-
Medical journals
- Career
Herpetic esophagitis in a 7-year-old immunocompetent patient
Authors: Jabandžiev P. 1,2; Jouza M. 1; Pecl J. 1; Urík M. 3; Papež J. 1; Pinkasová T. 1; Slabá K. 1; Trna J. 4,5; Kyclová J. 6; Vaculová J. 4; Kunovsky L. 4,7
Authors‘ workplace: Department of Pediatrics, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Central European Institute of Technology, Masaryk University, Brno, Czech Republic 2; Department of Pediatric Otorhinolaryngology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Gastroenterology and Internal Medicine, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 4; Department of Gastroenterology and Digestive Endoscopy, Masaryk Memorial Cancer Institute, Brno, Czech Republic 5; Department of Pathology, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 6; Department of Surgery, University Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic 7
Published in: Gastroent Hepatol 2020; 74(3): 233-237
Category: Digestive Endoscopy: Case Report
doi: https://doi.org/10.14735/amgh2020233Overview
Herpetic esophagitis is a disease diagnosed especially in immunocompromised patients. Although the disease is rare in immunocompetent individuals, the diagnosis should be considered in the presence of its acute triad of clinical symptoms – odynophagia, chest pain, and fever of unknown origin. Herpes simplex virus type 1 (HSV1) is the most common causative agent. In the majority of cases, the disease develops by re-activation of latent HSV1 infection or, rather rarely, by primo-infection. The basis of diagnosis is endoscopic examination of the esophagus with biopsy and direct detection of the virus in the bioptic sample. In immunocompromised patients, treatment with acyclovir, which is the first-line virostatic in this indication, is always indicated. In immunocompetent patients, this is a self-limiting disease, where in most cases merely symptomatic treatment is sufficient. This case report describes an immunocompetent patient with a suddenly occurring typical triad of symptoms caused by herpetic esophagitis. The diagnosis was confirmed by the presence of viral DNA as determined by polymerase chain reaction from a sample taken during endoscopic examination. Due to the more severe course of the disease, the patient was treated with acyclovir and the general condition and local endoscopic findings then quickly improved.
Keywords:
endoscopy – children – herpetic esophagitis – acyclovir
Introduction
Along with cytomegalovirus and candida, herpes simplex virus (HSV) is one of the possible causative agents of ulcerative esophagitis in immunocompromised patients (e. g., HIV patients, immunosuppressed patients, patients with severe systemic disease or severe burns, patients with long-term corticosteroid therapy) [1,2]. Despite the high prevalence of primary and recurrent HSV infection in the population [3], clinically manifested herpetic esophagitis (HE) is rare in immunocompetent patients [4]. Isolated cases of HE in immunocompetent children are nevertheless described in the literature [5–11].
Case report
A 7-year-old patient who had previously been healthy was transferred to the Department of Pediatrics of the University Hospital Brno from the pediatric department of a district hospital. He was a normally thriving boy with adequate psychomotor development who had been properly vaccinated with no adverse manifestations. He had had no physiological morbidities up to that time and no allergic disease. He had not taken any medication for a long time. Before the occurrence of the clinical symptoms, the boy had spent a month of summer holidays in Montenegro. Otherwise, the epidemiological history obtained on admission was negative. Four days before the admission to the hospital, the boy had had a fever that reached a maximum of 38.5 °C. The fever had responded well to antipyretic therapy. The boy had no other problems. On the second day of the fever, dysphagia and odynophagia had occurred. After examination by a GP for children and adolescents, proton pump inhibitor therapy in the usual dosage had been recommended. Despite the established treatment, the swallowing problems worsened over subsequent days and the boy had to be hospitalized in the children’s ward of the district hospital. The possibility of having ingested a foreign body was excluded by the patient and parents. There had been no previous signs of primary immunodeficiency or other signs of gastrointestinal disease in the patient.
There was significant elevation of CRP (110 mg/l) in the baseline blood samples, mild leukocytosis in the blood count, and significant ketonuria without glycosuria in the urine. During hospitalization, a chest CT scan was performed but did not show any specific pathology. Parenteral nutrition was initiated and symptomatic therapy (proton pump inhibitor, nonsteroidal anti-inflammatory drugs) continued. For persisting difficulties without significant improvement, the boy was transferred for further examination to the university hospital.
The initial clinical examination was without noteworthy changes. In a detailed supplement to the epidemiological history, it was found that 4 days prior to the onset of the patient’s complaints, his mother had had a significant eruption of herpes labialis. The patient himself had never had such symptoms. As a further step to the diagnosis, an esophagogastroduodenoscopic examination was performed. Significant inflammatory changes transitioning to longitudinal ulcerations in the distal third of the esophagus were revealed (Fig. 1A, B). In addition to the standard sampling of esophageal tissue, biopsy material was also sent for bacteriological and mycological examination and also for the detection of HSV and cytomegalovirus by polymerase chain reaction (PCR).
