usually vicryl mesh or dedicated plug, combination with tissue sealant, clipping or endoscopic suture is possible [55].
Specific application of endoscopy in the intraoperative and early postoperative anastomotic complications
Reconstruction after oesophageal resection
The incidence of leakage after oesophagectomy is up to 1-30% (most commonly reported as 10%) [56,57]. The leakage remains a very serious complication associated with significant morbidity and mortality of up to 40% of all postoperative deaths in thoracic anastomoses [58,59]. The diagnosis of leakage and its severity is based on the evaluation of sepsis symptoms and evaluation by imaging methods or endoscopies. It is essential to determine the viability of gastroplasty and to assess the leakage morphology.
In a number of studies, it has been shown that fluoroscopy (barium swallow) in the diagnosis of leakage up to 50% initially fails [60]. More accurate information is provided by CT with oral contrast with up to 40% increase in the number of leaks detected. Endoscopy is a very important modality to assess viability, but it has a relatively small leakage rate in routine usage.
The management of leaks should be individualized according to the extent of the defect and the severity of the symptoms. Asymptomatic leaks can be addressed conservatively by limiting oral intake, providing adequate nutritional support and, if needed, antibiotic therapy under continuous clinical monitoring, and developing inflammatory parameters and controlling local findings by any of the imaging methods.
Surgical therapy is the primary method of choice for large leaks, usually due to ischaemia of anastomosis, non-contained leaks and leaks due to severe sepsis, multiple organ dysfunction syndrome (MODS), or conservative therapy failure [56]. They differ significantly in cervical and thoracic anastomoses. Thus, in the case of thoracic anastomosis, surgery enables local debridement, revision of anastomosis, closure of the defect and perifocal drainage in less significant septic symptomatology after resection of the ischemic segment or the entire gastric conduit. It is suitable to overlap the reconstructed anastomosis with well-vascularized tissue (omentum, pleura, pericardium or intercostal periostomuscular flap). In the oesophageal diversion, the oral part of the conduit can be brought out as gastrostomy, the cervical oesophagus as cervical oesophagostomy with a delayed indication of passage reconstruction. Even in the case of early intervention and comprehensive therapy, surgical mortality reaches up to 50%. The procedure for cervical anastomosis is usually more accessible to surgical treatment, and despite a higher incidence of anastomotic complications (Fig. 7), they are better accessible to surgical management [60]. The surgical intervention includes revision of the cervical incision, debridement, drainage and secondary healing with controlled cervical fistula [27]. However, the condition entails a higher incidence of anastomotic strictures.
7. Anastomotic leak in cervical anastomosis following oesophagectomy. Obr. 7. Anastomotický leak v krční anastomóze po ezofagektomii. 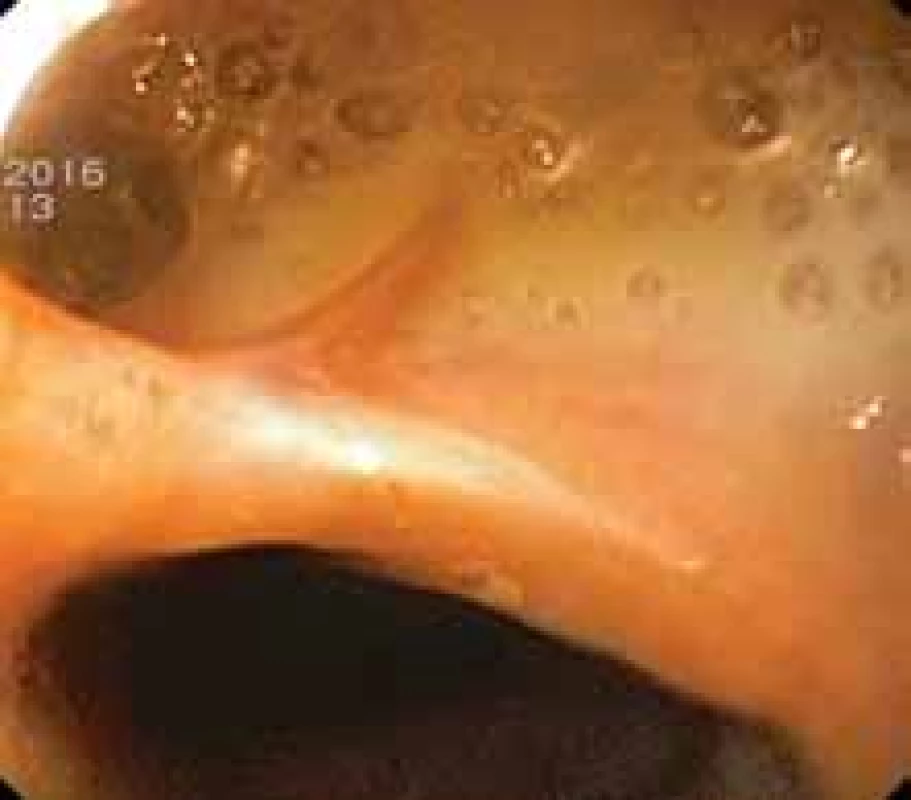
The oesophageal stent placement is the most common endoluminal therapeutic method in leak therapy after surgery with an intrathoracic anastomosis. The above-mentioned advantages are accompanied with possible complications, including migration, insufficient sealing of the stent with continuous leakage (Fig. 4), inability to extract the stent, decubital necrosis with fistulas to the surrounding organs and an increase in secondary obstructing granulation tissue [46] (Fig. 5). The results of the published studies have a relatively high success rate, but most of them have significant limitations, especially retrospective nature and lacking morphologic specification of the leak. Tuebergen [61] reports the success of leak healing in 85% of studies, mortality of 15% with 29% of significant complications. However, in its sample it indicates a leak size of 7.3 ± 5.5 mm (1–22 mm, median 5 mm), so it is questionable whether a number of these leaks could be healed conservatively. Also, the initiation of stent insertion is very discrepant in various studies. A further assessment was made by Van Boeckel [43] and shows 34% stent-related complications, 25% endoscopic reinterventions, and 13% cases of surgical therapy. Based on this experience, he recommends subsequent stent extraction 7 weeks after the introduction, at the latest. The total mortality is 13%. When introducing a stent with an impaired derivation of the void content in the leakage site, it is particularly necessary to consider an additional intervention, especially surgical or intervention drainage collection (up to 55%), or a more extensive intervention in the case of progression.
