-
Medical journals
- Career
Intra-abdominal candidiasis in surgical intensive care unit – epidemiology characteristics and trends
Authors: J. Ulrych 1; V. Adámková 2; J. Matek 1; M. Komarc 3; V. Frýba 1; D. Schmidt 1; P. Koželský 1; A. Studená 2; J. Bříza 1; Z. Krška 1
Authors‘ workplace: First Faculty of Medicine, Charles University in Prague and General University Hospital in Prague, Czech Republic ; Clinical Microbiology and ATB Center, Institute of Medical Biochemistry and Laboratory Diagnostics, General University Hospital, Charles University, Prague, Czech Republic 2; Institute of Biophysics and Informatics, First Faculty of Medicine, Charles University in Prague, Czech Republic 3; st Department of Surgery – Department of Abdominal, Thoracic Surgery and Traumatology 11
Published in: Epidemiol. Mikrobiol. Imunol. 69, 2020, č. 2, s. 57-63
Category: Original Papers
Overview
Objectives: Intra-abdominal candidiasis (IAC) is an invasive fungal infection representing the most common type of invasive Candida infection in surgical intensive care units (ICUs). Recently, decreased antifungal susceptibility and progressive shift in the aetiology of invasive candidiasis has been observed worldwide. We explored IAC epidemiology in surgical ICU.
Material and Methods: We retrospectively reviewed the records of 64 patients with IAC admitted at our surgical ICU over a 4-year period (2013-2016). IAC incidence, microbiological results, antifungal therapy, and mortality were analysed.
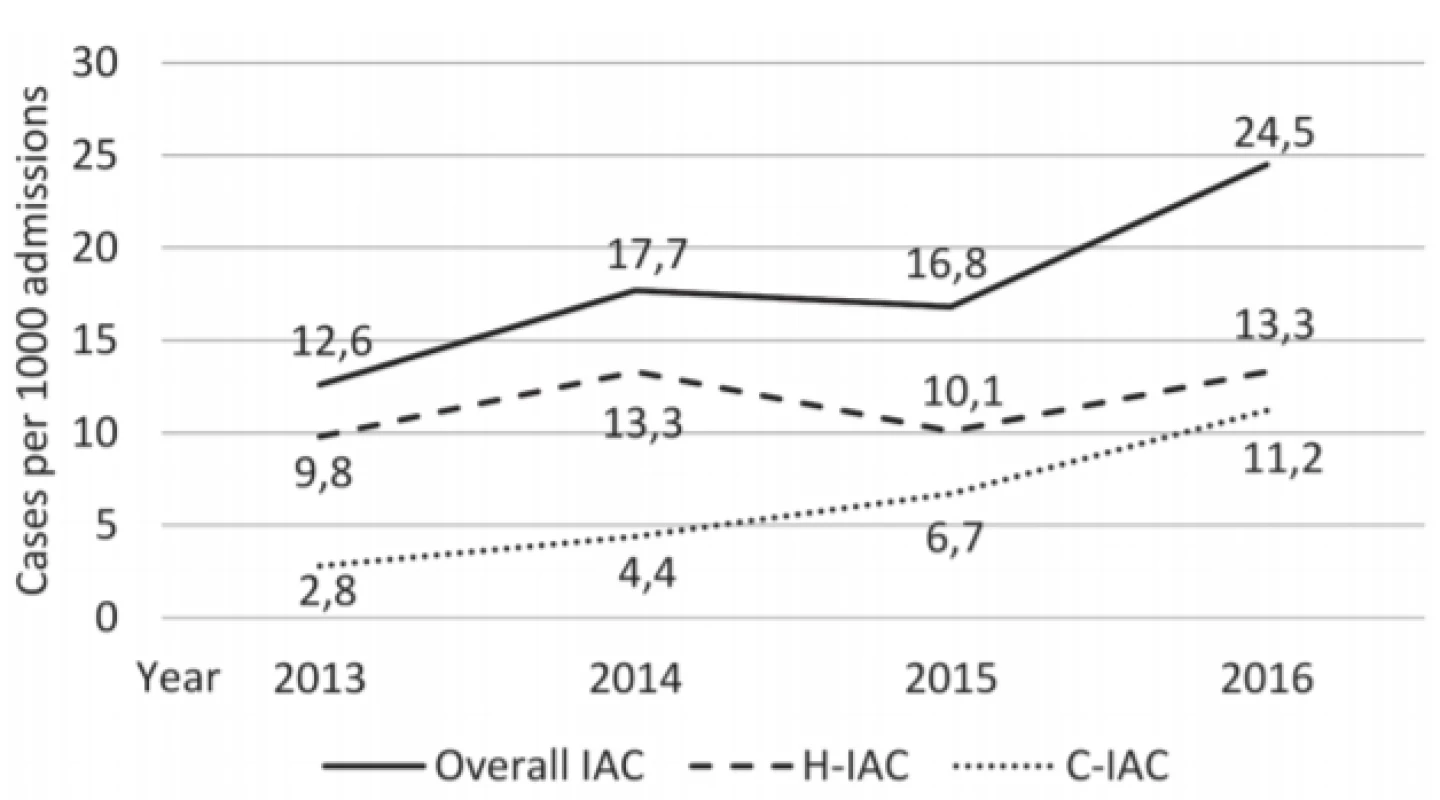
Results: The cumulative IAC incidence was 18.4 cases per 1000 admissions (2013 : 12.6; 2014 : 17.7; 2015 : 16.8; 2016 : 24.5), including hospital-acquired IAC incidence (2013 : 9.8; 2014 : 13.3; 2015 10.1; 2016 : 13.3) and community-acquired IAC incidence (2013 : 2.8; 2014 : 4.4; 2015 : 6.7; 2016 : 11.2). Candida albicans represented the most common species (n = 35, 50.0%) followed by Candida glabrata (n = 15, 21.4%), Candida tropicalis (n = 6, 8.6%) and other yeasts (each < 5.0%). Incidence rate of C. albicans (2013 : 7(78%); 2014 : 10(59%); 2015 : 6(35%); 2016 : 12(44%)) and incidence rate of C. non-albicans (2013 : 2(22%); 2014 : 7(41%); 2015 : 9(53%); 2016 : 14(52%)) were different in trend. All fungal isolates were susceptible to echinocandins, amphotericin B and voriconazole. Regarding fluconazole susceptibility, C. krusei (n = 3) was resistant and C. glabrata (n = 9) was susceptible-dose dependent (SDD). The ratio of SDD C. glabrata isolates to all isolated C. glabrata strains was 9/15 (60%) (2013 : 0/2; 2014 : 0/2; 2015 : 1/3; 2016 : 8/8). Decreased fluconazole susceptibility for C. glabrata isolates was reported in both community-acquired IAC (n = 3) and hospital-acquired IAC (n = 6). Overall 30-day mortality rate was 25.0% (16/64).
