-
Medical journals
- Career
EGFR in triple negative breast carcinoma: significance of protein expression and high gene copy number
Authors: Folakemi Sobande 1; Ladislav Dušek 2; Adéla Matějková 1; Tomáš Rozkoš 1; Jan Laco 1; Aleš Ryška 1
Authors‘ workplace: The Fingerland Department of Pathology, Charles University Faculty of Medicine and University Hospital, Hradec Králové, Czech Republic 1; Institute of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic 2
Published in: Čes.-slov. Patol., 51, 2015, No. 2, p. 80-86
Category: Original Article
Overview
Background:
As up to 60 % of triple negative breast carcinomas are reported to express EGFR, the receptor is a potential target for biological therapy. The exact role EGFR plays in triple negative breast carcinoma (TNBC) biology, however, remains uncertain. We aimed to discover associations between EGFR protein expression as well as gene copy number changes and clinico-pathologic TNBC characteristics.Methods:
We performed an immunohistochemical and dual in situ hybridization study on a set of 52 archive cases of pre-treatment TNBC in order to detect EGFR protein expression and EGFR gene copy number changes. Clinico-pathologic and follow up data were compared with EGFR status for determining possible links between EGFR and tumor characteristics and/or behavior.Results:
88.5 % of our cases showed EGFR expression. We found no significant links between EGFR expression and tumor grade (p = 0.204), lymph node status (p = 0.514) or p53 status (p = 0.078). Though EGFR gene amplification (EGFR gene:chromosome 7 ratio ≥ 2) was rare (1.9 % of all cases), a high gene copy number (≥ 4 copies per cell) was observed in 15.4 % of all cases. High EGFR gene copy number appeared to be more common in non-ductal, ‘special-type’ carcinomas than in ductal carcinomas. Neither EGFR expression nor EGFR gene copy number was associated with event-free survival.Conclusion:
EGFR changes do not appear to be associated with markers of aggressive behavior in TNBC. Further studies with much larger sample sizes are essential in understanding the role EGFR plays in TNBC biology in order to identify the patients that could benefit from EGFR targeted therapy.Keywords:
EGFR - triple negative breast cancer - prognosis - gene amplification
Breast cancer is a heterogeneous group of malignancies comprising phenotypically and genetically distinct entities. Up to 20 % of breast carcinomas are ‘triple negative’, that is, they lack estrogen and progesterone receptor expression and do not over-express HER2 and are therefore unlikely to respond to hormonal or HER2 targeted therapy (1,2). Triple negative breast carcinomas (TNBCs) are associated with poor prognosis and yet paradoxically, reports show that some of them respond very well to anthracyline-based neoadjuvant chemotherapy regimens (1-3). There is however no standard approach to management of these tumors or any widely accepted evidence-based and clinically relevant way to sub - -classify them.
EGFR has been put forward as a possible target molecule for biological therapy of TNBC as it is frequently expressed in this group of malignancies. Numerous clinical trials have investigated EGFR inhibitors for treatment of unselected cases of breast carcinoma and so far, results have not been satisfactory (1,4,5). The observed low success of anti-EGFR therapy in breast carcinoma could result from inadequate patient selection. In one phase II randomized trial, cetuximab, an anti-EGFR monoclonal antibody, blocked the activity of the EGFR pathway in only a minority of TNBCs (6). It is clear that it is necessary to discover reliable markers that will identify this group of TNBCs likely to respond to anti-EGFR therapy.
The purpose of this study was to assess and compare the immunohistochemical (IHC) expression of EGFR and in-situ hybridization detection of EGFR gene and chromosome 7 in cases of TNBC and to discover possible clinico-pathologic correlations.
Materials and methods
From the archives of the Fingerland Department of Pathology, we selected 52 consecutive cases of pre-treatment invasive TNBC diagnosed between 2005 and 2008, for which adequate material for further studies and sufficient clinical data were available. Triple negativity was defined as immunohistochemical negativity for estrogen and progesterone receptors (0% positive tumor cells) and a HER score = 0, 1+ or 2+ (Table 1) non-amplified by fluorescence in situ hybridization (FISH) or bright-field dual in situ hybridization (HER2 gene:chromosome 17 ratio < 2). In each case one representative formalin-fixed paraffin-embedded tissue block was selected for further studies.