The application of stents in a cervical anastomosis is very difficult for frequent migration and intolerance with significant dysphagic difficulties [62,63]. The assessment of the use of newly developed stents with up to 90% success rate requires further studies, yet appears to be a promising alternative or addition to surgical procedures.
OTSC clips offer the ability to address minor leaks, including intraoperatively detected leaks in poorly accessible sites. Growing technical and indication experience with this method make it an acceptable option in both perioperative and early postoperatively diagnosed leaks. Last but not least, other methods (EVAC, stent, etc.) may be the final method in the event of a successful application. However, it is difficult or impossible to use in cervical anastomoses.
Most of the studies on EVAC in anastomotic leaks (Figure 6) summarize the leak after esophagectomy and total gastrectomy [64–70]. In the analysis of groups of more than 5 patients [68], treatment of anastomotic leakage is associated with a success rate of 90% (107 out of 119 patients) with an average duration of treatment of 17 days (11–36) with 3–5-day replacement intervals and a follow-up duration of 235 days (106–383). Therefore, EVAC failure occurs in less than 10% of cases. Mortality reaches 0–6 % in groups without anastomotic leak selection and perforation. The causes of death are mainly complications of comorbidities. EVAC-associated deaths were caused by the bleeding due to aortooesophagal fistula or bleeding from a large mediastinal vessel, observed in three patients [67]. The risk can be diminished by CT verification of the sponge location and subsequent intraluminal application of EVAC. EVAC as a complementary therapy for surgical treatment was used in Kuehn‘s studies in 43% of patients [68,71], intraluminal EVAC was particularly useful in late diagnosis or large-scale defects. Even in advanced cases of mediastinitis, most patients can achieve oesophageal salvage.
Out of the late complications, the incidence of stenosis is relatively low. Laukoetter et al. showed in a study with 52 patients that four stenoses occurred in the follow-up [67].
Four studies compared EVAC with stent insertion [51,52,65,72]. Despite their retrospective nature, their results are similar. The observed mortality in the Schniewind study was 50 % for the surgical treatment, 17% for the stent and 12% for EVAC. Brangewitz, Menningen and Hwang reported closure of 84.4–100% for EVAC and 54–64% for stenting, with plausible mortality and higher incidence of strictures in the stent group.
The endoscopic suture using the Apollo OverstitchTM suturing device remains a less frequently used option for tackling leakage for the technical difficulty and a need for special equipment [73]. Individual case reports have been published with possible insuring by the subsequent stenting. Another possible indication is stent fixation.
The prevention of leakage remains a priority effort during surgery and perioperative care. The purpose is to identify the presence and predict the possibility of developing anastomotic complications. The contribution of intraoperative and early postoperative endoscopy in cervical anastomosis was studied by Fujiwara et al [74]. The first postoperative day was assessed by assessing change in mucosal colour; in addition to mucosal changes, the presence of leakage and mucosal defects was also evaluated a week after the procedure. When a mucosal change was found, 55.6 % of these patients developed leakage, compared with 2.6% of normal finding leaks in the first postoperative day. The main objection to early or intraoperative endoscopy is the fear of injury to anastomosis or conduit and an increase in possible dehiscence or leakage. This diagnostic method may be essential for early EVAC indications.
Neumann et al [75] offer the possibility of pre-emptive use prior to the development of leakage in ischemia found in intraoperative endoscopy, as evidenced by a small set of eight patients. Complete healing was achieved in 75% of patients; the development of two leaks was treated with endoscopic vacuum therapy. The EVAC application lasted an average of 16 days with an average of 5 exchanges per patient. Three patients developed stenosis. However, further clarification is needed on wider implementation. Oversewing of the stapler line [76] or omental reinforcement [77] are possible surgical additions.
The prevention of pylorospasm is another issue. Extramucosal pyloroplasty as conventional surgery possibility to decrease outlet obstruction and anastomosis leak risk could be replaced by botulinum toxin application [78] or dilatation [79,80].
