-
Medical journals
- Career
Atypical fibroxanthoma, rare and often unrecognized cutaneous soft tissue tumor – a case report and review of the literature
Authors: Zuzana Čierna 1; Rastislav Trška 2
Published in: Čes.-slov. Patol., 55, 2019, No. 3, p. 182-186
Category: Original Articles
Overview
Atypical fibroxanthoma (AFX) is a rare cutaneous soft tissue tumor typically occurring in the elderly on sun exposed skin. Histologically, it is composed of pleomorphic, atypical cells with multiple mitoses including atypical mitotic figures resembling undifferentiated malignant tumor. AFX is considered to be a benign tumor with almost uniformly excellent prognosis following conservative therapy if strict diagnostic criteria are applied. We present a case report of 68-year-old man with a skin tumor in the temporo-parietal region. Histomorphological and immunohistochemical analysis led us to the diagnosis of atypical fibroxanthoma. We offer a review of terminology and categorization of this tumor and an overview of immunohistochemical markers useful in differential-diagnostic process to rule out other malignant tumors, because AFX is a diagnosis of exclusion. The correct diagnosis prevents unnecessary overtreatment of the patient.
Keywords:
Atypical fibroxanthoma – undifferentiated pleomorphic sarcoma – immunohistochemistry.
Atypical fibroxanthoma (AFX) is a rare cutaneous soft tissue tumor typically occurring in the elderly on sun exposed skin. It comprises up to 0,2% of all skin tumors (1). AFX is considered to be benign tumor with excellent prognosis after complete surgical excision with free margins. However, the histological structure of AFX remarkably resembles an undifferentiated malignant tumor, that needs to be excluded in differential diagnostic process. Incorrect diagnosis may lead to unnecessary overtreatment of the patient.
CLINICAL HISTORY
68-year-old man with a skin tumor in the right temporo-parietal region was referred to the plastic surgeon by a dermatologist with the diagnosis of haemangioma with recorded trauma in that region in the past. Plastic surgeon´s clinical diagnosis was suspicious pyogenic granuloma.
MATERIALS AND METHODS
Formalin-fixed, paraffin-embedded tissue blocks were cut into 5-μm sections, stained with hematoxylin-eosin and analyzed immunohistochemically with different primary antibodies: AE1/3 (clone AE1/AE3, diluted 1 : 300, DAKO, Denmark), vimentin (clone V9, prediluted, DAKO, Denmark), SMA (smooth muscle actin, clone 1A4, diluted 1 : 200, DAKO, Denmark), desmin (clone D33, diluted 1 : 100, DAKO, Denmark), h-caldesmon (clone h-CD, diluted 1 : 100, DAKO, Denmark), beta-catenin (clone β-catenin-1, prediluted, DAKO, Denmark), S100 (clone Anti-S100, diluted 1 : 1000, DAKO, Denmark), Melan-A (clone A103, diluted 1 : 200, DAKO, Denmark), HMB45 (clone HMB45, diluted 1 : 100, DAKO, Denmark), CD10 (clone 56C6, prediluted, DAKO, Denmark), CD34 (clone QBEnd 10, diluted 1 : 100, DAKO, Denmark), CD68 (clone PG-M1, diluted 1 : 150, DAKO, Denmark), CD99 (clone 12E7, prediluted, DAKO, Denmark), CD117 (clone CD117, c-kit, diluted 1 : 800, DAKO, Denmark) and Ki-67 (clone MIB-1, diluted 1 : 100, DAKO, Denmark).
RESULTS
Grossly, the skin sample measured 26x13x12 mm and showed a large nodular tumorous mass significantly prominent above the level of the surrounding skin measuring 13x13x8 mm. The tumor was of firm-elastic consistency, light brown color with red spots, pale on cut surface.