Conclusions: We have revealed slowly raising of overall IAC incidence, more increasing trend in incidence of community-acquired IAC compared to rather steady incidence of hospital-acquired IAC. During period 2013–2016 we have observed a significant shift in the aetiology of IAC towards an increased proportion of non-albicans Candida species, particularly C. glabrata. Acquired decreased fluconazole susceptibility was related to C. glabrata isolates exclusively. Emergence of decreased antifungal susceptibility has been preceded by increase of non-albicans Candida isolates.
Keywords:
intra-abdominal candidiasis – Abdominal surgery – antifungal susceptibility – antifungal therapy – mortality
INTRODUCTION
Invasive fungal infections in critically ill patients in the intensive care unit (ICU) are associated with considerable morbidity and mortality. The predominant type of invasive candidiasis is candidemia [1]. However, intra-abdominal candidiasis (IAC) is a frequent invasive Candida infection in surgical patients, representing the most common type of invasive candidiasis in surgical ICUs [2, 3]. Candida strains are obtained from an intraoperative intra-abdominal specimen in approximately 20–50% of patients with intra-abdominal perforation non-appendicular aetiology [4, 5, 6]. C. albicans is the most common causative agent of invasive candidiasis, but a shift towards non-albicans Candida species has been recently observed worldwide [7, 8, 9, 10, 11]. Moreover, global epidemiology trend is characterised by an increased prevalence of triazole-resistant Candida species, such as C. glabrata and C. krusei.
In 2016 emergence of decreased antifungal susceptibility for Candida species has been observed in culture isolates of Candida species in our surgical patients after abdominal surgery [12]. Therefore, this retrospective study was initiated to reveal the IAC epidemiology characteristics and trends in our surgical ICU patients over the period 2013–2016.
MATERIAL AND METHODS
We performed a retrospective observational single-centre cohort study analysing patients which were hospitalized in the ICU of First Department of Surgery, First Faculty of Medicine, Charles University and General University Hospital in Prague within a period from January 1st 2013 till December 31st 2016. All patients admitted to ICU in the period mentioned above were identified via a search of the hospital medical database. Individuals with a positive culture for Candida species in the peritoneal fluid obtained during surgery or in the abdominal drain fluid during the post-operative period were included. A report form was completed for each study patient based on a review of the medical charts.
The following data were collected: demographic characteristics, underlying disease, antifungal therapy (antifungal agents, length of administration), length of stay in ICU, length of stay in hospital, 30-day mortality.