Oesophagojejunal anastomosis
According to large studies, total gastrectomy is burdened with a mortality of 2–13% [81,82]. A symptomatic leak in oesophagojejunal anastomosis is the cause of death in up to 26–30%, in case of fulminant progression with mediastinitis up to 65% [84,85]. There was no significant difference between the approach (open versus laparoscopic) techniques [86] or other techniques.
The importance of intraoperative endoscopic evaluation for the prevention of anastomotic complications has been evaluated in several studies with a relatively small number of patients [89]. The information on the incidence of severe anastomotic bleeding is poorly supported. Intraoperative endoscopy studies report the incidence of bleeding in less than 3% of cases (3/107) [17,87]. As a rule, the risk of technical complications is 1%, and the failure of a stapler suture (positive air leak or erroneous stapler launch), or occlusion of the lumen to interpolate the contralateral jejunal wall may be included. Especially in the mediastinal anastomosis, these complications may be missed.
The incidence of stenosis is 2.9% [84], but usually occurs after more than 30 days and usually requires pneumatic dilatation or stent introduction.
The treatment options for leakage include conservative, endoscopic and surgical therapy [26]. The radiological leak or leak in a stable patient is limited to the immediate vicinity of anastomosis, reaching less than 10% of the circumference, and can be treated conservatively [88,89].
Extensive leaks over 50% of the circumference, usually associated with hypovitality or necrosis of the anastomosis margins, which are manifested by mediastinitis and severe sepsis, require urgent and aggressive surgical treatment with the possibility of reresection to oesophagectomy and diversion [88,90], requiring complicated reconstruction possibilities.
The space for endoscopic interventions can especially be seen in leaks affecting 10–50% of the circumference with the absence of necrosis. Leaks need to be assessed radiologically (CT, water contrast fluoroscopy) or endoscopically, preferably by assessing mucosal viability and anastomosis, or later the presence of necrosis. The character and morphology of the leak then offer a choice of therapy.
The indication of additional suturing or reanastomosis by surgical approach is a basic option. However, achieving leak test negativity does not exclude future leakage, no matter if we use air leak test [10], methylene blue probe [91], or intraoperative endoscopy.
The use of clips is safe but limited by the quality of leak edges (duration, inflammatory changes). The success of using OTSC clips may be expected in the case of potential leaks diagnosed within 1 week after the procedure [92–96].
The introduction of a partially or completely coated plastic or metallic stent is an effective method, especially for larger leaks (30–50%, even 70% of the circumference) without the presence of necrosis [43,97,98]. The disadvantage of the absence of drainage, however, must be solved by additional drainage of present mediastinal or intra-abdominal collections. Despite relatively high success rates, especially in early leaks, the above-mentioned complications can be expected in up to 70% [43,93,94,97,98].
In the case of the discrepant dimension of the stent and the lumina, partially coated stents with a larger diameter [99] may be considered, or the stent may be fixed to the oesophagus or transnasally. In a prospectively conducted study [93], the effect of stent compared to other endoscopic treatment methods was demonstrated, but with more complications, however, new modalities (OTSC clips, EVAC) were not used in the file. As a rule, the stent loading time is recommended for 5–6 weeks.
The information on the isolated use of tissue adhesive is rare [96,100]. The combination with clip application [93] or Vicryl plug [100] may be considered. EVAC and its potential are discussed above in view of the inability to unambiguously differentiate the presented use in oesophagectomy and total gastrectomy. This achieves excellent results, albeit so far in limited sets.
Bariatric performances
The standardization and extension of surgical procedures for the treatment of obesity and metabolic syndrome offer a wide field for the development of methods with the potential to reduce postoperative complications. They can be divided into groups of sleeve gastrectomies and laparoscopic bypass procedures.
The incidence of complications in sleeve gastrectomy includes 1.06% of leaks (1–3.93% in primary and up to 10% in revision) and 4.07% of bleeding [101–103]. The most common bypass surgery is the laparoscopic Roux-Y gastric bypass. The incidence of leakage is 0.1–5.6% and bleeding is about 3.45% (1.9–4.4 %), but intraperitoneal and intraluminal haemorrhages are mostly included. Technical failure occurs in less than 1% of cases (0.78–0.98%), but the incidence may be underestimated [104].
Intraoperative or postoperative diagnosis and therapy should be provided as soon as possible when tissue quality and local conditions are the most appropriate for revisional surgery or endoscopy [103].
Sleeve gastrectomy
Intraoperative leak detection methods aim to reduce some of the leaks that are caused by technical perioperative difficulties (incorrect stapler launch, direct tissue damage, orogastric tube seizures, etc.) and intraluminal causes (gastric outlet obstruction) [105]. Intraoperative diagnosis with subsequent surgical treatment and with possible endoscopic navigation carries, in this case, a potential to prevent serious complications. These complications are usually presented within two days of primary performance [106,107].
For intraoperative leakage assessment, the methylene blue test (which has high sensitivity and specificity) [108], air-leak test or intraoperative endoscopy can be used [109]. The introduction of drainage does not completely fulfil its function. The signs of sepsis (tachycardia, fever, hypotension) are important in the early postoperative period.