Histologically, the tumor was located in the dermis, not exceeding the reticular dermis, well circumscribed from the surrounding subcutaneous tissue, with an expansive growth pattern. The surface squamous epithelium of epidermis created a collar-shaped rim around the tumor, and the epithelium covering the tumor was ulcerated. Solar elastosis was present in the surrounding dermis. The tumor was composed predominantly of spindle-shaped cells with pleomorphic oval nuclei and prominent nucleoli. Round to oval cells, large cells with giant bizarre nuclei and multinucleated cells were less numerous. Multiple mitoses (46 per 10 high-power fields), including atypical mitotic figures, were present (Fig. 1). Necrosis, bleeding, and vascular invasion were not detected in the tumor. Tumor cells were present at the resections margin.
1. Histological appearence of atypical fibroxanthoma. A. Tumor localized in dermis, surface epithelium forms collar-shaped rim around the tumor (arrow). B. The border between tumor and subcutaneous tissue is sharp. C, D. Tumor is composed predominantly of spindle-shaped cells with pleomorphic nuclei and multiple mitoses including atypical mitotic figures (arrows). HE, original magnification x40, x100, x400, x400. 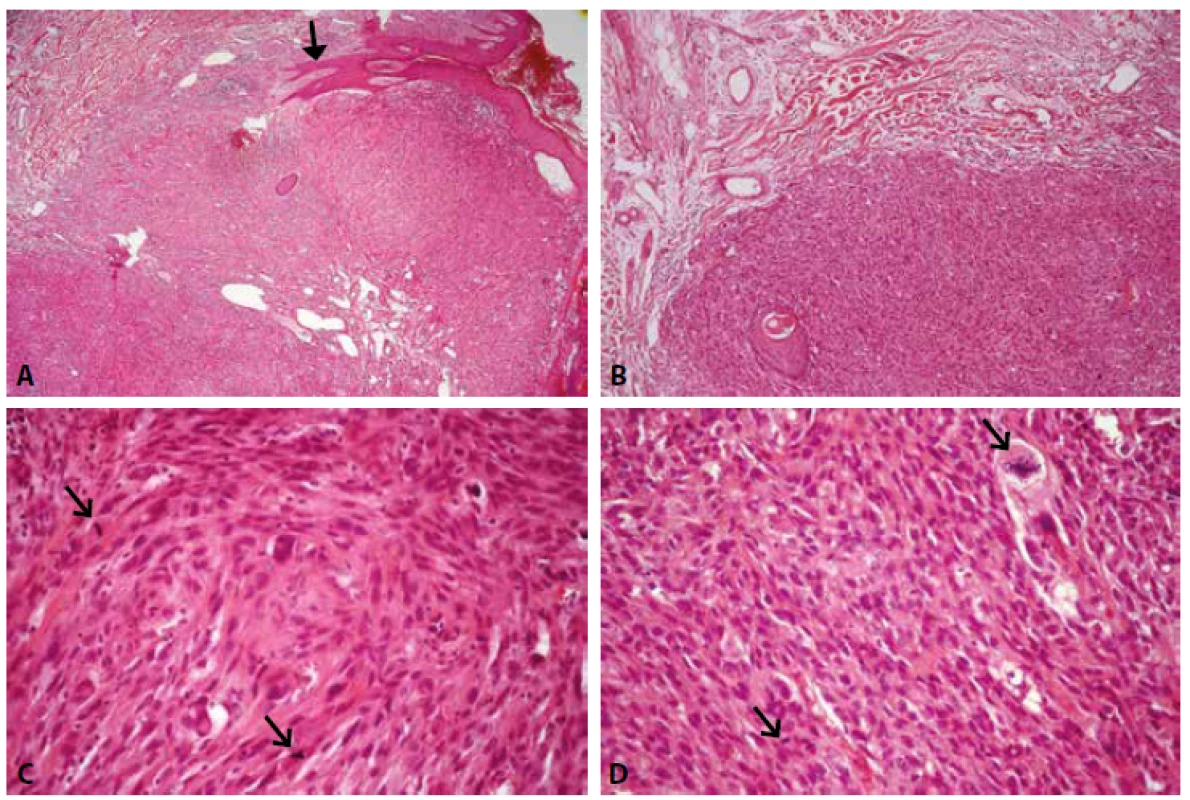
Immunohistochemically, the tumor cells showed diffuse cytoplasmic positivity with antibodies against vimentin, CD68, CD10, CD99 and SMA. H-caldesmon and beta-catenin were focally positive (H-caldesmon cytoplasmic and beta-catenin strong nuclear and weak cytoplasmic), CD117 was weakly positive in the cytoplasm of sporadic cells. Tumor cells were negative with antibodies against AE1/3, desmin, S100, melanA, HMB45 and CD34. CD34 positivity was recorded in endothelial cells of the vessels. Proliferation activity was high, with Ki-67 nuclear positivity in approximately 50% of cells (Fig. 2). The Ki-67 antigen-labeling index was determined by counting percentage of Ki-67-positive cells/500 tumor cells.