The mycological and microbiological results of cultured peritoneal fluid and drain fluid were recorded. Microbiological techniques applied for culture and identification of bacteria and fungi, as well as antibiotic susceptibility testing, were those routinely used in the microbiology laboratory. In the laboratory, culture for aerobic, anaerobic bacteria and fungi was performed. For bacterial isolation blood agar and chromogenic agar UriSelect4 (BioRad) was used. The plates were incubated at 36 ± 1 °C for 24–48 hours. Cultures positive for bacterial growth were identified by MALDI-TOF mass spectrometer (Bruker). An antimicrobial susceptibility test of the identified bacteria was performed using the Kirby Bauer Disc Diffusion method following CLSI guidelines [13]. Sabouraud dextrose agar (SDA), as selective medium, was used for isolation of fungi and incubation was done at 36 ± 1 °C for 48 hours. Positive cultures on SDA were identified by MALDI-TOF mass spectrometer (Bruker). Antifungal susceptibility tests were done by using an E-test on RPMI 1640 medium supplemented with glucose to a final concentration of 2% following CLSI criteria [14].
In accordance with recently proposed criteria, Candida isolation was classified as intra-abdominal candidiasis in patients with clinical evidence of intra-abdominal infection and isolation of Candida species in a sample collected from an intra-abdominal site or from abdominal drains that were in place < 24 hours [15, 16]. Community-acquired IAC (C-IAC) was defined as intra-abdominal Candida infection characterised by onset of clinical abdominal manifestation before hospital admission or ≤ 48 hours after admission. Hospital-acquired IAC (H-IAC) was defined as Candida infection of abdominal cavity not present on admission that became evident > 48 hours after admission in hospitalised patients.
Statistical analysis
Continuous normally distributed data are expressed as the mean and standard deviation (SD) and compared using independent group t test. Categorical data are expressed as the number and percentage and compared using χ2 or the Fisher’s exact tests. In all comparisons, p-values bellow 0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS version 23 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographics, basic characteristics of study group and microbiological results