1. A. Endoscopic imaging – view of distal esophagus. Ulceration covered by mucus, mucosa fragile to the touch, contact bleeding.
Obr. 1A. Endoskopický obraz – pohled na distální jícen. Ulcerace kryté hlenem, sliznice kâehká na dotek, kontaktně krvácející.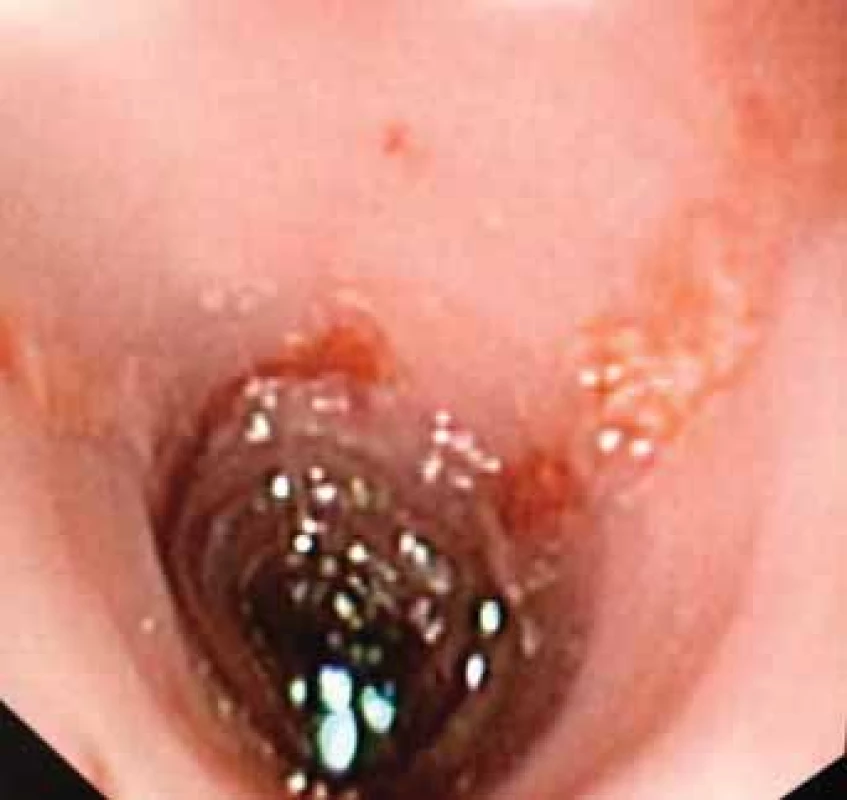
Fig. 1B. Endoscopic imaging – ulceration of the distal esophagus.
Obr. 1B. Endoskopický obraz – ulcerace distálního jícnu.
Given that this was a relatively unusual finding in a pediatric patient, empirical therapy with antibiotics (cefuroxime), antifungal agent (fluconazole), and antiviral drug (acyclovir) were administered intravenously. Over the next two days, there was a significant subjective improvement in symptoms. The patient began to tolerate fluids and subsequently a semi-solid diet, and the fever disappeared.
The result of HSV DNA detection by PCR from the esophageal mucosa was positive. Histological examination of the tissue taken from the affected area of the esophagus confirmed the presence of nonspecific ulceration (necrosis and granulation tissue) (Fig. 2), but the pathognomonic features of herpesvirus infection (inclusion, multinucleated keratinocytes) were not found in the sample. Immunohistochemistry (anti-CMV antibody) and special staining (PAS, Grocott silvering) excluded the presence of cytomegalovirus and fungal infection.