2. Immunohistochemical staining of atypical fibroxanthoma. A. Diffuse strong cytoplasmic CD68 positivity. B Diffuse strong cytoplasmic CD10 positivity. C. Diffuse strong cytoplasmic SMA positivity. D. Ki-67 positivity in approximately 50% of nuclei. DAB, original magnification x200. 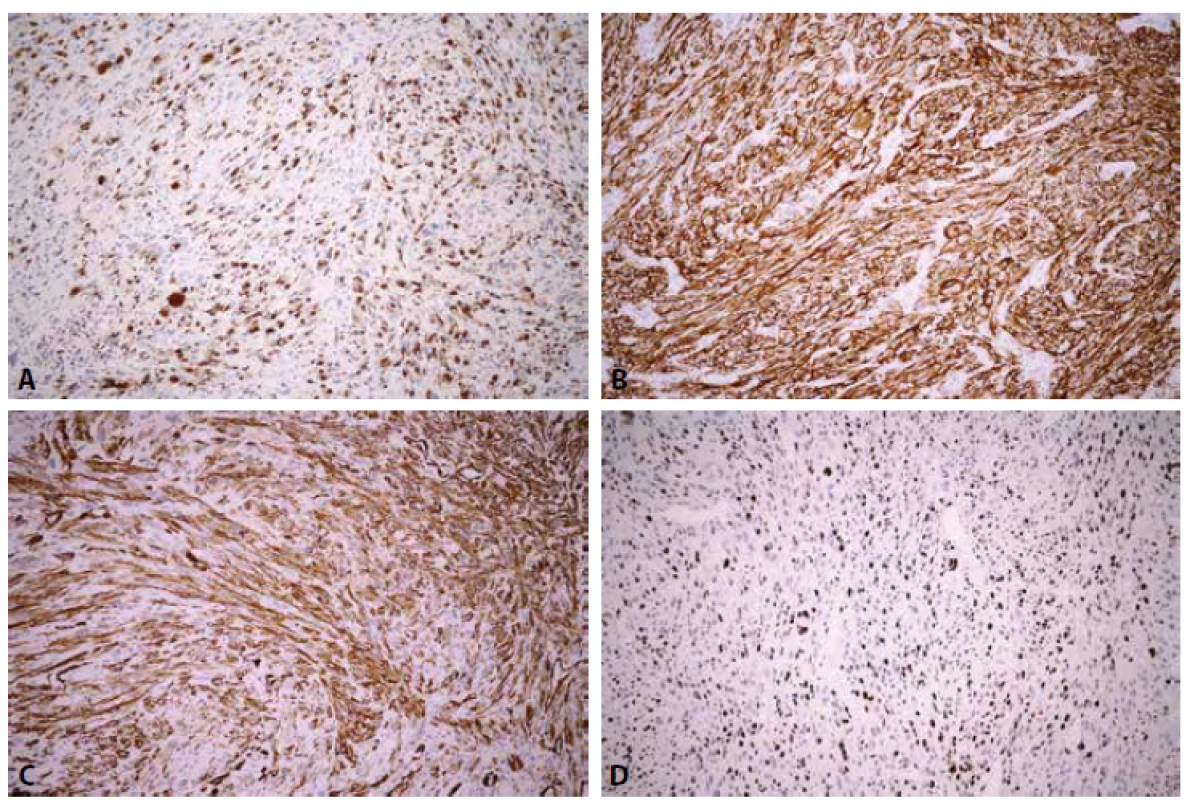
DISCUSSION
Atypical fibroxanthoma is a rare cutaneous soft tissue tumor described for the first time by Helwig in 1961 (2). It occurs typically in the elderly, mostly in the 7th to 8th decades of life, predominantly in men, on skin exposed to sunlight (head, neck). UV radiation, radiation therapy and immunosuppression play a major role in the etiology (3). Changes of actinic damage including solar elastosis, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) often occur in the surrounding skin.