1. Details of antibodies used. 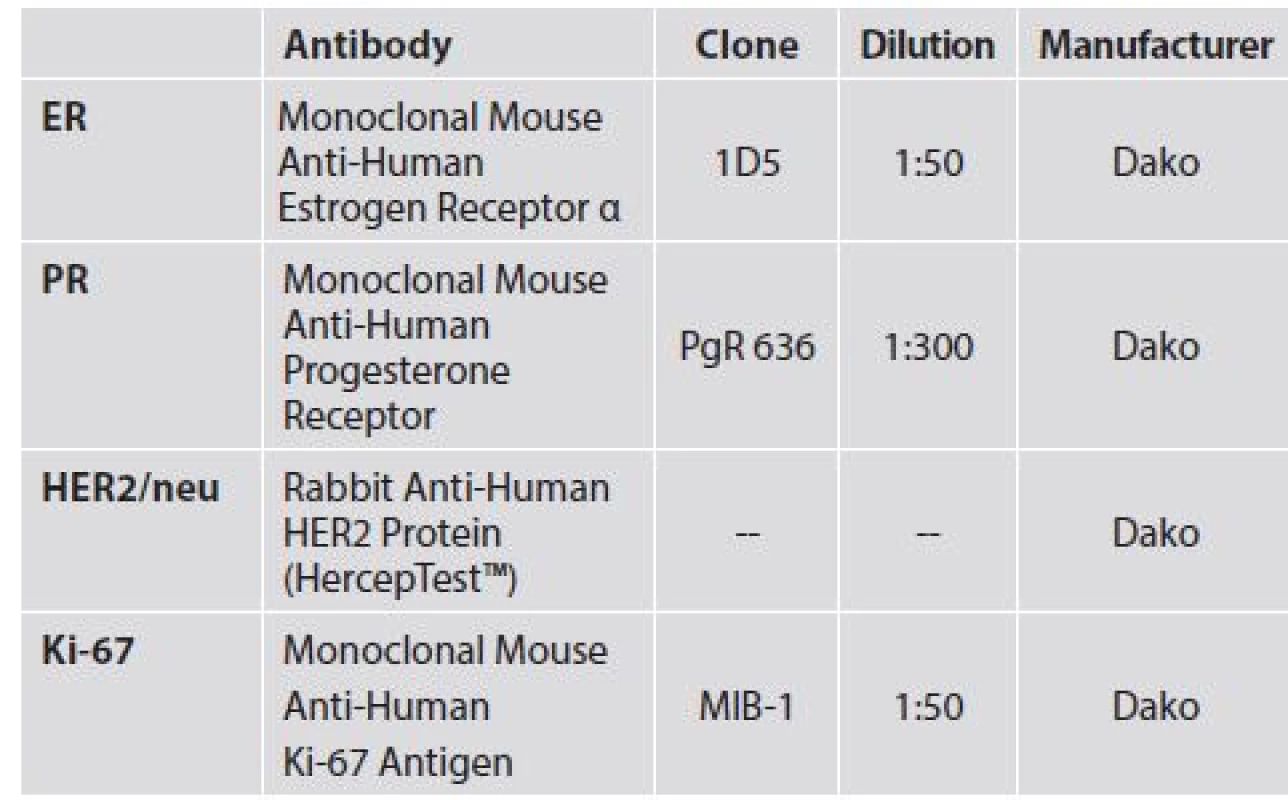
From the pathology reports the following information was obtained in each case: patient age at diagnosis, tumor type and histological grade as well as immunohistochemically detected expression of Ki-67 and p53. Positive nuclear staining for Ki-67 in ≥20% of tumor cells was used to define high proliferative activity. P53 positivity was defined as nuclear staining in ≥ 50% of tumor cells.
A clinical chart review was also performed to determine the clinical course and outcome at the end of the follow-up period. Where available, the pathological stage at time of diagnosis (pTNM) was also obtained. For the cases in which the patients received neoadjuvant chemotherapy, clinical stage (TNM) at the time of diagnosis was recorded instead. TNM stage IV tumors were excluded from our study. Event-free survival was used as the clinical end point (event: presence of residual local breast cancer, recurrence of breast cancer, development of distant metastasis and death, regardless of cause).
Immunohistochemical (IHC) staining in order to detect EGFR was performed using the EGFR pharmDx™ Kit (DAKO, Glostrup, Denmark) according to the manufacturer’s guidelines. A semiquantitiative method for scoring EGFR expression was used employing a combination of staining intensity and the percentage of positive tumor cells. Intensity was graded on four levels – 0 (no membrane staining), 1 (low), 2 (moderate) and 3 (high). The percentage of positive cells was scored as follows 1 (≤ 10 %), 2 (11 - 50 %), 3 (51 - 80 %) and 4 (> 80 %). These 2 parameters were then combined to give a final EGFR score (0-12).