In the period 2013–2016, we identified altogether 101 patients with a positive culture for fungi from the peritoneal fluid collected during abdominal surgery or from abdominal drain postoperatively. Criteria for IAC were met in 64 patients. Basic characteristics, site of IAC origin, in hospital characteristics and mortality are reported in Table 1.
1. Basic characteristics, site of IAC origin, in hospital characteristics and mortality 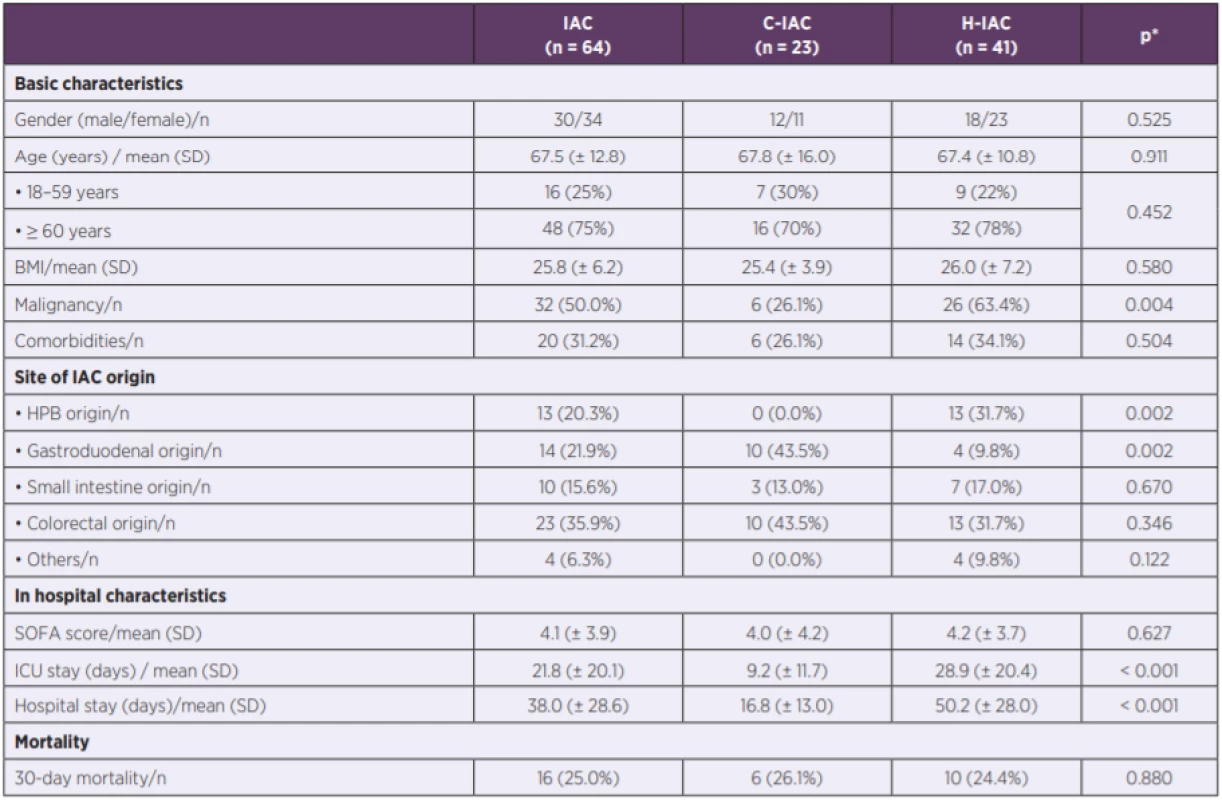
Legends: n – number of patients; BMI – body mass index; HPB – hepatopancreatobiliary; IAC – intra-abdominal candidiasis (C – community-acquired, H – hospital-acquired); ICU – intensive care unit; p value – statistically significant < 0.05; SD – standard deviation; * C-IAC vs. H-IAC. In the 64 samples, 70 fungi isolates were identified. Candida species (n = 67) was the most frequent species followed by Saccharomyces (n = 3). Although Candida albicans (CAAL) was the most frequent Candida pathogen, the incidence rate of non-albicans Candida species (non-CAAL) was nearly the same (CAAL 50.0%; non-CAAL 45.7%). Yeast species were the only organism cultured in 14% cases. Bacterial co-infection was present in 85.9% patients. In addition to fungi, 142 bacteria isolates were identified in the microbiological specimens of 55 patients with up to six different species per patient. The mean number of isolated bacteria was 2.2 isolates per sample. Detailed microbiological results, including the comparison of microbial aetiology in C-IAC and H-IAC, are reported in Table 2.
2. Microbiological results in IAC, including C-IAC and H-IAC 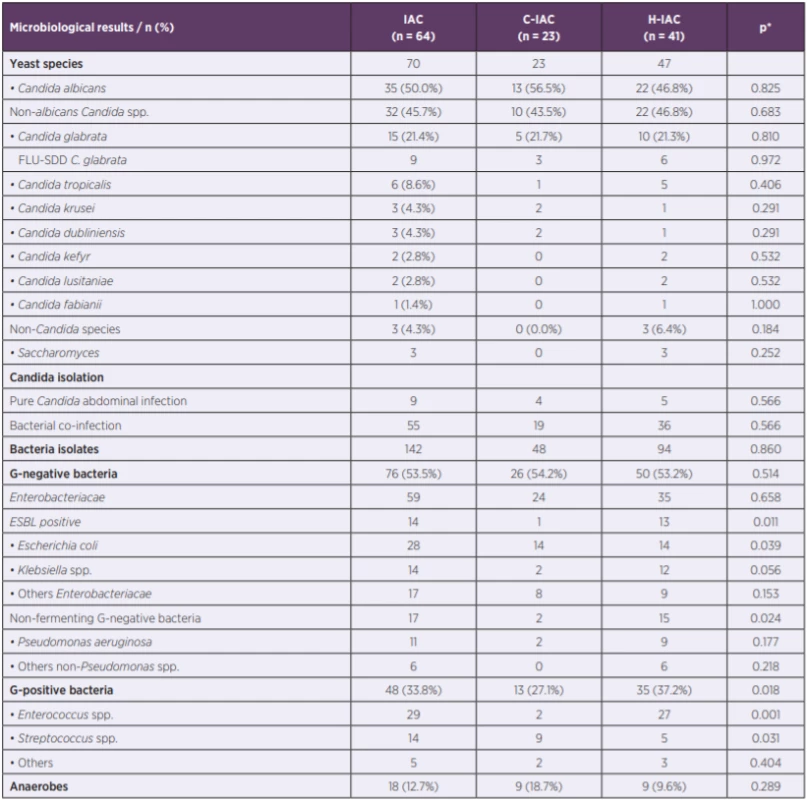
Legends: ESBL – extended spectrum beta-lactamase; FLU-SDD – fluconazole susceptible-dose dependent; IAC – intra-abdominal candidiasis (C – community-acquired, H – hospital-acquired); n – number of patients; p value – statistically significant bellow 0.05; *C-IAC vs. H-IAC. Incidence
In the period 2013–2016, altogether 3487 patients were admitted to surgical ICU and there were a total 64 episodes of IAC. The cumulative IAC incidence in ICU was 18.4 cases per 1000 admissions. The trend in incidence of IAC in abdominal surgery patients, including C-IAC and H-IAC, is shown in Figure 1.