Macroscopically, AFX is a solitary nodular or polypoid tumor of reddish-pink color, mostly not more than 2 cm in diameter, often ulcerated and bleeding. In most cases, it is a rapidly growing tumor, clinically resembling a pyogenic granuloma. BCC or SCC can be in the differential diagnosis, especially in the case of surface ulceration (4).
Histologically, AFX is a dermal circumscribed tumor composed of spindle-shaped, epithelioid and multinucleated cells arranged in sheets and fascicles. The nuclei are pleomorphic, hyperchromatic or vesicular, with prominent and often multiple nucleoli, numerous mitoses including atypical mitotic figures (1). Haemorrhage with haemosiderin deposition may occur. Solar elastosis is typical finding in adjacent dermis (4). Based on the histological composition, different variants of AFX are distinguished - spindle cell, clear cell, granular cell, myxoid and pigmented. Rare variants include AFX with osteoclast-like giant cells, keloid-like areas, osteoid production and chondroid differentiation (5-14).
A specific positive diagnostic immunohistochemical marker of AFX is not known. Tumor cells show positivity for CD68, CD10, CD99 and CD117, but none of these markers are specific for AFX. Markers of epithelial, melanocytic, myogenic, and vascular differentiation, such as keratins, p40, p63, SOX10, S100, desmin, h-caldesmon, myogenin, CD34, CD31, or ERG protein should be negative in AFX (3). However, SMA may be focally/weakly positive, indicating myofibroblastic differentiation, confirmed also by electron microscopy (15).
The terminology and categorization of AFX have gradually changed. In the past AFX has been referred as pseudosarcoma of the skin, paradoxical fibrosarcoma, pseudosarcomatous dermatofibroma, or pseudosarcomatous reticulohistiocytoma (16). AXF is considered by some authors not to be a homogeneous entity, but rather a heterogeneous group of mesenchymal and epithelial lesions. Others believe that the term refers to a relatively homogeneous group of superficial fibrohistiocytic neoplasms (16). Some authors considered AFX to be a SCC variant, however, multiple studies ruled out this interpretation. CD10 is positive in almost all cases of AFX and also in the vast majority of well-differentiated SCCs, but the expression is lost with dedifferentiation (17).
The view on histogenesis and dignity of AFX varies and the categorization of the tumor into a particular class of tumors is not uniform. In the 4th edition of Enzinger and Weiss´s Soft Tissue Tumors, AFX was included in the category of benign fibrohistiocytic tumors because of its almost uniformly excellent prognosis. However, considering its histological similarity with malignant fibrous histiocytoma (MFH), AFX was regarded as a superficial form of that tumor and was discussed in the chapter of malignant fibrohistiocytic tumors (16). From the 5th edition, AFX was included in the category of MFH (18). In the most recent 6th edition, MFH was renamed to undifferentiated pleomorphic sarcoma (UPS) and, analogically, the term “undifferentiated pleomorphic sarcoma of the skin” was added as a synonym of AFX (19).