2. Histological examination – base of ulceration consisting of granulation tissue with mixed inƪ ammatory infiltrate.
Obr. 2. Histologické vyšetření – spodina ulcerace tvořená smíšeně zánětlivě celulizovanou granulační tkání.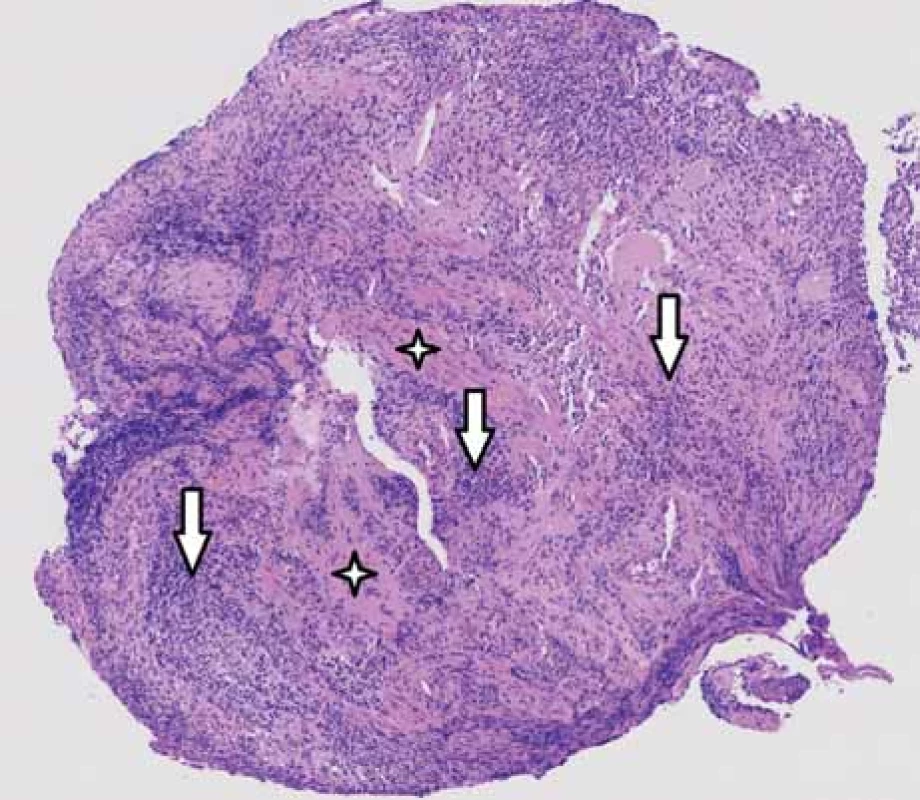
The presence of smooth muscle bundles verifies the depth of ulceration to the muscularis propria. Hematoxylin-eosin staining, magnification 30-times. Stars indicate smooth muscle bundles, vertical arrows indicate inflammatory infiltrate.
Přítomnost snopců hladké svaloviny dokumentuje hloubku ulcerace až ke svalovině muscularis propria. Barvení hematoxylin-eozinem, zvětšení 30krát. Hvězdičky – snopce hladké svaloviny, svislé šipky – zánětlivý infiltrát.Due to the relatively unusual diagnosis, an immunological examination was performed. This did not indicate any impairment of immunity. After completing the results of the microbiological examination, the antimicrobial treatment was revised. Antiviral therapy was maintained until the result of the control esophagogastroduodenoscopy, which was performed 3 weeks after the diagnosis. Endoscopic and histopathological examination confirmed the complete disappearance of inflammatory changes in the esophagus.
Discussion
HE was first described in 1940 by Johnson [12] and confirmed histopathologically by the Pearce team 3 years later [13]. HE is regarded today as an opportunistic infection that usually affects patients with immune disorders [1]. The first larger set of endoscopic findings of immunocompromised patients in which the diagnosis was based on virus culture was described by McBan in 1991 [14]. In 2000, Ramathan et al published an overview of HE described in immunocompetent patients, including several children [4].
HE can be caused by human HSV types 1 and 2. Type 2 is, however, rarely the causative agent [6]. Nevertheless, 20% of adult HE patients have been reported to have concomitant oropharynx and genital involvement [4]. The HSV rarely attacks internal organs. In such cases as it does so, the esophagus is often affected but the lungs and kidneys very rarely. Cases with stomach involvement also have been described [15,16]. In a series of 1,307 autopsies performed in adults, a study from Japan found an incidence of HE in 1.8% patients [17]. HE can develop as a result of direct expansion in the primary infections of the orofacial region or as a result of reactivation of latent infection when invasion of the esophagus mucosa by anterograde neuronal migration had occurred via the vagus nerve [1].
One of the predisposing factors of HE in immunocompetent patients may be esophageal mucosal trauma. This predisposition may be gastroesophageal reflux disease, previous esophageal instrumental examination, long--term insertion of a nasogastric tube, or swallowing of a foreign body [5]. HE is typically manifested by a triad of symptoms: odynophagia, chest pain, and fever of unknown origin. These symptoms are usually accompanied by weight loss [4,14].