The first choice in the diagnosis is abdominal CT with peroral contrast with the diagnostic accuracy of 86% [107], or endoscopic verification [110]. The reliability of the radiologic study (water-soluble contrast swallow) is considered insufficient for the diagnosis of leakage, but it may provide additional information on the presence of stricture and anatomic changes [111,112].
The effectiveness of prophylactic stapler oversewing is the subject of controversial discussions [113]. However, randomized studies do not demonstrate the benefits of reinforcement for leak prevention [23,114], but rather for the prevention of postoperative bleeding.
In unstable patients or in case of early post-operative symptom development, a suture of the stapling line defect is indicated for an urgent surgical revision, including lavage, drainage, or debridement, and in case of a favourable finding [115]. The conservative approach (antibiotics, proton pump inhibitors, nutritional support, percutaneous drainage) can be chosen for later leaks. In case of conservative failure and fistula persistence, intraoperative endoscopy can be used to elucidate and possibly to drain the defect in rendez-vous approach. Endoscopy is also recommended after an ineffective 2-week conservative therapy [116,117].
OTSC clips allow the closure of minor mucosal defects and are unreliable in inflammatory and edematous tissue, but more than 80% of closure success can be achieved when correctly indicated [118,119].
The use of tissue adhesives based on fibrin or cyanoacrylate has limited success when used alone. The indications for the usage of stents were gradually extended from the original treatment of stenoses to the treatment of leaks, especially the upper and middle parts of the stomach [117,120]. The main advantage is the possibility of oral intake and dimension, but the risk of migration is up to 30% [121]. The duration of healing reaches an average of 6 weeks, removal is recommended within 2 months, at the latest (Fig. 9). Bége [122] then recommends endoscopic treatment in three steps – primarily endoscopic lavage and drainage, followed by stent insertion and definitive closure by tissue glue or clips.
8. Minor leak in esophagojejunal anastomosis following total gastrectomy. Prolonged healing required endoscopic vacuum assisted closure therapy.
Obr. 8. Malý leak ezofagojejunální anastomózy po totální gastrektomii. Prodloužené hojení vyžadovalo využití endoskopické vakuové terapie.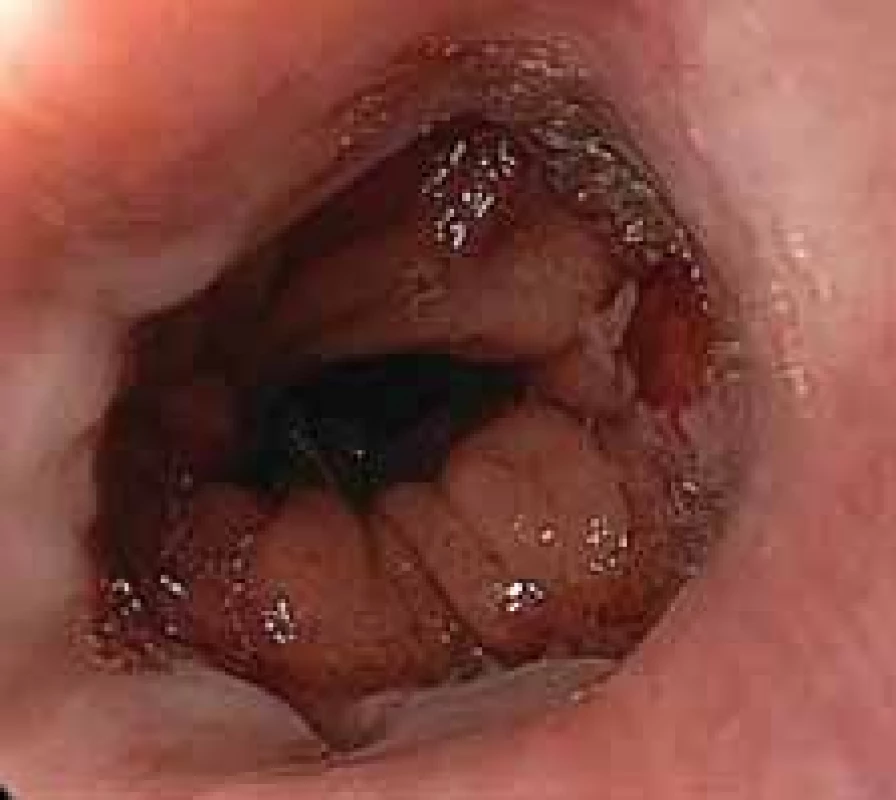
9. Persistent leakage following stent extraction in post-sleeve gastrectomy condition. Obr. 9. Perzistující leak po extrakci stentu při stavu po sleeve gastrektomii. 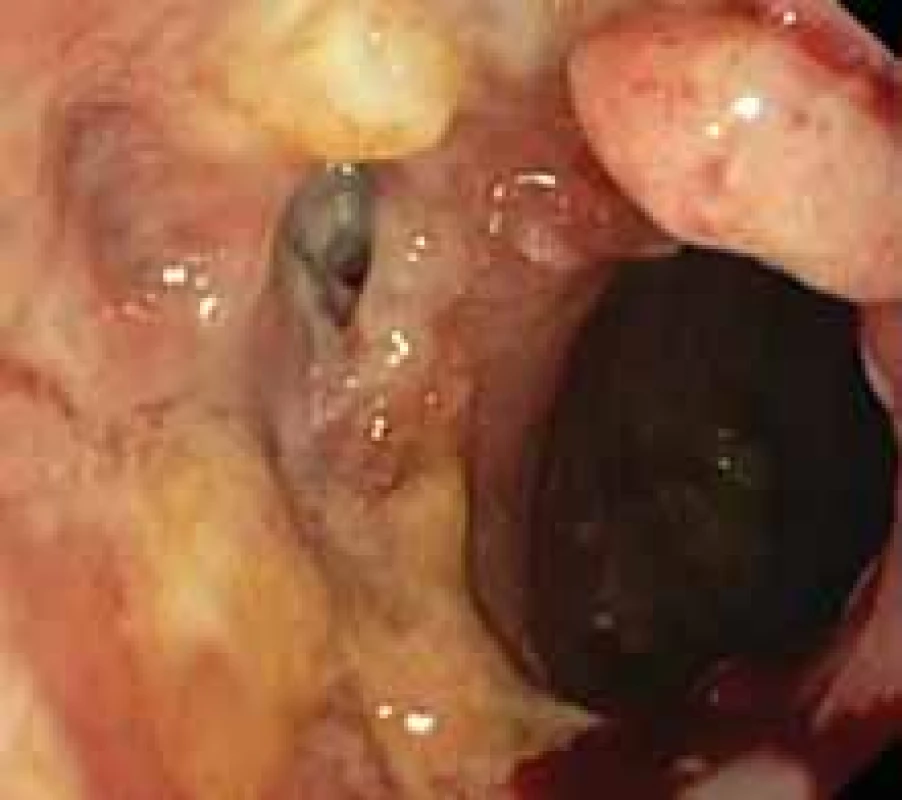
So far, EVAC has been used in smaller groups of patients with an average of 10.3 procedures per patient and an average duration of 50 days [123]. The duration of therapy supports the use of EVAC as a back-up method for gastrectomy. Another alternative may be internal drainage with good success [34].
In patients with these failures, surgical intervention should be considered including consideration of conversion to gastric bypass or fistula overlap using jejunal loop sec. Roux. The salvage may require the completion of total gastrectomy with oesophagojejunal anastomosis [124].