Antifungal susceptibility testing
All fungal isolates were susceptible to echinocandins, amphotericin B and voriconazole, however antifungal susceptibility testing revealed decreased fluconazole susceptibility in 12 (17.1%) Candida isolates. All Candida albicans isolates were susceptible to fluconazole. The three C. krusei isolates were resistant to fluconazole (intrinsic resistance) and the nine C. glabrata isolates had fluconazole MIC 32 mg/l, thus, reported as susceptible-dose dependent (SDD). Other non-albicans Candida species were susceptible to fluconazole. Of all tested Candida glabrata isolates, 60% (9/15) were fluconazole SDD.
Microbiological results and trends
Detailed analysis of Candida species incidence during the observed period is reported in Table 3. The ratio of fluconazole SDD C. glabrata isolates to all isolated C. glabrata strains was reported in 4-year period (2013 : 0/2, 0.0%; 2014 : 0/2, 0.0%; 2015 : 1/3, 33.3%; 2016 : 8/8, 100.0%). Statistically significant increased incidence of C. glabrata isolates with decreased susceptibility to fluconazole was reported in the period 2015–2016 (9/11, 81.8%) compared to the period 2013–2014 (0/4, 0.0%) (p = 0.006). Moreover, in 2016 all isolates of C. glabrata (8/8) were SDD to fluconazole and decreased fluconazole susceptibility for C. glabrata isolates was reported in both C-IAC (3/3) and H-IAC (5/5).
3. Incidence of yeast species in the period 2013–2016 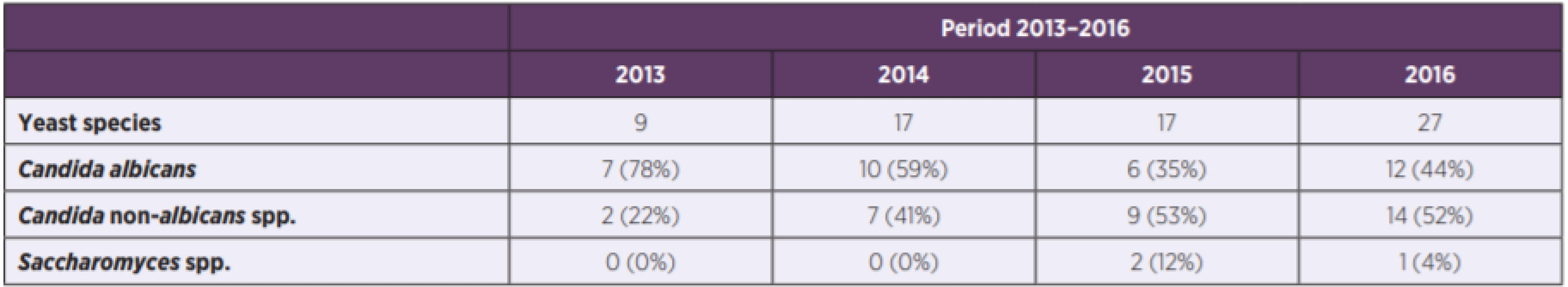
Antifungal therapy and mortality
Only 39.1% (25/64) of patients with IAC received antifungal therapy. However, antifungal therapy was more frequently administered in cases of H-IAC (21/42, 51.2%) compared to cases of C-IAC (4/23, 17.4%) (p = 0.008). In 25 patients, empiric monotherapy was administered using fluconazole (n = 19), voriconazole (n = 1), anidulafungin (n = 4) and micafungin (n = 1). In four cases de-escalation therapy was performed, echinocandin was replaced by fluconazole. Per the Candida species identification and susceptibility results, empiric antifungal treatment appeared as inadequate in 20.0% (5/25) of patients. Inadequate antifungal therapy was administered more frequently in the period 2015–2016 (5/14; 35.7%) compared to the period 2013–2014 (0/11; 0.0%).
Overall 30-day mortality rate was 25.0% (16/64), including mortality of 32.0% (8/25) in the period 2013–2014 and mortality of 20.5% (8/39) in the period 2015–2016 (p = 0.300). The mortality in the subgroups of patients with C-IAC and H-IAC was 26.1% (6/23) and 24.4% (10/41), respectively (p = 0.880).