The diagnosis of HE consists of an endoscopic examination, exclusion of other causes (from infection, especially cytomegalovirus and candida), and confirmation of HSV’s presence. Bioptic examination almost always shows inflammatory changes of the mucosa, but only in some cases can the typical diagnostic signs of herpesvirus infection (i.e., intranuclear inclusion in the epithelium of the esophageal mucosa, either eosinophilic [Cowdry A type] or ground-glass [Cowdry B type]) be found [18]. Distinguishing of HSV and varicella-zoster virus is not possible with the use of light microscopy. In the past, the virus has been proven by a combination of immunohistochemical methods and cultivation [4,14]. Presently, due to its indisputable advantages, the detection of viral DNA by PCR is used [19].
The diagnosis is therefore usually based on clinical signs, upper gastrointestinal endoscopy, histology, microbiological examination, and then PCR, respectively. Serological examination may be another marker for diagnosis [20].
Other esophageal infections (cytomegalovirus, varicella-zoster virus, candida, or various bacterial agents), trauma, esophageal burns, and inflammatory diseases such as Crohn’s disease or Behcet’s disease may be considered in the differential diagnosis of ulcerative esophagitis [21].
Acyclovir, famciclovir, and valacyclovir are used to treat HE in immunocompromised patients [22].
HE in immunocompetent patients appears generally to be self-limiting [23]. The reasons for administering antiviral therapy may include expectation to reduce the length of the period of infection or to treat or prevent complications.
Primary HSV infection is a common disease in childhood. Positive antibodies to HSV 1 occur in as many as 90% of all adolescents. The highest incidence of the disease with clinical manifestation is around 2 years of age, and gingivostomatitis is the most common clinical form of the disease [5]. During primo-infection, HE can occur rather frequently and thus be underdiagnosed. It is very likely that undiagnosed esophagitis may also be suffered by patients with severe primo-infection (i.e., herpetic gingivostomatitis or pharyngitis), as well as patients with gastroesophageal reflux disease treated with proton pump inhibitors. HE in immunocompetent children is mostly represented by primo-infection, but it may also follow reactivation of latent infection. Although HSV infections of the visceral organs are the result of viremia, esophagitis as such is rather the result of a direct spread of oropharyngeal involvement. Boys are more affected than girls in a ratio of 3.4/1.
The described patient had been in contact with his mother who had symptoms of labial herpes 4 days before the onset of his symptoms. It can be assumed, therefore, that this was a primary HSV infection that then spread to the esophagus. This would correspond to the initial negative serological examination of HSV antibodies in the patient. Also, our patient showed no other clinical signs of immunodeficiency and the results of the immunological examination werenormal.
The clinical manifestations described in the literature (which means the triad of odynophagia, chest pain, and fever) were also present in this case.
Submitted/ Doručeno: 27. 3. 2020
Accepted/ Přijato: 29. 3. 2020
Lumír Kunovský, M.D., Ph.D.
Department of Gastroenterology and Internal Medicine, University Hospital Brno,
Faculty of Medicine, Masaryk University
Jihlavská 20
625 00 Brno, Czech Republic
e-mail: lumir.kunovsky@gmail.com
Sources
1. Buss DH, Scharyj M. Herpesvirus infection of the esophagus and other visceral organs in adults. Incidence and clinical significance. Am J Med 1979; 66 (3): 457–462. doi: 10.1016/0002-9343 (79) 91068-4.
2. Jetté-Côté I, Ouellette D, Béliveau C et al. Total dysphagia after short course of systemic corticotherapy: herpes simplex virus esophagitis. World J Gastroenterol 2013; 19 (31): 5178–5181. doi: 10.3748/wjg.v19.i31.5178.
3. Xu F, Sternberg MR, Kottiri BJ et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296 (8): 964–973. doi: 10.1001/jama.296.8.964.
4. Ramanthan J, Rammouni M, Baran J Jr et al. Herpes simplex virus esophagitis in the immunocompetent host: an overview. Am J Gastroenterol 2000; 95 (9): 2171–2176. doi: 10.1111/j.1572-0241.2000.02299.x.
5. Al-Hussaini AA, Fagih AM. Herpes simplex ulcerative esophagitis in healthy children. Saudi J Gastroenterol 2011; 17 (5): 353–356. doi: 10.4103/1319-3767.84496.
6. Bastian JF, Kaufman LA. Herpes simplex esophagitis in a healthy 10-year-old boy. J Pediatr 1982; 100 (3): 426–427. doi: 10.1016/ s0022-3476 (82) 80451-4.
7. Desigan G, Schneider RP. Herpes simplex esophagitis in healthy adults. South Med J 1985; 78 (9): 1135–1137. doi: 10.1097/00007611-198 509000-00025.
8. Stillman AE. Herpes esophagitis in normal children. J Pediatr 1986; 109 (3): 563–564. doi: 10.1016/s0022-3476 (86) 80148-2.