AFX was included in the WHO (World Health Organization) classification of soft tissue tumors for the first time in 2013 (20,21). According to this classification, atypical fibroxanthoma is considered to be a benign tumor, but it is included in the category of tumors with uncertain differentiation (3).
Despite the fact that AFX is a skin tumor, it was not mentioned in 2006 WHO classification of skin tumors (22). In the most recent 2018 WHO classification of skin tumors, AFX is categorized as a separate diagnostic entity with ICD-O code and is included among tumors of uncertain differentiation, similarly to 2013 WHO soft tissue tumors classification (23).
The histological and immunohistochemical features of AFX are indistinguishable from superficial UPS. Both tumors are composed of pleomorphic predominantly spindle-shaped cells with admixture of epithelioid and multinucleated cells with nuclei exhibiting characteristics of a malignant tumor. The epithelium over the tumor is often ulcerated and the surrounding preserved epithelium forms collarette around the tumor. Signs favoring AFX diagnosis over UPS are tumor size not more than 2 cm, localization on actinically damaged skin, localization in the dermis without infiltration of the subcutaneous tissue and deeper structures (fascia, muscle), pushing growth instead of infiltrative one, absence of necrosis and lack of angioinvasion and perineural invasion (19). It follows from the above, that the definite diagnosis cannot be made from superficial samples not including the base of the tumor (4).
The biological behavior of AFX and UPS is significantly different. AFX is considered to be a benign tumor with almost uniformly excellent prognosis following conservative therapy if strict diagnostic criteria are applied. The risk of local recurrence is low and results from insufficiently wide resection margins. Metastases of AFX have been described rarely, but it is thought, that most of the metastasized tumors were other entities misdiagnosed as AFX (19). Satter et al. published a case report of AFX that through metastatic spread to lungs led to the death of the patient. In that case, the tumor was greater than 2 cm, and focally infiltrated into the superficial part of the subcutaneous tissue, without signs of angioinvasion or necrosis. However, the authors expressed theirs uncertainty in the diagnosis, and included a superficial variant of UPS in the differential diagnosis (24). However, cases of indisputable AFX with cutaneous, lymph node, parotid area or other site metastases were published (25-28). In contrast to AFX with mostly benign behaviour, UPS is usually intermediate or high grade malignancy, with pulmonary metastases and recurrences developing in 30 – 40% of cases. Other metastatic sites are liver, bone and lymph nodes (27). Based on immunohistochemical, FISH (fluorescence in-situ hybridizatioin) and NGS (next-generation-sequencing) analyses, Helbig et al. hypothesized that AFX is an UV-induced non-infiltrating precursor of cutaneous UPS (29).
AFX is a diagnosis of exclusion, and several malignant tumors, especially poorly differentiated carcinoma (spindle-cell SCC), spindle-cell malignant melanoma (desmoplastic melanoma), pleomorphic leiomyosarcoma and angiosarcoma have to be ruled out. Carcinomas show positivity of epithelial markers such as AE1/3 (cytokeratin cocktail), CK5/6, 34betaE12 (high molecular cytokeratins), p63 or p40. However epithelial markers, especially the commonly used AE1/3 cocktail may be negative in poorly differentiated spindle cell carcinomas, which may lead to incorrect diagnosis of AFX and undertreatment of the patient, as presented in Benoit et al. case report (30). p63 seems to be a useful and sensitive immunohistochemical marker for cutaneous spindle cell carcinomas (31,32). p40 is even more specific marker for cutaneous poorly differentiated SCC compared to p63 (33,34). In leiomyosarcoma, positivity of at least two of the three typical markers – SMA, desmin, and h-caldesmon should be present. SMA can be positive in AFX, which may lead to misdiagnosis of leiomyosarcoma. Negativity of other more specific muscle markers (desmin, h-caldesmon) excludes diagnosis of leiomyosarcoma. Spindle-cell melanoma may be a diagnostic pitfall because of negativity of typical melanocytic markers such as Melan-A and HMB45 in most cases. However S100 protein and SOX10 are positive in spindle cell melanoma in contrast to AFX (35). Rare cases of AFX with HMB45 and/or Melan-A positive giant cells or HMB45 positive clear cell variant of AFX (in the absence of S100 labeling) were reported (36-38). Angiosarcoma is in the differential diagnosis, especially in the case of haemorrhage and haemosiderin deposition in AFX, and may be diagnosed with the use of endothelial markers, such as CD31, CD34, or ERG. A high index of suspicion and immunohistochemical analysis with antibodies against vascular markers will prevent misdiagnosis of AFX instead of angiosarcoma (39). Moreover, in the case of AFX variants, other diseases have to be taken into consideration, e. g. for clear cell variant of AFX – balloon cell melanoma, sebaceous carcinoma, pleomorphic liposarcoma, clear cell SCC or metastatic renal cell carcinoma (40,41).