Bypass procedures
The safety of endoscopy during bariatric procedures has been repeatedly demonstrated in large patient populations [6]. A possible reduction of complications when using stapler suture reinforcement in case of air leakage positivity is, according to Haddad‘s study, quantified to 1.9% (3.2% versus 1.3%) by additional suture with possible omental enhancement or fibrin glue [125].
The navigation of gastrointestinal haemorrhage usually proves the origin in gastrojejunal anastomosis (90 %) [126]. In Alasfar‘s study [127], the incidence of haemorrhage was 3.45%, most patients were treated with additional suturing of the bleeding site under endoscopic control. Intraoperative endoscopic haemorrhage therapy [126,128], especially in the case of bleeding directly from constructed anastomosis or later detected marginal ulcer, includes the possibility of applying adrenaline and polidocanol, coagulation, or endoclips to a visible vessel stump [129]. Despite the possible need for control endoscopy, a successful endoscopic intervention can be achieved, 100% in the case of the Jamil study [22].
Leak management is very similar to sleeve gastrectomy [130]. Stent placement is successful in as many as 87.8% of cases [39,131], but studies have few distinctions between bariatric procedures. The main problem is again the incidence of stent migration, reaching 16.9%, and the inability of stent extraction in 8.4% of cases.
The use of tissue sealant is most often used in combination with stents [128,129,132]. The application techniques used can be in the form of a fibrin glue plug [133] or in the form of an injector [134]. The use of EVAC is still in its infancy. The reference so far comes from an animal study [135], so far only clinical use has been reported for leaks after sleeve gastrectomy.
Conclusion
Endoscopic therapeutic methods for anastomotic complications are of increasing use and tumultuous. Increasing possibilities of successful endoscopic interventions can offer a targeted and effective variant in a surgically poorly accessible locality especially in the case of oesophageal anastomoses including oesophagojejunal anastomoses.
Intraoperative endoscopic assistance or therapy is safe, and highly effective if properly indicated. Besides the use of established methods (stents), it is necessary to consider the introduction of new endoscopic methods (especially endoscopic vacuum therapy or OTSC clips) into the portfolio of endoscopic and surgical workplaces. The approach and strategy of the endoscopic therapy of anastomotic complications should be spread among both surgeons and endoscopists. In the future, we can expect the expansion of the evidence of individual methods and their introduction into wider practice.
Submitted/Doručeno: 31. 3. 2020
Accepted/Přijato: 1. 4. 2020
MUDr. Martin Stašek, Ph.D.
Department of Surgery I,
University Hospital Olomouc
I.P. Pavlova 185/6
77900 Olomouc
Czech Republic
e-mail: martin.stasek@fnol.cz
Sources
1. Takeshita N, Ho KY. Endoscopic closure for full-thickness gastrointestinal defects: available applications and emerging innovations. Clin Endosc 2016; 49 (5): 438–443. doi: 10.5946/ce.2016.104.
