-
Medical journals
- Career
Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
Authors: J. Holoubek 1,2; M. Knoz 1,2,3; B. Lipový 1,2; J. Bartošková 1; I. Kocmanová 4; M. Hanslianová 4; M. Krtička 2,5; T. Kubek 2,3
Authors‘ workplace: Department of Burns and Plastic Surgery, University Hospital Brno, Czech Republic 1; Faculty of Medicine, Masaryk University Brno, Czech Republic 2; Department of Plastic and Aesthetic Surgery, St. Ann‘s University Hospital, Brno, Czech Republic 3; Department of Clinical Microbiology, University Hospital Brno, Czech Republic 4; Department of Traumatology, University Hospital Brno, Czech Republic 5
Published in: Epidemiol. Mikrobiol. Imunol. 69, 2020, č. 2, s. 81-86
Category:
Overview
Nowadays, free flap reconstruction in devastating lower limb trauma is a standard procedure in reconstructive surgery. The greatest factor directly affecting limb salvage is still the risk of infectious complications, whether local or systemic. Fungal wound infections are not among the most common infection complications in surgery, but their low incidence is compensated for by their fulminant and serious course, as well as severe local tissue destruction and strong angio-invasive potential together with the possibility of dissemination. In this case study, we present an example of a devastating lower leg injury, solved using latissimus free flap reconstruction, with subsequent difficult and prolonged healing, due to an invasive filamentous fungi infection. In the final part of the article, we focus briefly on the occurrence of similar cases in the literature.
Keywords:
reconstruction – infection – filamentous fungi – free flap
INTRODUCTION
The foot and ankle are prone to injuries and diseases because of insufficient soft tissue [1]. Complex soft tissue defects of the foot and ankle, caused by trauma, infection, tumorous cancers or diabetes, are common and can be accompanied by exposed tendons, neurovascular bundles and bone [2]. Microsurgical free-tissue transfer is the standard care option for open fractures of the lower extremity accompanied by significant soft tissue trauma. These microsurgical techniques have enabled the salvage of limbs previously managed by amputation, with reported free flap success rates of 91–92% in traumatic lower limb injuries [3, 4].
Although there exist a number of factors likely to contribute to the increased incidence of complications in lower extremity free flaps, one particular area of controversy involves infection. For decades, infectious complications have been one of the major complications of surgical wound healing, and although bacteria are still among the most dominant infectious complications, we have recorded a year-on-year increase in the number of isolated micromycetes [5]. We present herein a case of post-traumatic filamentous fungi wound infection in an immunocompetent patient and discuss the diagnosis and treatment with a review of the relevant literature.
CASE REPORT
In this case study, we present a 50-year-old previously healthy man who was admitted for a devastating left foot injury due to a car accident. The primary image was dominated by subluxation in the ankle joint, luxation in the Chopart’s joint and a multiple fracture of cuboideum bone. All of this was accompanied with significant axial limb deformity and extensive soft tissue damage in the crural and tarsal areas. The patient was operated on acutely, to stabilise and reposition the fracture, using an external fixator. During initial treatment, the surrounding tissues began to show signs of tissue ischemia, and their vitality was considerably controversial. As a result of the magnitude and location of the injury, the maximum restraining procedure was chosen to minimise further limb trauma.
In the next few days, repeated revisions and necrectomy of the demarcating necrosis of skin and soft tissue were performed. Due to the progression of tissue devitalisation, a CT angio was undertaken, which did not show pathology in the vascular supply of the distal limb.
Surgical care
Eleven days after the injury, the patient was transferred to our clinic, where we continued with revisions and the necrectomy of devitalised tissues. On the nineteenth day following the injury, the affected area was stripped of all devitalised tissue and the resulting defect reconstructed using a latissimus free flap. The reconstructive part of the surgery was preceded by external fixator correction. A Schantz pin from the calcaneus was removed to allow flap application to the injured area. Two Kirschner wires were then inserted from the calcaneus to the distal tibia, in order to maintain the talocrural joint in the right position. Next, the flap was harvested along with the skin marker, and the tibialis posterior artery and vein were dissected as recipient vessels. Shortly after suturing the anastomosis, there was a repeated thrombotic closure at the site of the arterial anastomosis, which required a shortening of the vessel and suture end-to-side anastomosis in the proximal direction. After reperfusion flap showed good signs of vitality and the skin marker had a positive capillary return.
On the fifth day after the operation, the vitality of the flap was still suitable, but unfortunately there was a further devitalisation of the edge of the tissue in the heel area, which required further necrectomy in this area (Figure 1).