The recommended therapeutic procedure of AFX is Mohs surgery with safety resection margins ≥ 2 cm (42). Recently, electronic brachytherapy following shave biopsy was reported as an effective and safe treatment for AFX, with recurrence rate 0% for debulked lesions (43).
CONCLUSION
Atypical fibroxanthoma is a diagnosis of exclusion, mostly with excellent prognosis with negligible risk of recurrence and metastasis. Correct diagnosis based on stringent diagnostic criteria enables to prevent overtreatment of the patient. However, the fact, that recurrence and metastases can occur in different interval from primary tumor underscores the importance of long-term clinical evaluation of patients with a history of AFX.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Zuzana Cierna, M.D.,
Department of Pathology,
Comenius University, Faculty of Medicine,
and University Hospital, Sasinkova 4,
811 08 Bratislava, Slovak Republic,
tel.: +421259357588
e-mail: ciernaz@gmail.co
Sources
1. Koch M, Freundl AJ, Agaimy A, et al. Atypical fibroxanthoma - histological diagnosis, immunohistochemical markers and concepts of therapy. Anticancer research 2015; 35(11): 5717-5735.
2. Helwig EB. Atypical fibroxanthoma. In: Tumor Seminar Proceedings of 18th Annual Tumor Seminar of San Antonio Society of Pathologists. Tex State J Med 1963; 59 : 664-667.
3. Calonje JE, Brenn T, Komminoth P. Atypical fibroxanthoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone (4th ed). Lyon: International Agency for Research on Cancer (IARC); 2013 : 202-203.
4. Lopez L, Velez R. Atypical fibroxanthoma. Arch Pathol Lab Med 2016; 140(4): 376-379.
5. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol 2010; 37(3): 301-309.
6. Wright NA, Thomas CG, Calame A, Cockerell CJ. Granular cell atypical fibroxanthoma: case report and review of the literature. J Cutan Pathol 2010; 37(3): 380-385.
7. Patton A, Page R, Googe PB, King R. Myxoid atypical fibroxanthoma: a previously undescribed variant. J Cutan Pathol 2009; 36(11): 1177-1184.
8. Rudisaile SN, Hurt MA, Santa Cruz DJ. Granular cell atypical fibroxanthoma. J Cutan Pathol 2005; 32(4): 314-317.
9. Pastor-Nieto MA, Kilmurray LG, Requena L. Pigmented or hemosiderotic atypical fibroxanthoma. Actas Dermosifiliogr 2009; 100(4): 321-324.
10. Requena L, Sangueza OP, Sanchez Yus E, Furio V. Clear-cell atypical fibroxanthoma: an uncommon histopathologic variant of atypical fibroxanthoma. J Cutan Pathol 1997; 24(3): 176-182.