Dual in situ hybridization (dual ISH) assay was performed using the Ventana silver in situ hybridization (SISH) detection kit (Ventana, Arizona, USA) for the EGFR gene and the Ventana Alk Phos Red ISH detection kit (Ventana, Arizona, USA) for chromosome 7 centromere.
For each case, the number of copies of the EGFR gene and chromosome 7 was counted and recorded in 40 different tumor cell nuclei. The average number of copies of the gene (G) and chromosome (C) for each case was recorded and the gene-chromosome copy ratio was calculated. A G:C ratio ≥ 2 was considered EGFR gene amplification.
Standard descriptive statistics were applied in the analysis: absolute and relative frequencies for categorical variables and a median supplemented by 5th - 95th percentile range for continuous variables. The statistical significance of differences in categorical variables between groups of patients was tested using the maximum-likelihood chi-square test.
Survival outcomes were visualized using Kaplan-Meier methodology; statistical significance of differences among groups of patients was tested using log-rank test. Potential predictors of overall and event-free survival were tested using a Cox proportional hazards model.
A value of α = 0.05 was adopted as the level of statistical significance in all analyses. Statistical analysis was computed using SPSS 22 (IBM Corporation, 2013).
Results
All the patients were women aged 28 - 84 years at the time of diagnosis (average: 55 ± 13 years, median: 56 years). The group as a whole was characterized by high tumor grade, high proliferative activity, and p53 positivity. Ductal NOS (not otherwise specified) was the most common histological subtype accounting for 88.5% of all cases. Clinico-pathologic characteristics of all cases are shown in Table 2. The patients received varied combinations of loco-regional and systemic therapy. Seven patients did not receive any surgical treatment for various reasons (contraindications due to comorbidities, patient refusal of surgical treatment, patients lost to follow up during the course of neoadjuvant therapy).
2. Baseline characteristics of sample data set (N = 52). 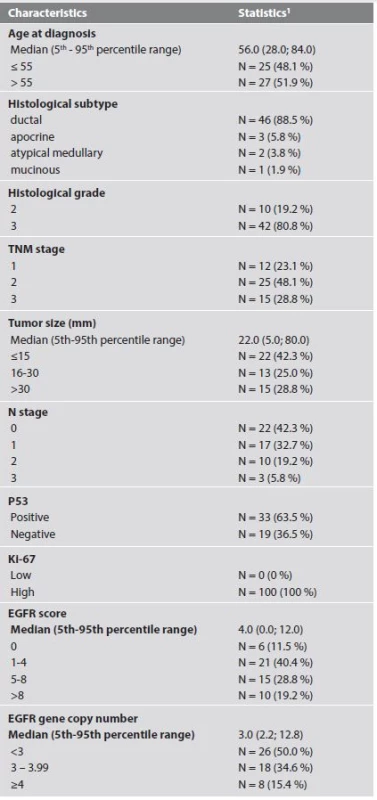
1 absolute and relative frequencies for categorical variables The average duration of follow up was 58 ± 28 months (range: 3 - 96 months, median: 60 months). At the end of the follow up period 39/52 (75.0%) patients had no sign of residual breast carcinoma (Fig. 1). Two patients developed metachronous breast carcinoma in the contralateral breast 16 and 83 months after the primary diagnosis. One of these 2 patients was completely disease free at the end of the follow up period. Two of the 52 patients had loco-regional residual breast disease at the end of the follow up period. These two women were lost to follow-up after 6 and 3 months respectively. Eight of the 52 (15.4 %) patients developed distant metastasis, all within 37 months of initial diagnosis (average: 21 months, range: 12 - 37 months). All but one of the patients that developed distant metastases had regional lymph node involvement at the time of diagnosis. The only patient with pN0 cancer at diagnosis that developed metastasis had a pT2 grade 3 atypical medullary carcinoma. The most frequent metastatic sites were the lung (3/8), bone (3/8), lymph nodes (3/8) and the brain (3/8). Other locations for metastatic deposits were the liver (2/8), pleura (2/8), and meninges (1/8). One of the patients that developed lung and brain metastasis was alive and disease-free 67 months after the initial breast cancer diagnosis. The clinico-pathologic characteristics of cases that developed distant metastasis are shown in Table 3.