1. 5th day after free flap reconstruction, newly created defect in heal region 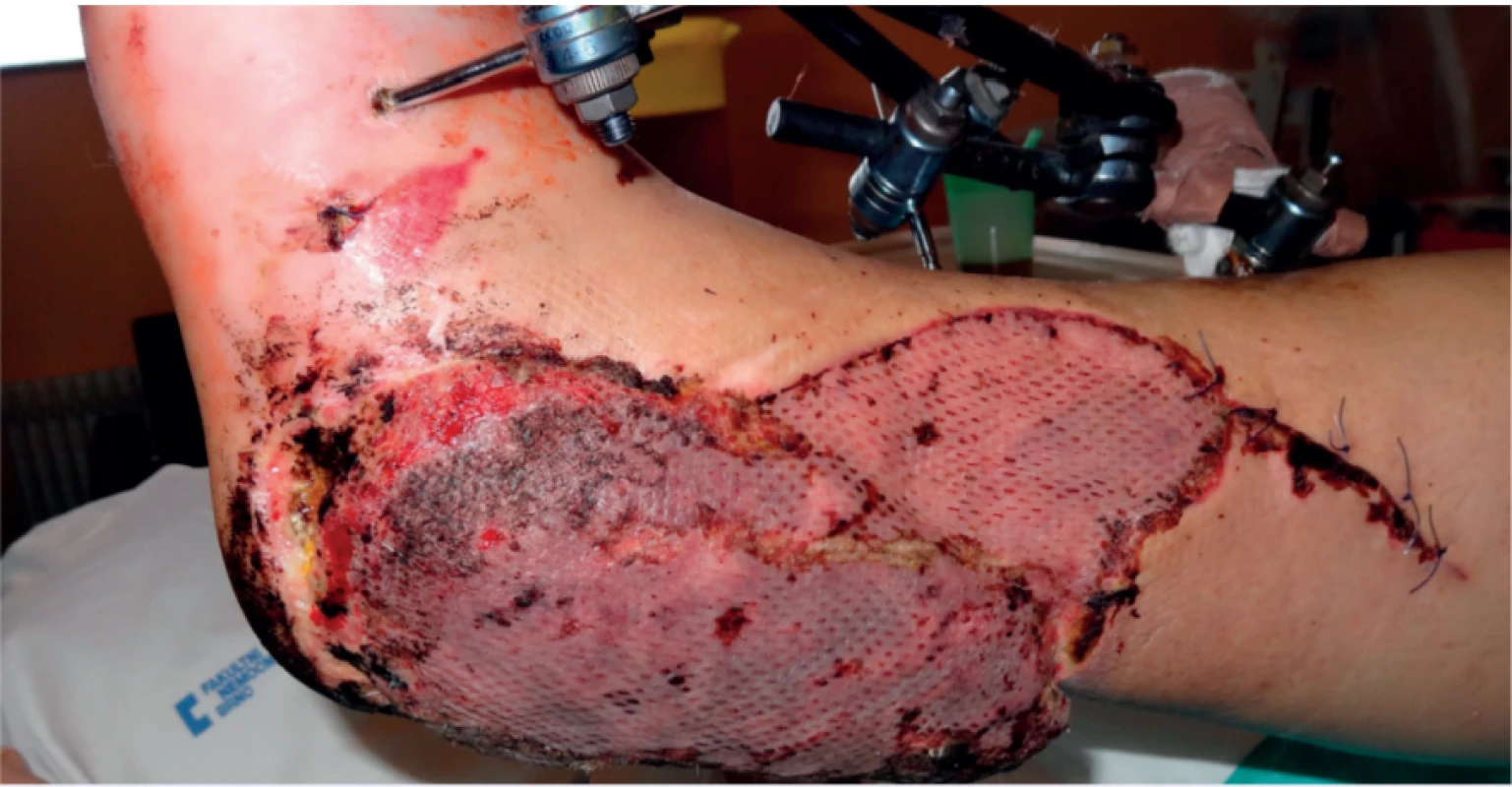
Microbiology
Following admission, a prophylactic triple combination of antibiotics was applied to the patient (methicillin 1 g every 6 hours + gentamicin 240 mg every 24 hours + metronidazol 500 mg every 8 hours) as a standart truama protocol. This was adjusted according to the results of precise microbiological surveillance during each surgical procedure.
All microbiological tests were performed in Department of Microbiology, University Hospital Brno. Collected materials from the wound were cultured aerobic, anaerobic and fungal (Sabouraud’s agar, incubation temperature – 25, 30 and 37 °C). Based on routine clinical practice, specimens were collected by a print and swab method, so direct microscopy could not be performed.