2. Winder JS, Pauli EM. Comprehensive management of full-thickness luminal defects: the next frontier of gastrointestinal endoscopy. World J Gastrointest Endosc 2015; 7 (8): 758–768. doi: 10.4253/wjge.v7.i8.758.
3. Goto T, Kawasaki K, Fujino Y et al. Evaluation of the mechanical strength and patency of functional end-to-end anastomoses. Surg Endosc 2007; 21 (9): 1508–1511. doi: 10.1007/s00464-006-9131-6.
4. Kawasaki K, Fujino Y, Kanemitsu K et al. Experimental evaluation of the mechanical strength of stapling techniques. Surg Endosc 2007; 21 (10): 1796–1799. doi: 10.1007/s00464-007-9265-1.
5. Causey MW, Fitzpatrick E, Carter P. Pressure tolerance of newly constructed staple lines in sleeve gastrectomy and duodenal switch. Am J Surg 2013; 205 (5): 571–575. doi: 10.1016/j.amjsurg.2012.12.008.
6. Haddad A, Tapazoglou N, Singh K et al. Role of intraoperative esophagogastroenteroscopy in minimizing gastrojejunostomy-related morbidity: experience with 2,311 laparoscopic gastric bypasses with linear stapler anastomosis. Obes Surg 2012; 22 (12): 1928–1933. doi: 10.1007/s11695-012-0757-2.
7. Bingham J, Lallemand M, Barron M et al. Routine intraoperative leak testing for sleeve gastrectomy: is the leak test full of hot air? Am J Surg; 211 (5): 943–947. doi: 10.1016/j.amjsurg.2016.02.002.
8. Bingham J, Kaufman J, Hata K et al. A multicenter study of routine versus selective intraoperative leak testing for sleeve gastrectomy. Surg Obes Relat Dis 2017; 13 (9): 1469–1475.
doi: 10.1016/j.soard.2017.05.022.
9. Varban OA, Cassidy RB, Sheetz KH et al. Technique or technology? Evaluating leaks after gastric bypass. Surg Obes Relat Dis 2016; 12 (2): 264–272. doi: 10.1016/j.soard.2015.07.013.
10. Kanaji S, Ohyama M, Yasuda T et al. Can the intraoperative leak test prevent postoperative leakage of esophagojejunal anastomosis after total gastrectomy? Surg Today 2016; 46 (7): 815–820. doi: 10.1007/s00595-015-1243-y.
11. Urbanavičius L, Pattyn P, Van de Putte D et al. How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 2011; 3 (5): 59–69. doi: 10.4240/wjgs.v3.i5.59.
12. Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 2016; 401 (6): 767–775. doi: 10.1007/s00423-016-1400-9.
13. Servais EL, Rizk NP, Oliveira L et al. Real-time intraoperative detection of tissue hypoxia in gastrointestinal surgery by wireless pulse oximetry. Surg Endosc 2011; 25 (5): 1383–1389. doi: 10.1007/s00464-010-1372-8.
14. Anegg U, Lindenmann J, Maier A et al. Influence of route of gastric transposition on oxygen supply at cervical oesophagogastric anastomoses. Br J Surg 2008; 95 (3): 344–349. doi: 10.1002/bjs.5997.
15. Myers C, Mutafyan G, Petersen R et al. Real-time probe measurement of tissue oxygenation during gastrointestinal stapling: mucosal ischemia occurs and is not influenced by staple height. Surg Endosc 2009; 23 (10): 2345–2350. doi: 10.1007/s00464-009-0342-5.
16. Bludau M, Hölscher AH, Vallböhmer D et al. Ischemic conditioning of the gastric conduit prior to esophagectomy improves mucosal oxygen saturation. Ann Thorac Surg 2010; 90 (4): 1121–1126. doi: 10.1016/j.athoracsur.2010.06.003.
17. Lieto E, Orditura M, Castellano P et al. Endoscopic intraoperative anastomotic testing may avoid early gastrointestinal anastomotic complications. A prospective study. J Gastrointest Surg 2011; 15 (1): 145–152. doi: 10.1007/s11605-010-1371-z.
18. Ferreira LE, Song LM, Baron TH. Management of acute postoperative hemorrhage in the bariatric patient. Gastrointest Endosc Clin N Am 2011; 21 (2): 287–294. doi: 10.1016/j.giec.2011.02.002.
19. Kim KH, Kim MC, Jung GJ et al. Endoscopic treatment and risk factors of postoperative anastomotic bleeding after gastrectomy for gastric cancer. Int J Surg 2012; 10 (10): 593–597. doi: 10.1016/j.ijsu.2012.09.026.
20. Lee JG. What is the value of early endoscopy in upper gastrointestinal bleeding? Nat Clin Pract Gastroenterol Hepatol 2006; 3 (10): 534–535. doi: 10.1038/ncpgasthep0609.
21. Mayer G, Lingenfelser T, Ell C. The role of endoscopy in early postoperative haemorrhage. Best Pract Res Clin Gastroenterol 2004; 18 (5): 799–807. doi: 10.1016/j.bpg.2004.06.002.