DISCUSSION
Invasive fungal infection in ICUs ranges from 4.7 to 28.0 episodes per 1000 admissions; however, most studies have reported the incidence of candidemia while the incidence of intra-abdominal candidiasis has remained unknown [2, 3, 17, 18, 19]. In the Czech Republic multicentre study assessing epidemiology of invasive candidiasis in 2012–2015 authors analysed only episodes of candidemia [20]. We have revealed cumulative incidence of IAC of 18.4 cases per 1000 admissions in our surgical ICU. It means that IAC incidence in surgical ICUs is comparable to the incidence of candidemia in medical ICUs (6.7–34.3 episodes per 1000 ICU admissions) [17, 18, 21, 22]. In addition, we observed a trend towards slow increase in IAC incidence during period. It should be mentioned that significant difference in incidence trend was observed in C-IAC compared to H-IAC. Incidence rate of H-IAC was steady within the whole period while C-IAC demonstrated increasing incidence during this 4-year period. In 2016 C-IAC incidence reached nearly same level as H-IAC incidence. Based on this data, Candida species should be considered in aetiology of hospital-acquired intra-abdominal infections as well as in aetiology of community acquired intra-abdominal infections. There is no doubt that healthcare exposure is the most important risk factor for IAC, including recent abdominal surgery, anastomotic leaks, or necrotizing pancreatitis. However, there is a lack of data regarding risk factors for C-IAC. Increasing incidence of C-IAC supports the theory that the abdomen may be an important reservoir of Candida species in community. Unfortunately, the fungal colonization of otherwise healthy hosts and characteristic of human gut mycobiome have not been yet completely explored. We need to better identify and define the significant risk factors for C-IAC particularly.
Many different Candida species are known to be aetiological agents of invasive fungal infection, however, 90% of invasive infections are caused by C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei [19, 23, 24]. Our epidemiology study is consistent with the literature, C. albicans followed by C. glabrata and C. tropicalis were the most frequently isolated species from the peritoneal specimens. It is well recognized that just C. glabrata predominates as the second most frequent pathogen of invasive candidiasis in the North and Centre of Europe [25, 26, 27]. Moreover, non-CAAL species, including C. glabrata, C. tropicalis and C. krusei, were repeatedly reported in older patients in association with recent major abdominal surgery [25, 28, 29]. Regarding epidemiology trends, shift in the aetiology of IAC was observed in our surgical ICU. The first half of the period (2013–2014) was characterized by a predominance of C. albicans species. In the second half of the period (2015–2016) C. albicans decreased proportionally and non--CAAL species accounted for more than 50% of isolates.
Antifungal susceptibility/resistance is really important epidemiology characteristic. Generally, azole resistance for Candida species is more frequent than echinocandin resistance. In Europe the reported rate of Candida species with reduced susceptibility to fluconazole is 11–28% in patients with IAC [2, 16, 30]. Echinocandins demonstrate excellent activity for Candida species, accounting < 4% Candida isolates resistant to echinocandins worldwide [31, 32]. Bassetti et al. observed in 481 patients included in a multicentre study of abdominal candidiasis a total of 98% of Candida strains susceptible to echinocandins [16]. Candida species exhibit varying rates of susceptibility to the azole agents. C. albicans and some non-CAAL species, such as C. tropicalis, C. parapsilosis, C. lusitaniae, C. kefyr and C. dubliniensis, remain highly susceptible to fluconazole (susceptibility rate > 98.0% and > 90.0%, respectively) [33, 34]. By contrast, decreased fluconazole susceptibility for C. glabrata (susceptibility rate 92–68.7%) has been reported and C. krusei is associated with intrinsic fluconazole resistance [33, 34]. Concerning antifungal resistance pattern in our surgical ICU, decreased antifungal susceptibility to azole drugs and excellent activity of echinocandins have been demonstrated. Prior fluconazole exposure and echinocandin sparing strategy may explain this shift towards to decreased fluconazole susceptibility. Acquired decreased fluconazole susceptibility was reported for C. glabrata exclusively. We have observed reduced susceptibility to fluconazole in association with increased prevalence of non-CAAL species. Moreover, aetiology shift towards to increase of non-CAAL isolates preceded sudden emergence of decreased fluconazole susceptibility in 2016. Therefore, we propound that changing antifungal susceptibility was predictable based on the shift in aetiology. Interestingly, increased prevalence of fluconazole SDD C. glabrata has been observed in H-IAC as well as in C-IAC. Prior fluconazole exposure explains the emergence of decreased fluconazole susceptibility in H-IAC. However, reason of emergence of fluconazole SDD C. glabrata in C-IAC is obscure.