On the fifth day after free flap reconstruction, we observed the first positive isolation of filamentous fungi. This finding was confirmed by further culture positive sampling (positive at day 5, 8 and 21 of post-operative hospitalization) and histopathologically. The first isolate was categorized as Mucor circinelloides by macroscopic and microscopic appearance (Figure 2). Samples from the twenty-first day of hospitalization reveald a mixed culture – beside Mucor sp. Fusarium sp. was identified. Samples from following days identified only this Fusarium sp. Additional identification at species level at that time was not clinically relevant.
2. A – Mucor circinelloides (left), B – Fusarium sp. (right) both under microscope Enlarged 40x. 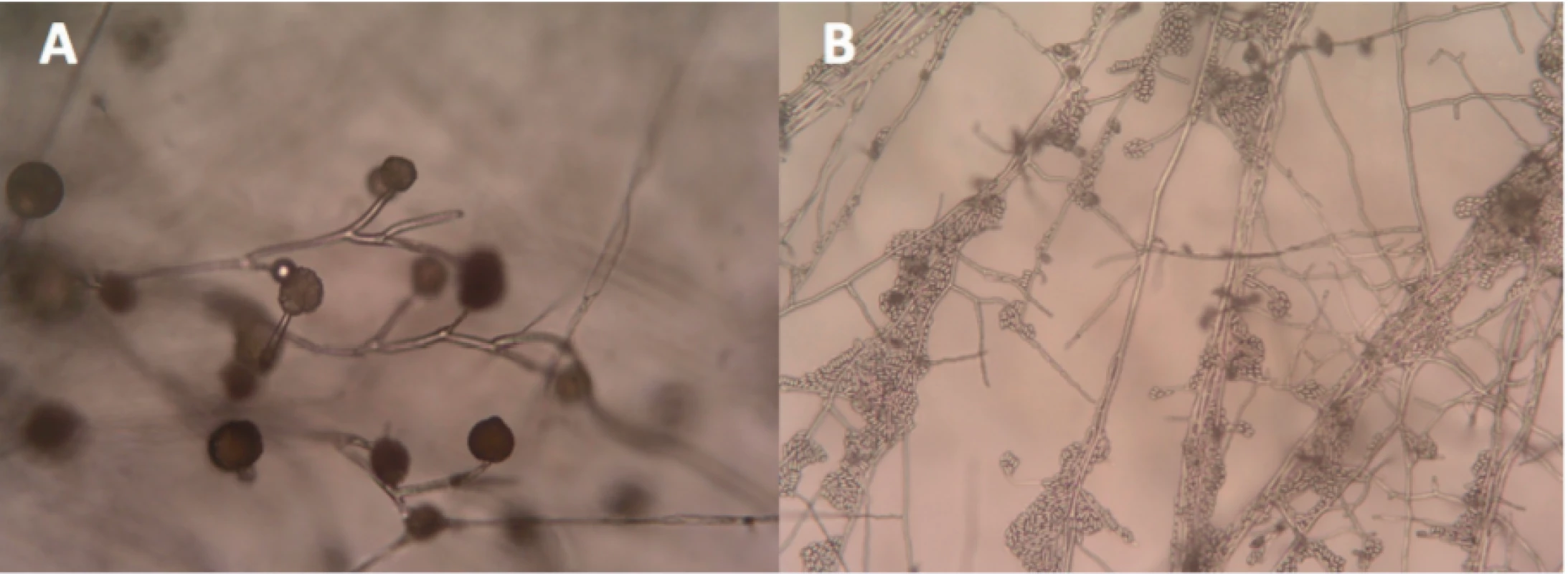
In both isolates, MIC (minimum inhibitors of concentration) was determined for selected antifungal agents using the Etest method (with RPMI agar 1640 containing MOPS and 2% glucose and were read after incubation at 34–36 °C for 24 h for Mucor sp. and 24–48 h for Fusarium sp.). For Mucor sp. were MICs for amphotericin B and posaconazol 0.064 and 2.0 µg/ml, respectively. For Fusarium sp. were MICs for amphotericin B and voriconazol 0.5 and 0.25 µg/ml, respectively. Unfortunately, the MIC gradually increased for Fusarium sp. (MICs for amphotericin and voriconazol were 8.0 and 2.0 µg/ml, respectively). Based on the new MIC results and previous therapy, voriconazol (200 mg every 24 h) was chosen as the most suitable systemic antifungal agent. Breakpoints have not been established for Fusarium spp., so we used epidemiologic cut-off values (ECV). ECV of voriconazol is 4–16 µg/ml, according Fusarium species, however, the epidemiological cut-off values will not categorize a fungal isolate as susceptible or resistant [6].