11. Kim J, McNiff JM. Keloidal atypical fibroxanthoma: a case series. J Cutan Pathol 2009; 36(5): 535-539.
12. Zheng R, Ma L, Bichakjian CK, Lowe L, Fullen DR. Atypical fibroxanthoma with lymphomatoid reaction. J Cutan Pathol 2011; 38(1): 8-13.
13. Tongdee E, Touloei K, Shitabata PK, Shareef S, Maranda EL. Keloidal atypical fibroxanthoma: case and review of the literature. Case Rep Dermatol 2016; 8(2): 156-163.
14. Němejcová K, Dundr P. Granular cell variant of atypical fibroxanthoma. A case report. Cesk Patol 2014; 50(1): 34-37.
15. Ito A, Yamada N, Yoshida Y, Morino S, Yamamoto O. Myofibroblastic differentiation in atypical fibroxanthomas occurring on sun-exposed skin and in a burn scar: an ultrastructural and immunohistochemical study. J Cutan Pathol 2011; 38(8): 670-676.
16. Weiss SW, Goldblum JR. Malignant fibrohistiocytic tumors. In: Strauss M, ed. Enzinger and Weiss´s Soft Tissue Tumors (4th ed). USA: Mosby; 2001 : 535-569.
17. Fernandez-Flores A. Cutaneous squamous cell carcinoma of different grades: variation of the expression of CD10. Cesk Patol 2008; 44(4): 100-102.
18. Weiss SW, Goldblum JR. Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma). In: Schmitt W, ed. Enzinger and Weiss’s Soft Tissue Tumors (5th ed). Philadelphia, PA: Mosby Elsevier; 2008 : 403-428.
19. Goldblum JR, Weiss SW, Folpe AL. Undifferentiated pleomorphic sarcoma. In: Schmitt WR, ed. Enzinger and Weiss’s Soft Tissue Tumors (6th ed). Philadelphia, PA: Elsevier Saunders; 2014 : 421-442.
20. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer 2014; 120(12): 1763-1774.
21. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014; 46(2): 95-104.
22. LeBoit PE, Burg G, Weedon D, Sarasin A. WHO Classification of Tumours. Pathology and Genetics of Skin Tumours (3th ed). Lyon: International Agency for Research on Cancer (IARC); 2006 : 357 p.
23. Beer TW, Calonje E, Wick MR. Tumours of uncertain differentiation. Atypical fibroxanthoma and variants. In: Elder DE, Massi D, Scolyer RA, Willemze R, eds. WHO Classification of Skin Tumours (4th ed). Lyon: International Agency for Research on Cancer (IARC); 2018 : 368-369.
24. Satter EK. Metastatic atypical fibroxanthoma. Dermatol Online J 2012; 18(9): 3.
25. Nergard J, Glener J, Reimer D, Greenwald JS. Atypical fibroxanthoma of the scalp with recurrent and multiple regional cutaneous metastases. JAAD Case Rep 2016; 2(6): 491-493.
26. New D, Bahrami S, Malone J, Callen JP. Atypical fibroxanthoma with regional lymph node metastasis: report of a case and review of the literature. Arch Dermatol 2010; 146(12): 1399-1404.
27. Miettinen M. Fibroblastic and myofibroblastic neoplasms with malignant potential. In: Miettinen M, ed. Modern Soft Tissue Pathology. Tumors and Non-neoplastic Conditions. New York: Cambridge University Press; 2010 : 348-392.
28. Helwig EB, May D. Atypical fibroxanthoma of the skin with metastasis. Cancer 1986; 57(2): 368-376.
29. Helbig D, Ihle MA, Putz K, et al. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget 2016; 7(16): 21763-21774.
30. Benoit A, Wisell J, Brown M. Cutaneous spindle cell carcinoma misdiagnosed as atypical fibroxanthoma based on immunohistochemical stains. JAAD Case Rep 2015; 1(6): 392-394.