22. Jamil LH, Krause KR, Chengelis DL et al. Endoscopic management of early upper gastrointestinal hemorrhage following laparoscopic Roux-en-Y gastric bypass. Am J Gastroenterol 2008; 103 (1): 86–91. doi: 10.1111/j.1572-0241.2007.01588.x.
23. Parikh M, Issa R, McCrillis A et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg 2013; 257 (2): 231–237. doi: 10.1097/SLA.0b013e31826cc714.
24. Clavien PA, Barkun J, de Oliveira ML et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250 (2): 187–196. doi: 10.1097/SLA.0b013e3181b13ca2.
25. Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: endoscopic vacuum therapy and over-the scope clips. World J Gastroenterol 2014; 20 (24): 7767–7776. doi: 10.3748/wjg.v20.i24.7767.
26. Carboni F, Valle M, Federici O et al. Esophagojejunal anastomosis leakage after total gastrectomy for esophagogastric junction adenocarcinoma: options of treatment. J Gastrointest Oncol 2016; 7 (4): 515–522. doi: 10.21037/jgo.2016.06.02.
27. Schuchert MJ, Abbas G, Nason KS et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010; 148 (4): 831–840. doi: 10.1016/j.surg.2010.07.034.
28. Wiggins T, Markar SR, Arya S et al. Anastomotic reinforcement with omentoplasty following gastrointestinal anastomosis: a systematic review and meta-analysis. Surg Oncol 2015; 24 (3): 181–186. doi: 10.1016/j.suronc.2015.06.011.
29. Sekhar N, Torquati A, Lutfi R et al. Endoscopic evaluation of the gastrojejunostomy in laparoscopic gastric bypass. A series of 340 patients without postoperative leak. Surg Endosc 2006; 20 (2): 199–201. doi: 10.1007/s00464-005-0118-5.
30. Shin RB. Intraoperative endoscopic test resulting in no postoperative leaks from the gastric pouch and gastrojejunal anastomosis in 366 laparoscopic Roux-en-Y gastric bypasses. Obes Surg 2004; 14 (8): 1067–1069. doi: 10.1381/0960892041975613.
31. Mohos E, Schmaldients E, Richter D et al. Examination of the efficacy and safety of intraoperative gastroscopic testing of the gastrojejunal anastomosis in laparoscopic Roux Y gastric bypass surgery. Obes Surg 2011; 21 (10): 1592–1596. doi: 10.1007/s11695-011-0428-8.
32. Stašek M, Aujeský R, Vrba R et al. Indications and benefits of intraoperative esophagogastroduodenoscopy. Wideochir Inne Tech Maloinwazyjne 2018; 13 (2): 164–175. doi: 10.5114/wiitm.2018.72740.
33. Slim R, Smayra T, Noun R. Biliary endoprosthesis in the management of gastric leak after sleeve gastrectomy. Surg Obes Relat Dis 2013; 9 (: 485–486. doi: 10.1016/j.soard.2012.12.008.
34. Donatelli G, Dumont JL, Cereatti F et al. Endoscopic internal drainage as first-line treatment for fistula following gastrointestinal surgery: a case series. Endosc Int Open 2016; 04 (6): E647–E651. doi: 10.1055/s-0042-105206.
35. Soufron J. Leak or fistula after sleeve gastrectomy: treatment with pigtail drain by the rendezvous technique. Obes Surg 2015; 25 (10): 1979–1980. doi: 10.1007/s11695-015-1804-6.
36. Barreca M, Nagliati C, Jain VK et al. Combined endoscopic-laparoscopic T-tube insertion for the treatment of staple-line leak after sleeve gastrectomy: a simple and effective therapeutic option. Surg Obes Relat Dis 2015; 11 (2): 479–482. doi: 10.1016/j.soard.2014.12.018.
37. Court I, Wilson A, Benotti P et al. T-tube gastrostomy as a novel approach for distal staple line disruption after sleeve gastrectomy for morbid obesity: case report and review of the literature. Obes Surg 2010; 20 (4): 519–522. doi: 10.1007/s11695-009-9898-3.
38. Chang J, Sharma G, Boules M et al. Endoscopic stents in the management of anastomotic complications after foregut surgery: new applications and techniques. Surg Obes Relat Dis 2016; 12 (7): 1373–1381. doi: 10.1016/j.soard.2016.02.041.
39. Yimcharoen P, Heneghan HM, Tariq N et al. Endoscopic stent management of leaks and anastomotic strictures after foregut surgery. Surg Obes Relat Dis 2011; 7 (5): 628–636. doi: 10.1016/j.soard.2011.03.017.
40. Dasari BV, Neely D, Kennedy A et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014; 259 (5): 852–860. doi: 10.1097/SLA.0000000000000564.
41. El Hajj II, Imperiale TF, Rex DK et al. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc 2014; 79 (4): 589–598. doi: 10.1016/j.gie.2013.08.039.
42. Repici A, Rando G. Stent for nonmalignant leaks, perforations, and ruptures. Techniques Gastrointest Endosc 2010; 12 (4): 237–245. doi: 10.1016/j.tgie.2011.02.004.