3. Clinico-pathologic characteristics of metastasizing tumors. 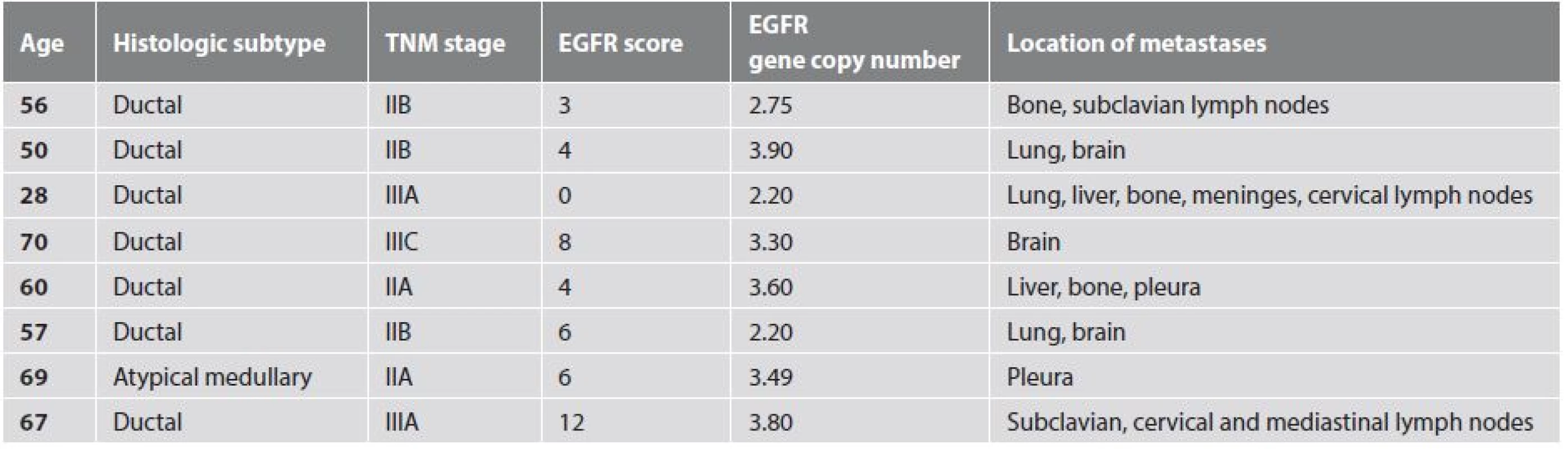
Fig. 1. Survival of all patients. 
Two patients died as a result of complications of metastatic disease 22 and 36 months after diagnosis. Two other patients died, both 3 months after primary diagnosis, of possible complications of treatment. The last death that occurred in our cohort was as a result of generalization of a non-breast (pancreatic) malignancy 60 months after diagnosis of the breast cancer; she was breast cancer free at the time of death.
Only patient age, tumour size and lymph node involvement showed significant correlation with event-free survival (Tab. 4, Fig. 2). Results of IHC and dual ISH staining are summarized in Table 5 with selected clinic-pathologic correlations shown in Table 6. Forty-six (88.5 %) of the tumors in our sample showed some degree of EGFR expression. High EGFR expression (EGFR score 12) is shown in Figure 3 while weak to moderate expression (EGFR score 4) is shown in Figure 4. We found no statistically significant relationships between EGFR score/EGFR gene copy number and tumor grade, lymph node status or p53 status.