Invasive fungal infections led to further devitalisation of the tissue and the formation of a large defect in the heel region (Figure 3). Applied systemic antimycotic therapy, along with effective local antisepsis (combination alcohol and PVP-Iodine solution) and thorough surgical debridement, led to the eradication of the micromycetes. Antigen testing (galactomannan and glucan) was not performed.
3. Local sign of invasive fungal infection 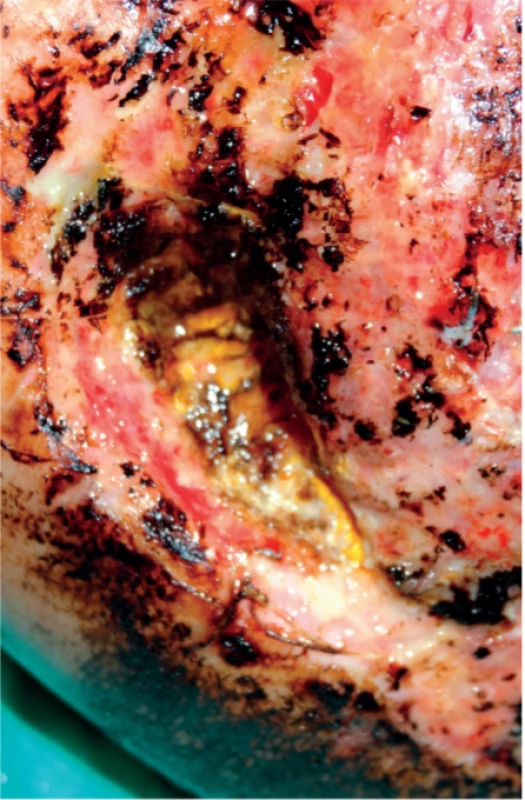
Unfortunately, prolonged healing of the newly developed defect in the heel region required re-hospitalization of the patient, further surgical therapy using the VAC system and more necrectomy of the defect (Figure 4). Systemic therapy by voriconazol (200 mg every 24 h) togehert with local wound care with amphotericin B was used. The concentration of amphotericin B was 50 mg in 500 ml of aqua purifica (0.1 mg in 1 ml). The resulting solution was then applied to the defect in the form of wet drapes and changed once every 24 hours. After successful eradication of the pathogen, the defect was closed by autotransplantation.
4. Defect after repeated debridement and necrectomy before final autotransplantation 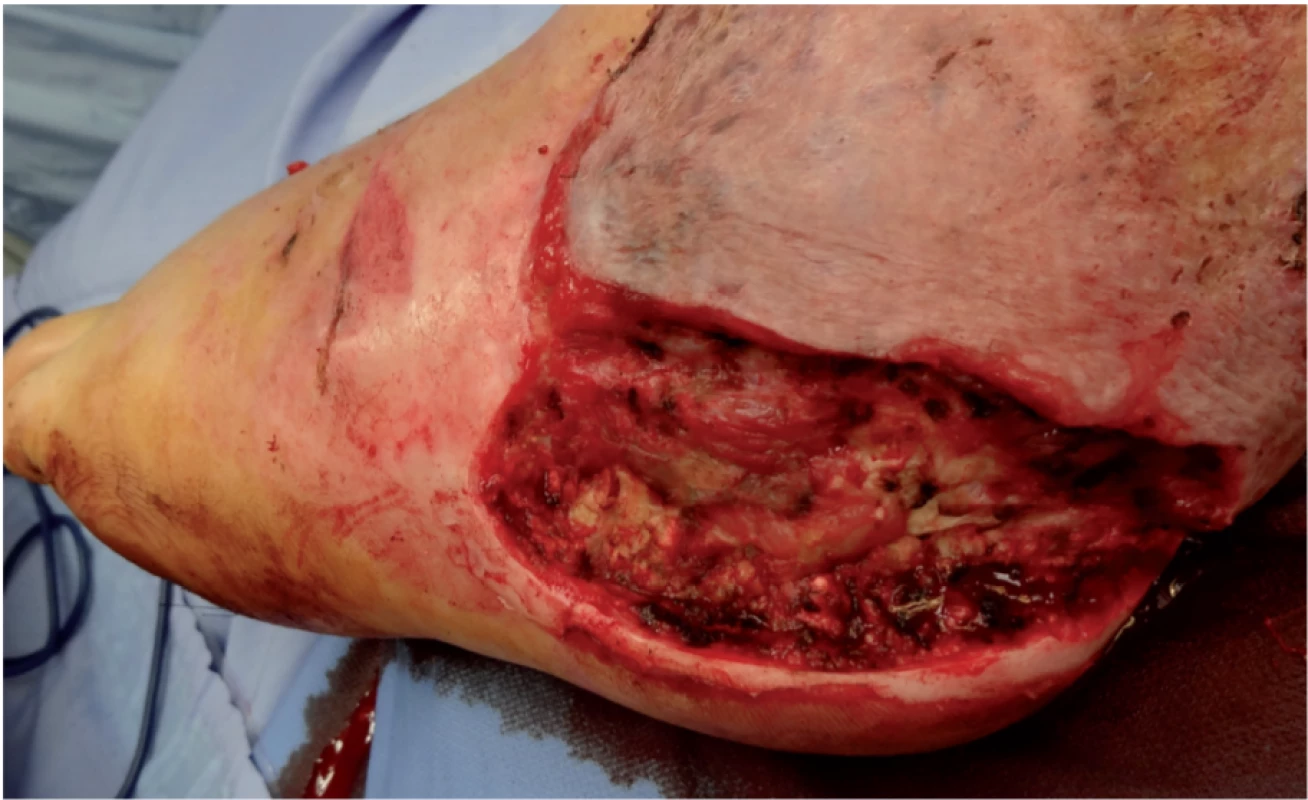
DISCUSSION
Moulds such as Mucorales, Aspergillus spp., Fusarium spp. and Scedosporium spp. are the main opportunistic filamentous fungi (FF) that can cause serious and rapidly fatal infections in immune-compromised patients such as those receiving chemotherapy for malignancies, the recipients of bone marrow or solid organ transplants and patients with neutrophil defects, especially chronic granulomatous disease [7, 8]. Lately, increasing numbers of fungal infection have been reported in burns patients, due to the loss of skin cover continuity along with the presence of necrotic tissue, thereby creating an ideal entry point for a bacterial or a mycotic infection [5]. On the other hand, only a few reports have described FF skin and soft tissue infections complicating post-surgical [9–11] or post-traumatic wounds [12–14] in non-neutropenic adult patients.
Recently there has been several major publication dealing with severe invasive fungal infection in trauma patients. Zahoor et al. in 2016 published broad literature review focusing on surgical treatment and antimycotic treatment of severe mucormycosis as a result of penetrative trauma. In total they went through 36 reports which 18 were case based. The conclusion of their work is clear that only radical and early surgical approach, even at the expense of disfigurement, is necessary to reduce mortality in the setting of cutaneous mucormycosis that results from penetrating trauma [15]. In 2017 Kroner et al. presented paper dealing with invasive fungal infections secondary to traumatic injury focusing epidemiology, natural history, mycology, risk factors, diagnosis, treatment, and outcomes in military and civilian populations. Mucorales is the major pathogen in both groups, and