43. van Boeckel PG, Sijbring A, Vleggaar FP et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011; 33 (12): 1292–1301. doi: 10.1111/j.1365-2036.2011.04663.x.
44. Freeman RK, Ascioti AJ, Giannini T et al. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg 2012; 94 (3): 959–965. doi: 10.1016/j.athoracsur.2012.05.047.
45. Rodella L, Laterza E, De Manzoni G et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy 1998; 30 (5): 453–456. doi: 10.1055/s-2007-1001307.
46. Dabizzi E, De Ceglie A, Kyanam Kabir Baig KR et al. Endoscopic „rescue“ treatment for gastrointestinal perforations, anastomotic dehiscence and fistula. Clin Res Hepatol Gastroenterol 2016; 40 (1): 28–40. doi: 10.1016/j.clinre.2015.04.006.
47. Sandmann M, Heike M, Faehndrich M. Application of the OTSC system for the closure of fistulas, anastomosal leakages and perforations within the gastrointestinal tract. Z Gastroenterol 2011; 49 (8): 981–985. doi: 10.1055/s-0029-1245972.
48. Haito-Chavez Y, Law JK, Kratt T et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014; 80 (4): 610–622. doi: 10.1016/j.gie.2014.03.049.
49. Wedemeyer J, Schneider A, Manns MP et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008; 67 (4): 708–711. doi: 10.1016/j.gie.2007.10.064.
50. Loske G, Müller C. Vacuum therapy of an esophageal anastomotic leakage – a case report. Zentralbl Chir 2009; 134 (3): 267–270. doi: 10.1055/s-0028-1098764.
51. Schniewind B, Schafmayer C, Voehrs G et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 2013; 27 (10): 3883–3890. doi: 10.1007/s00464-013-2998-0.
52. Brangewitz M, Voigtländer T, Helfritz FA et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013; 45 (6): 433–438. doi: 10.1055/s-0032-1326435.
53. Dent B, Griffin SM, Jones R et al. Management and outcomes of anastomotic leaks after oesophagectomy. Br J Surg 2016; 103 (8): 1033–3038. doi: 10.1002/bjs.10175.
54. Lippert E, Klebl FH, Schweller F et al. Fibrin glue in the endoscopic treatment of fistulae and anastomotic leakages of the gastrointestinal tract. Int J Colorectal Dis 2011; 26 (3): 303–311. doi: 10.1007/s00384-010-1104-5.
55. Ribeiro MS, de Barros RA, Wallace MB. Vicryl patch and fibrin glue as treatment of an esophageal leak. Gastrointest Endosc 2015; 82 (2): 402. doi: 10.1016/j.gie.2015.03.1910.
56. Crestanello JA, Deschamps C, Cassivi SD et al. Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005; 129 (2): 254–260. doi: 10.1016/j.jtcvs.2004.10.024.
57. Briel JW, Tamhankar AP, Hagen JA et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004; 198 (4): 536–542. doi: 10.1016/j.jamcollsurg.2003.11.026.
58. Lerut T, Coosemans W, Decker G et al. Anastomotic complications after esophagectomy. Dig Surg 2002; 19 (2): 92–98. doi: 10.1159/000052018.
59. Nederlof N, de Jonge J, de Vringer T et al. Does routine endoscopy or contrast swallow study after esophagectomy and gastric tube reconstruction change patient management? J Gastrointest Surg 2017; 21 (2): 251–258. doi: 10.1007/s11605-016-3268-y.
60. Kayani B, Jarral OA, Athanasiou T et al. Should oesophagectomy be performed with cervical or intrathoracic anastomosis? Interact Cardiovasc Thorac Surg 2012; 14 (6): 821–827. doi: 10.1093/icvts/ivs036.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2020 Issue 3-
All articles in this issue
- Editorial
- Kvíz z klinické praxe
- Impact of the COVID-19 pandemic on endoscopy practice in the Czech Republic – survey research
- Disconnected pancreatic duct syndrome – a neglected complication of acute pancreatitis
- Motorized spiral enteroscopy – our first experience
- Eosinophilic esophagitis – current overview of diagnostic and treatment modalities
- Projekt „Endoskopická centra“v České republice
- Current scientific background of Crohn’s Disease Exclusion Diet (CDED)
- Budesonide MMX in the treatment of ulcerative colitis
- An unusual case of upper type dysphagia
- Renal illness in patients with inflammatory bowel disease
- In memory of assoc. prof. Jan Kotrlík
- MUDr. Marek Beneš died on June 18, 2020 at the age of 44
- The selection from international journals
- Správná odpověď na kvíz: Ischemická kolitida na podkladě trombózy dolní mezenterické žíly
- Perioperative oesophagogastroduodenoscopy in the prevention and therapy of anastomotic complications – a review
- Herpetic esophagitis in a 7-year-old immunocompetent patient
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Eosinophilic esophagitis – current overview of diagnostic and treatment modalities
- MUDr. Marek Beneš died on June 18, 2020 at the age of 44
- An unusual case of upper type dysphagia
- Motorized spiral enteroscopy – our first experience
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