Characteristics of patients and IAC risk factors were statistically analysed just for the purpose of comparisons of C-IAC and H-IAC. Both subgroups of patients were comparable in most characteristics (gender, age, BMI, comorbidity, etc.) (see Table 1). Targeted analysis of risk factor correlations was out of the interest. Concerning antifungal therapy and mortality, there is limited evidence of study outcomes due to the retrospective design of the study and missing considerable variables. Except many variables, mismatched aetiology is major limitation of analysis of therapy, mortality and prognosis in IAC. The clinical significance of Candida species recovered from intra-abdominal peritoneal fluid is the most controversial factor. The role of Candida species in the pathophysiology of polymicrobial peritonitis has not been conclusively defined. The differentiation between contamination, colonization and infection remains the main diagnostic challenge. Colonisation index and Candida score are used for identifying high risk patients for IAC and non-culture-based methods including (1→3)-β-d-glucan, mannan antigen, anti-mannan antibodies are recommended for detection of Candida species [35]. However, more studies are needed to assess the optimal criteria in high-risk patients and the efficiency of fungal biomarkers in the differentiation of colonisation and infection. This is a keystone of the unconvincing outcomes of most IAC studies. Empirical antifungal therapy has been demonstrated to decrease mortality significantly in a specific patient population. Bassetti et al. reported association between the absence of an adequate initial antifungal therapy and increased mortality in patients with adequate source control of IAC and septic shock [16]. Similarly, in a retrospective multi-centric analysis of 258 patients with intra-abdominal candidiasis in Spain, ICU patients that did not receive antifungal therapy for an IAC episode had a ∼6-fold increase in mortality; however, the benefit of antifungal therapy was not observed in non-ICU patients [36]. Conversely, the current evidence does not demonstrate efficacy of antifungal therapy in improving outcomes in patients with IAC caused by perforated peptic ulcer [37]. Antifungal therapy in these patients may be considered overtreatment altering the outcomes. Prospective multicentre study and multivariable analysis are required for identification of IAC risk factors and determination of causative relationships of antifungal therapy and mortality.
Considering this study, the main limitation is the low number of included patients with IAC, which has resulted in a lower power of statistical significance of statistical analysis. However, our study outcomes underscore the importance of knowledge of the local epidemiology and resistance patterns for Candida species. Local epidemiology is crucial for guiding therapeutic decision making.
CONCLUSIONS
We have revealed slowly raising of overall IAC incidence. Interestingly, more increasing trend in incidence of C-IAC compared to rather steady incidence of H-IAC has been discovered. During period 2013–2016 we have observed a significant shift in the aetiology of IAC towards to an increased proportion of non-CAAL species, particularly C. glabrata. Acquired decreased susceptibility to fluconazole was related to C. glabrata isolates exclusively. Emergence of decreased antifungal susceptibility has been preceded by increase of incidence of non-CAAL isolates. Therefore, we assume that emergence of antifungal resistance may be predictable based on the shift in aetiology. In terms of antifungal susceptibility, significant risk of antifungal resistance may be related to H-IAC as well as C-IAC. Knowledge of local epidemiology is crucial for guiding therapeutic decision making.
Declaration
This work was supported by Ministry of Health, Czech Republic (Conceptual development of research organization 64165, General University Hospital in Prague).
Do redakce došlo dne 22. 1. 2020.
Adresa pro korespondenci:
MUDr. Jan Ulrych, Ph.D.
I. chirurgická klinika – hrudní, břišní a úrazové chirurgie 1. LF UK a VFN
U Nemocnice 2
128 00 Praha 2
e-mail: Jan.Ulrych@vfn.cz
Sources
1. Carneiro HA, Mavrakis A, Mylonakis E. Candida peritonitis: an update on the latest research and treatments. World J Surg, 2011;35(12):2650–2659.
2. Aguilar G, Delgado C, Corrales I, et al. Epidemiology of invasive candidiasis in a surgical intensive care unit: an observational study. BMC Research Notes, 2015;8 : 491.
3. Vergidis P, Clancy CJ, Shields RK, et al. Intra-Abdominal Candidiasis: The Importance of Early Source Control and Antifungal Treatment. PLoS One, 2016;11(4):e0153247.
4. Sandven P, Qvist H, Skovlund E, et al. Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit Care Med, 2002;30(3):541–547.
5. de Ruiter J, Weel J, Manusama E, et al. The epidemiology of intra-abdominal flora in critically ill patients with secondary and tertiary abdominal sepsis. Infection, 2009;37(6):522–527.
6. Jindal N, Arora S, Pathania S. Fungal Culture Positivity in Patients with Perforation Peritonitis. J Clin Diagn Res, 2015;9(6):DC01–DC03.
7. Méan M, Marchetti O, Calandra T. Bench-to-bedside review: Candida infections in the intensive care unit. Crit Care, 2008;12(1):204.
8. Leroy O, Gangneux JP, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med, 2009;37(5):1612–1618.
9. Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence, 2014;5(1):161–169.
10. Shields RK, Nguyen MH, Press EG, et al. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother, 2014;58(12):7601–7605.
11. Maseda E, Rodríguez-Manzaneque M, Dominguez D, et al. Intraabdominal candidiasis in surgical ICU patients treated with anidulafungin: A multicenter retrospective study. Rev Esp Quimioter, 2016;29(1):32–39.
12. Adámková V, Vaňková A, Ulrych J, et al. Characterisation of Candida sp. isolated from patients after abdominal surgery. Rozhl Chir, 2017;96(10):426–431.