the risk factors are, of course, very different due to battlefield injuries and field conditions. Similarly, diagnosis and therapy are often very limited in the event of a war conflict, and this must also have an impact on mortality. Here again, however, there is a clear conclusion that only adequal surgical therapy along with effective antimycotic medication can yield a satisfactory result [16]. Last report from Loganathan et al. published in 2018 dealing with Invasive fungal infection in 5 patients with open fractures. The conclusion of all the work is quite similar and the greatest emphasis is placed on correctly timed and adequate surgical therapy. An important part of treatment is then targeted antifungal treatment. Amphotericin B is nowadays still the most used preparation, but it is more and more necessary to treat the newer broad spectrum antimycotics of the II. generation of triazole. In all these works, the need for further research and data collection such as further illumination of risk factors, improved diagnostic methods, and optimal treatment regimens is strongly emphasized for future time [17].
However, in post-traumatic FF infections, acidosis due to severe soft tissue damage and lack of tissue viability, associated with local immune-depression, could explain the pathogenicity of fungal infections in patients without underlying conditions, as described by Wichmann et al. [18]. In addition, the presence of large amounts of spores in soil contributes to wound contamination and the development of infection. Finally, a key feature of wound-related fungal infection is the direct entry of the fungi into the skin. The characteristic feature of filamentous fungi infection, especially in the case of Mucorales, is their local angiovinase, which can lead easily to dissemination throughout the body. As a result, it is essential to prevent fast progression by performing rapid diagnostics, appropriate surgical debridement and antifungal therapy [19].
Since there was repeated isolation of the fungi from the same site during surgical procedures (including necrectomy), local infection in the patient was highly suspected. The only way to distinguish these two was to perform a histopathological examination (skin biopsy), in order to detect fungal invasiveness. Direct mycological examination is based on the cultivation and microscopy of sterile materials such as blood, normally sterile body fluids, deep tissue and organs. According the EORTC/MSG (European Organization for Research and Treatment of Cancer/Mycoses Study Group) criteria, we identified this case as a proven fungal infection (positive fungal culture with histopathological evidence and with clinical signs of infection) [20]. However, in some cases these criteria are not particularly applicable. In some cases we have to rely solely on cultivation capture and clinical picture, because further material collection would lead to further devastation of the affected area [21].