9. Ashenburg C, Rothstein FC, Dahms BB. Herpes esophagitis in the immunocompetent child. J Pediatr 1986; 108 (4): 584–587. doi: 10.1016/s0022-3476 (86) 80842-3.
10. Moore DJ, Davidson GP, Binns GF. Herpes simplex oesophagitis in young children. Med J Aust 1986; 144 (3): 716–717.
11. Altamimi EM, Alorjani MS, Alquran WY. Herpetic esophagitis in immunocompetent child. Pediatr Gastroenterol Hepatol Nutr 2019; 22 (3): 298–302. doi: 10.5223/pghn.2019.22.3.298.
12. Johnson HN. Visceral lesions associated with varicella. Arch Pathol 1940; 30 : 292–307.
13. Pearce J, Dagradi A. Acute ulceration of the esophagus with associated intranuclear inclusion bodies. Report of four cases. Arch Pathol 1943; 35 (6): 889–897.
14. McBane RD, Gross JB Jr. Herpes esophagitis: clinical syndrome, endoscopic appearance, and diagnosis in 23 patients. Gastrointest Endosc 1991; 37 (6): 600–603. doi: 10.1016/ s0016-5107 (91) 70862-6.
15. Depew WT, Prentice RS, Beck IT et al. Herpes simplex ulcerative esophagitis in a healthy subject. Am J Gastroenterol 1977; 68 (4): 381–385.
16. al-Samman M, Zuckerman MJ, Verghese A et al. Gastric ulcers associated with herpes simplex esophagitis in a nonimmunocompromised patient. J Clin Gastroenterol 1994; 18 (2): 160. doi: 10.1097/00004836-199403000-00017.
17. DiPalma JA, Brady CE 3rd. Herpes simplex esophagitis in a nonimmunosuppressed host with gastroesophageal reflux. Gastrointest Endosc 1984; 30 (1): 24–25. doi: 10.1016/s0016-5107 (84) 72289-9.
18. Wang HW, Kuo CJ, Lin WR et al. Clinical characteristics and manifestation of herpes esophagitis: one single-center experience in Taiwan. Medicine (Baltimore) 2016; 95 (14): e3187. doi: 10.1097/MD.0000000000003187.
19. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J 2014; 11 : 83. doi: 10.1186/1743-422X-11-83.
20. Mârginean CO, Meliţ LE, Mocan S et al. An uncommon case of herpetic esophagitis in a small child with allergic rhinitis: a case report and literature review (CARE Compliant). Medicine (Baltimore) 2019; 98 (20): e15601. doi: 10.1097/MD.0000000000015601.
21. Galbraith JC, Shafran SD. Herpes simplex esophagitis in the immunocompetent patient: report of four cases and review. Clin Infec Dis 1992; 14 (4): 894–901. doi: 10.1093/clinids/14.4.894.
22. Kajzrlíková IM, Buriánová A, Hořava V. ml. et al. Torpidní průběh herpetické ezofagitidy u imunokompetentní pacientky – videokazuistika. Gastroent Hepatol 2015; 69 (3): 201–203. doi: 10.14735/amgh2015201.
23. Solammadevi SV, Patwardhan R. Herpes esophagitis. Am J Gastroenterol 1982; 77 (1): 48–50.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2020 Issue 3-
All articles in this issue
- Editorial
- Kvíz z klinické praxe
- Impact of the COVID-19 pandemic on endoscopy practice in the Czech Republic – survey research
- Disconnected pancreatic duct syndrome – a neglected complication of acute pancreatitis
- Motorized spiral enteroscopy – our first experience
- Eosinophilic esophagitis – current overview of diagnostic and treatment modalities
- Projekt „Endoskopická centra“v České republice
- Current scientific background of Crohn’s Disease Exclusion Diet (CDED)
- Budesonide MMX in the treatment of ulcerative colitis
- An unusual case of upper type dysphagia
- Renal illness in patients with inflammatory bowel disease
- In memory of assoc. prof. Jan Kotrlík
- MUDr. Marek Beneš died on June 18, 2020 at the age of 44
- The selection from international journals
- Správná odpověď na kvíz: Ischemická kolitida na podkladě trombózy dolní mezenterické žíly
- Perioperative oesophagogastroduodenoscopy in the prevention and therapy of anastomotic complications – a review
- Herpetic esophagitis in a 7-year-old immunocompetent patient
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Eosinophilic esophagitis – current overview of diagnostic and treatment modalities
- MUDr. Marek Beneš died on June 18, 2020 at the age of 44
- An unusual case of upper type dysphagia
- Motorized spiral enteroscopy – our first experience
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