31. Gleason BC, Calder KB, Cibull TL, et al. Utility of p63 in the differential diagnosis of atypical fibroxanthoma and spindle cell squamous cell carcinoma. J Cutan Pathol 2009; 36(5): 543-547.
32. Ha Lan TT, Chen SJ, Arps DP, et al. Expression of the p40 isoform of p63 has high specificity for cutaneous sarcomatoid squamous cell carcinoma. J Cutan Pathol 2014; 41(11): 831-838.
33. Alomari AK, Glusac EJ, McNiff JM. p40 is a more specific marker than p63 for cutaneous poorly differentiated squamous cell carcinoma. J Cutan Pathol 2014; 41(11): 839-845.
34. Henderson SA, Torres-Cabala CA, Curry JL, et al. p40 is more specific than p63 for the distinction of atypical fibroxanthoma from other cutaneous spindle cell malignancies. Am J Surg Pathol 2014; 38(8): 1102-1110.
35. Palla B, Su A, Binder S, Dry S. SOX10 expression distinguishes desmoplastic melanoma from its histologic mimics. Am J Dermatopathol 2013; 35(5): 576-581.
36. Smith-Zagone MJ, Prieto VG, Hayes RA, Timperman WW, Jr., Diwan AH. HMB-45 (gp103) and MART-1 expression within giant cells in an atypical fibroxanthoma: a case report. J Cutan Pathol 2004; 31(3): 284-286.
37. Thum C, Hollowood K, Birch J, Goodlad JR, Brenn T. Aberrant Melan-A expression in atypical fibroxanthoma and undifferentiated pleomorphic sarcoma of the skin. J Cutan Pathol 2011; 38(12): 954-960.
38. Cai JP, Randall B. HMB-45 expression in a clear cell variant of atypical fibroxanthoma. J Cutan Pathol 2006; 33(2): 186-188.
39. Gonzalez CD, Hawkes JE, Bowles TL. Recurrent cutaneous angiosarcoma of the ear masquerading as atypical fibroxanthoma. JAAD Case Rep 2016; 2(6): 445-447.
40. Crowson AN, Carlson-Sweet K, Macinnis C, et al. Clear cell atypical fibroxanthoma: a clinicopathologic study. J Cutan Pathol 2002; 29(6): 374-381.
41. Nguyen CM, Chong K, Cassarino D. Clear cell atypical fibroxanthoma: a case report and review of the literature. J Cutan Pathol 2016; 43(6): 538-542.
42. Wollina U, Schonlebe J, Ziemer M, et al. Atypical fibroxanthoma: a series of 56 tumors and an unexplained uneven distribution of cases in southeast Germany. Head Neck 2015; 37(6): 829-834.
43. Doggett S, Brazil J, Limova M, Press L, Smith S, Peck J. Electronic brachytherapy management of atypical fibroxanthoma: report of 8 lesions. J Contemp Brachytherapy 2017; 9(2): 158-160.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2019 Issue 3-
All articles in this issue
- Monitor aneb nemělo by vám uniknout, že...
- Pituitary adenomas – practical approach to the diagnosis and the changes in the 2017 WHO classification
- Cytological examination of cerebrospinal fluid
- Histopathological assessment of the intensity and activity of the inflammation in inflammatory bowel diseases: An important addition to the endoscopy, or a pointless effort?
- The changes of angiogenesis and immune regulations in stromal microenvironment of cutaneous melanomas
- Neuronal ceroid lipofuscinosis with cardiac involvement
- Metanephric adenoma. A case report and literature review
- Atypical fibroxanthoma, rare and often unrecognized cutaneous soft tissue tumor – a case report and review of the literature
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Cytological examination of cerebrospinal fluid
- Neuronal ceroid lipofuscinosis with cardiac involvement
- Pituitary adenomas – practical approach to the diagnosis and the changes in the 2017 WHO classification
- Atypical fibroxanthoma, rare and often unrecognized cutaneous soft tissue tumor – a case report and review of the literature
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