Until 2002, the only option to counter the threat of systemic filamentous fungal infection was to use amphotericin B and itraconazole. Newly discovered drugs, such as voriconazole and posaconazaloze, as well as new echinocandins, such as caspofungin, have brought new therapeutic options into systemic micromycetal infections, especially on Aspergillus-invasive infections [22]. However, therapeutic options for the remaining FF continue to be limited in the form of liposomal amphotericin B and posaconazole for Mucorales and voriconazole for Fusarium spp. and Scedosporium spp. Therapy consists of initial, adequate surgical debridement and then adequate antifungal therapy. As a result of the large variety of possible agents, and their changing sensitivities to different antifungal agents, empirical therapy is hard to identify. Therefore, the identification of the fungus, using a culture or polymerase chain reaction (PCR), is necessary. The choice of antifungal therapy is dependent on the fungal species, the condition and immune status of the patient and the extent of the infection [23].
We were able to find ten articles linking to a total of 11 patients with free flap reconstruction who had also presented with filamentous fungi infection [24–32] since 2001. These observations are of cases with mucormycosis infection or retrospective studies of reconstruction by free flaps, in which some of the indications concerned zygomycosis [24]. In total, six patients were evaluated as immune-competent, and the infection was a result of a high-energy trauma. The remaining five patients were enrolled in different stages of immune-compromisation (haematological malignancy, extreme burning and an advanced stage of autoimmune disease). All patients required serial debridement of the necrotic tissue, accompanied by significant morbidity and the loss of soft tissue or even bone. All patients survived. In total, there were three maxillectomies, two exenterations, one mandibulectomy and one complete loss of a limb. The range of free flaps used varied. The only multiple-use flaps were anterolateral thigh flaps (ALT) and latissimus dorsi flaps, both of which were used twice. Three osteocutaneous free flaps were used in reconstruction (capular osteocutaneous free flap, a serratus anterior combined with the seventh rib and a vascularised fibular osteocutaneous flap). All reconstructions were successful, and there was zero flap mortality. Only in one case, as well as in our case, was there a complication with thrombosis or anastomosis. The most frequently used antimycotic agent was amphotericin B alone (six cases). In other cases, it was supplemented with or replaced by posaconazole or voriconazole.
Adequate and sufficiently long antimycotic therapy is essential for successful defect closure, especially when taking into account vascular tropism caused by Zygomycetes, which is a source of thrombosis and vascular dissections [24]. In summary, on the diagnosis and treatment of rare fungal infections, we can refer to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) of the Fungal Infection Study Group (EFISG) and the European Confederation of Medical Mycology (ECMM) 2013 Emerging fungal diseases [33].
CONCLUSION
Fungal post-traumatic infections after extensive soft tissue damage are a rare but severe complication. All clinical cases require significant surgical debridement and extensive surgical reconstruction. Early and accurate identification of the fungus is also important to minimising the consequences and overall survival of the patient. The high risk of dissemination and any associated increasing lethality should always be a signal of aggressive surgical and systemic therapy. Research concerning mucormycosis, secondary to penetrating trauma, is in its infancy. High quality non-anecdotal studies have yet to be performed or reported. We should foster the development of new targets for intervention, including surgical treatment protocols and additional pharmacological therapies in addition to possibly re-examining endeavours of therapy that have failed previously. Greater clinical suspicion in trauma patients is strongly suggested to identify and manage invasive fungal infection expediently and satisfactorily [15].
Do redakce došlo dne 28. 6. 2019.
Adresa pro korespondenci:
MUDr. Martin Knoz
Klinika plastické a estetické chirurgie
LF MU a FN u sv. Anny
Pekařská 664/53
656 91 Brno-střed
e-mail: martinknoz@gmail.com
Sources
1. Hollenbeck ST, Woo S, KOMATSU I, et al. Longitudinal Outcomes and Application of the Subunit Principle to 165 Foot and Ankle Free Tissue Transfers. Plastic [online], 2010;125(3):924–934 [cit. 2018-04-29]. DOI: 10.1097/PRS.0b013e3181cc9630. ISSN 00321052.
2. Li X, Cui J, Maharjan S, et al. Reconstruction of the Foot and Ankle Using Pedicled or Free Flaps: Perioperative Flap Survival Analysis. PLoS ONE [online], 2016;11(12):1–13 [cit. 2018-04-29]. DOI: 10.1371/journal.pone.0167827. ISSN 19326203.
3. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Clinics in Plastic Surgery [online], 1986;13(4):619–620 [cit. 2018-04-29]. ISSN 00941298.
4. Khouri RK, Shaw WW. Reconstruction of the lower extremity with microvascular free flaps: A 10-year experience with 304 consecutive cases. Journal of Trauma – Injury, Infection and Critical Care [online], 1989;29(8):1086–1094 [cit. 2018-04-29]. ISSN 15298809.
5. Suleyman G, Alangaden G. Nosocomial Fungal Infections. Epidemiology, Infection Control, and Prevention. Infectious Disease Clinics of North America [online], 2016;30(4): 1023–1052 [cit. 2018-04-29]. DOI: 10.1016/j.idc.2016.07.008. ISSN 08915520.
6. Espinel-Ingroff A, Colombo A, Cordoba S, et al. International Evaluation of MIC Distributions and Epidemiological Cut-off Value (ECV) Definitions for Fusarium Species Identified by Molecular Methods for the CLSI Broth Microdilution Method. Antimicrobial Agents And Chemotherapy [online], 2015;60(2):1079–1084 [cit. 2019-04-06]. DOI: 10.1128/AAC.02456-15. ISSN 10986596.
7. Roden M, Zaoutis T, Buchanan WL, et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clinical Infectious Diseases [online], 2005;41(5):634–653 [cit. 2018-04-29]. ISSN 10584838.
8. Young R, Bennett J, Vogel C, et al. Aspergillosis, the spectrum of the disease in 98 patients. Am J Med, 1970;49 : 147–173.
9. Sawyer R, Schenk W, Adams R, et al. Aspergillus flavus wound infection following repair of a ruptured duodenum in a non-immunocompromised host. Scandinavian Journal of Infectious Diseases [online], 1992;24(6):805–809 [cit. 2018-05-09]. ISSN 00365548.
10. Bryce E, Walker M, Scharf S, et al. An outbreak of cutaneous aspergillosis in a tertiary-care hospital. Infection Control And Hospital Epidemiology [online], 1996;17(3):170–172 [cit. 2018-05-09]. ISSN 0899823X.
11. Carlson G, Mughal M, Birch M, et al. Aspergillus wound infection fol-lowing laparostomy. The Journal Of Infection [online], 1996;33(2):119–121 [cit. 2018-05-09]. ISSN 01634453.
12. Myers J, Dunn A. Aspergillus infection of the hand. JAMA, 1930;95 : 794–796.
13. Böhler K, Metze D, Poitschek C. et al. Cutaneous aspergillosis. Clinical And Experimental Dermatology [online], 1990;15(6):446–450 [cit. 2018-05-09]. ISSN 03076938.
14. Harmon C, Su WP, Peters M. Cutaneous aspergillosis complicating pyoderma gangrenosum. Journal Of The American Academy Of Dermatology [online], 1993;29(4):656–658 [cit. 2018-05-09]. ISSN 01909622.
15. Zahoor B, Kent S, Wall D. Review: Cutaneous mucormycosis secondary to penetrative trauma. Injury [online], 2016;47(7):1383–1387 [cit. 2019-04-06]. DOI: 10.1016/j.injury.2016.03.011. ISSN 00201383.
16. Kronen R, Liang S, Bochicchio G, et al. Review: Invasive Fungal Infections Secondary to Traumatic Injury. International Journal of Infectious Diseases [online], 2017;62 : 102–111 [cit. 2019-04-06]. DOI: 10.1016/j.ijid.2017.07.002. ISSN 12019712.
17. Loganathan S, Ajay G, Thyagarajan U. et al. Invasive fungal infection in immunocompetent trauma patients – A case series. Journal of Clinical Orthopaedics and Trauma [online], 2018;9:S10–S14 [cit. 2019-04-06]. DOI: 10.1016/j.jcot.2017.10.005. ISSN 22133445.
18. Wichmann M, Ayala A, Chaudry I. Severe depression of host immune functions following closed-bone fracture, soft-tissue trauma, and hemorrhagic shock. Critical Care Medicine [online], 1998;26(8):1372–1378 [cit. 2018-05-09]. ISSN 00903493.
19. Vitrat-Hincky V, Lebeau B, Bozonnet E, et al. Severe filamentous fungal infections after widespread tissue damage due to traumatic injury: Six cases and review of the literature. Scandinavian Journal of Infectious Diseases [online], 2009;41(6/7):491–500 [cit. 2018-05-09]. DOI: 10.1080/00365540902856537. ISSN 00365548.
20. De Pauw B, Walsh T, Donnelly J, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases: An Official Publication Of The Infectious Diseases Society Of America [online], 2008;46(12):1813–1821 [cit. 2018-05-09]. DOI: 10.1086/588660. ISSN 15376591.