13. CLSI. Method for antifungal disk diffusion susceptibility testing of yeasts; Approved Guideline – Second Edition. CLSI document M44-A2. Wayne, Pa: Clinical and Laboratory Standards Institute, 2009.
14. CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved standard – Third Edition. CLSI document M27-A3. Wayne, Pa: Clinical and Laboratory Standards Institute, 2008.
15. Bassetti M, Marchetti M, Chakrabarti A, et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med, 2013;39(12):2092–2106.
16. Bassetti M, Righi E, Ansaldi F, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med, 2015;41(9):1601–1610.
17. Tortorano AM, Dho G, Prigitano A, et al. Invasive fungal infections in the intensive care unit: a multicentre, prospective, observational study in Italy (2006–2008). Mycoses, 2012;55(1):73–79.
18. Montagna MT, Caggiano G, Lovero G, et al. Epidemiology of invasive fungal infections in the intensive care unit: results of a multicenter Italian survey (AURORA Project). Infection, 2013;41(3):645–653.
19. Klingspor L, Tortorano AM, Peman J, et al. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008). Clin Microbiol Infect, 2015;21(1):87.
20. Kocmanová I, Lysková P, Chrenkova V, et al. Nosocomial candidemia in the Czech Republic in 2012–2015: results of a microbiological multicentre study. Epidemiol Mikrobiol Imunol, 2018;67(1):3–10.
21. Bougnoux ME, Kac G, Aegerter P, et al. Candidemia and candiduria in critically ill patients admitted to intensive care units in France: incidence, molecular diversity, management and outcome. Intensive Care Med, 2008;34(2):292–299.
22. González de Molina FJ, León C, Ruiz-Santana S, et al. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care, 2012;16(3):R105.
23. Pfaller MA, Moet GJ, Messer SA, et al. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother, 2011;55(2):561–566.
24. Orasch C, Marchetti O, Garbino J, et al. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs. old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect, 2014;20(7):698–705.
25. Quindós G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol, 2014;31(1):42–48.
26. Presterl E, Daxböck F, Graninger W, et al. Changing pattern of candidaemia 2001–2006 and use of antifungal therapy at the University Hospital of Vienna, Austria. Clin Microbiol Infect, 2007;13(11):1072–1076.
27. Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect, 2013;19(8):E343–353.
28. Arendrup MC. Candida and candidaemia. Susceptibility and epidemiology. Dan Med J, 2013;60(11):B4698.
29. Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candidabloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect, 2014;20(4):O245–254.
30. Montravers P, Mira JP, Gangneux JP, et al. A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin Microbiol Infect, 2011;17(7):1061–1067.
31. Pham CD, Iqbal N, Bolden CB, et al. Role of FKS Mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother, 2014;58(8):4690–4696.
32. Montagna MT, Lovero G, Coretti C, et al. Susceptibility to echinocandins of Candida spp. strains isolated in Italy assessed by European Committee for Antimicrobial Susceptibility Testing and Clinical Laboratory Standards Institute broth microdilution methods. BMC Microbiol, 2015;15 : 106.
33. Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol, 2010;48(4):1366–1377.
34. Cleveland AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis, 2012;55(10):1352–1361.
35. León C, Ostrosky-Zeichner L, Schuster M. What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med, 2014;40(6):808–819.
36. Lagunes L, Rey-Pérez A, Martín-Gómez MT, et al. Association between source control and mortality in 258 patients with intra-abdominal candidiasis: a retrospective multi-centric analysis comparing intensive care versus surgical wards in Spain. Eur J Clin Microbiol Infect Dis, 2017;36(1):95–104.
37. Huston JM, Kreiner L, Ho VP, et al. Role of Empiric Anti-Fungal Therapy in the Treatment of Perforated Peptic Ulcer Disease: Review of the Evidence and Future Directions. Surg Infect (Larchmt), 2019;20(8):593–600.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2020 Issue 2-
All articles in this issue
- The significance of p16 protein expression in oral squamous cell carcinoma
- Barriers to treatment of infectious and other somatic comorbidity in drug users
- Q fever and prevention
- Smuteční oznámení: zemřel profesor MUDr. Miroslav Votava, CSc.
- Blahopřání RNDr. Marii Brůčkové, CSc.
- Intra-abdominal candidiasis in surgical intensive care unit – epidemiology characteristics and trends
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
- Neonatal pneumonia caused by Trichomonas vaginalis
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- The significance of p16 protein expression in oral squamous cell carcinoma
- Q fever and prevention
- Neonatal pneumonia caused by Trichomonas vaginalis
- Vzácná invazivní fungální infekce Mucor circinelloides a Fusarium u imunokompetentního pacienta po devastačním poranění dolní končetiny s rekonstrukcí volným lalokem m. latissimus dorsi
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career