21. Schaal J, Leclerc T, Soler C, et al. Epidemiology of filamentous fungal infections in burned patients: A French retrospective study. Burns: Journal Of The International Society For Burn Injuries [online], 2015;41(4):853–863 [cit. 2018-05-09]. DOI: 10.1016/j.burns.2014.10.024. ISSN 18791409.
22. Kauffman C. Clinical efficacy of new antifungal agents. Current Opinion In Microbiology [online], 2006;9(5):483–488 [cit. 2018-05-09]. ISSN 13695274.
23. Hajdu S, Obradovic A, Presterl E, et al. Invasive mycoses following trauma. Injury [online], 2009;40(5):548–554 [cit. 2018-05-09]. ISSN 00201383.
24. Reinbold C, Derder M, Hivelin M, et al. Using free flaps for reconstruction during infections by mucormycosis: A case report and a structured review of the literature. Annales de Chirurgie Plastique et Esthetique [online], 2016;61(2):153–161 [cit. 2018-05-09]. DOI: 10.1016/j.anplas.2015.05.006. ISSN 1768319X.
25. Shand J, Albrecht R, Burnett H, et al. Invasive fungal infection of the midfacial and orbital complex due to Scedosporium apiospermum and mucormycosis. Journal of Oral And Maxillofacial Surgery: Official Journal Of The American Association Of Oral And Maxillofacial Surgeons [online], 2004;62(2):231–234 [cit. 2018-05-09]. ISSN 02782391.
26. Sham E, Bruscino-Raiola F, Leung M. The serratus anterior free flap revisited. Journal Of Plastic, Reconstructive [online], 2010;63(2):e184–185 [cit. 2018-05-09]. DOI: 10.1016/j.bjps.2009.02.052. ISSN 18780539.
27. Seitz I, Adler N, Odessey E, et al. Latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap reconstruction in composite defects of the scalp: case series and review of literature. Journal Of Reconstructive Microsurgery [online], 2009;25(9):559–567 [cit. 2018-05-09]. DOI: 10.1055/s-0029-1236834. ISSN 10988947.
28. Murphy A, Williamson P, Vesely M. Case report: Reconstruction of an extensive peri-orbital defect secondary to mucormycosis in a patient with myelodysplasia. Journal of Plastic, Reconstructive [online], 2013;66(3):e69 [cit. 2018-05-09]. DOI: 10.1016/j.bjps.2012.11.032. ISSN 17486815.
29. Moran S, Strickland J, Shin A. Upper-extremity mucormycosis infections in immunocompetent patients. The Journal Of Hand Surgery [online], 2006;31(7):1201–1205 [cit. 2018-05-09]. ISSN 03635023.
30. Metzen D, Böhm H, Zimmermann M, et al. Mucormycosis of the head and neck. Journal of Cranio-Maxillofacial Surgery [online], 2012;40(8):e321 [cit. 2018-05-09]. DOI: 10.1016/j.jcms.2012.01.015. ISSN 10105182.
31. Antonetti J, Killyon G, Chang, et al. Microvascular transfer of burned tissue for mandibular reconstruction. Journal Of Burn Care [online], 2009;30(3):536–539 [cit. 2018-05-09]. DOI: 10.1097/BCR.0b013e3181a28e5f. ISSN 1559047X.
32. Odessey E, Cohn A, Beaman K, et al. Invasive mucormycosis of the maxillary sinus: extensive destruction with an indolent presentation. Surgical Infections [online], 2008;9(1):91–98 [cit. 2018-05-09]. DOI: 10.1089/sur.2006.039. ISSN 10962964.
33. Cornely O, Cuenca-Estrella M, Meis J, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clinical Microbiology And Infection: The Official Publication Of The European Society Of Clinical Microbiology And Infectious Diseases [online], 2014;20(Suppl 3):1–4 [cit. 2019-10-31]. DOI: 10.1111/1469-0691.12569. ISSN 14690691.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2020 Issue 2-
All articles in this issue
- The significance of p16 protein expression in oral squamous cell carcinoma
- Barriers to treatment of infectious and other somatic comorbidity in drug users
- Q fever and prevention
- Smuteční oznámení: zemřel profesor MUDr. Miroslav Votava, CSc.
- Blahopřání RNDr. Marii Brůčkové, CSc.
- Intra-abdominal candidiasis in surgical intensive care unit – epidemiology characteristics and trends
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
- Neonatal pneumonia caused by Trichomonas vaginalis
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The significance of p16 protein expression in oral squamous cell carcinoma
- Q fever and prevention
- Neonatal pneumonia caused by Trichomonas vaginalis
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