4. EGFR expression and other clinic-pathologic characteristics in association with event-free survival – univariate analysis. 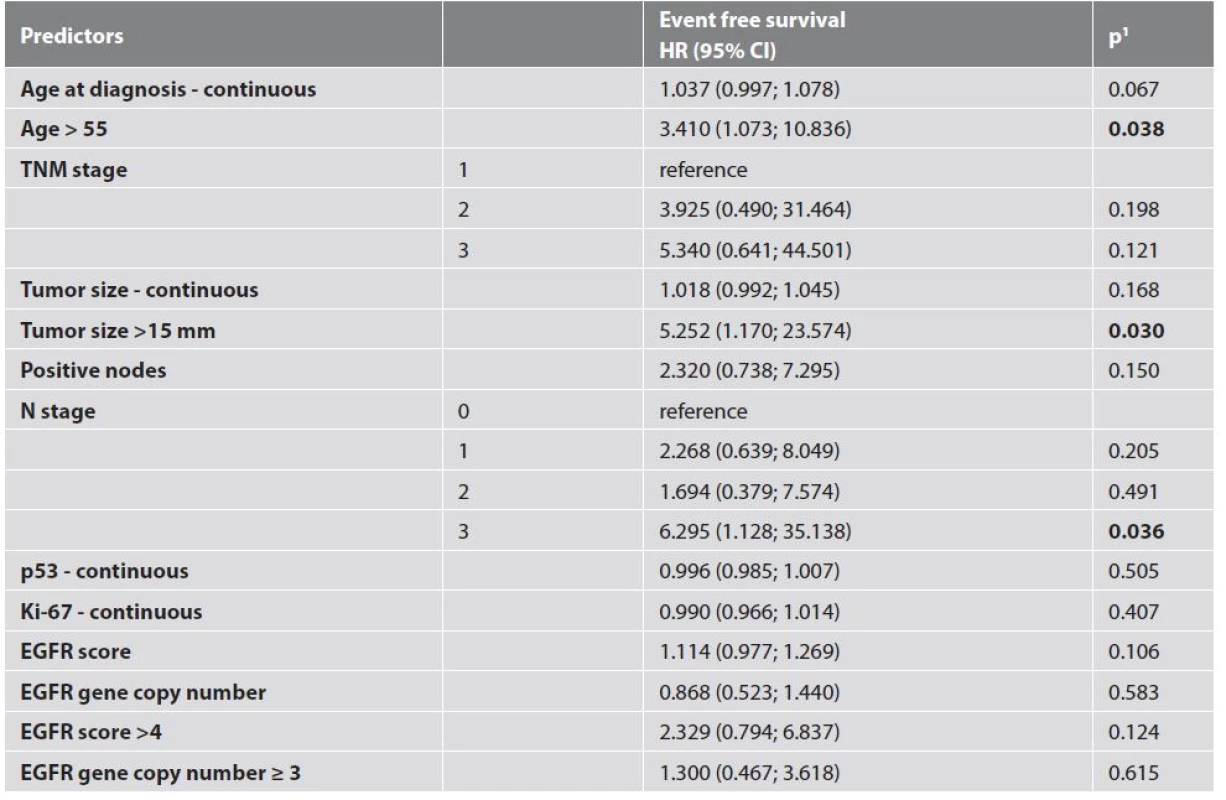
5. EGFR gene copy number and immunohistochemical EGFR score. 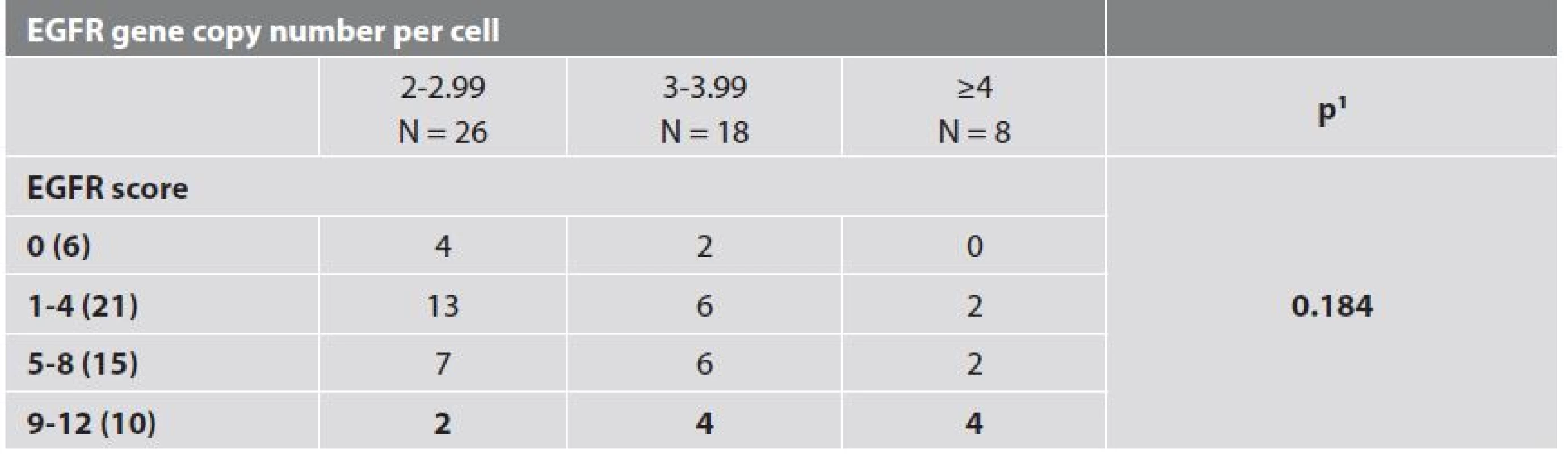
1 maximum likelihood chi-square test 6. EGFR expression and selected clinico-pathologic characteristics. 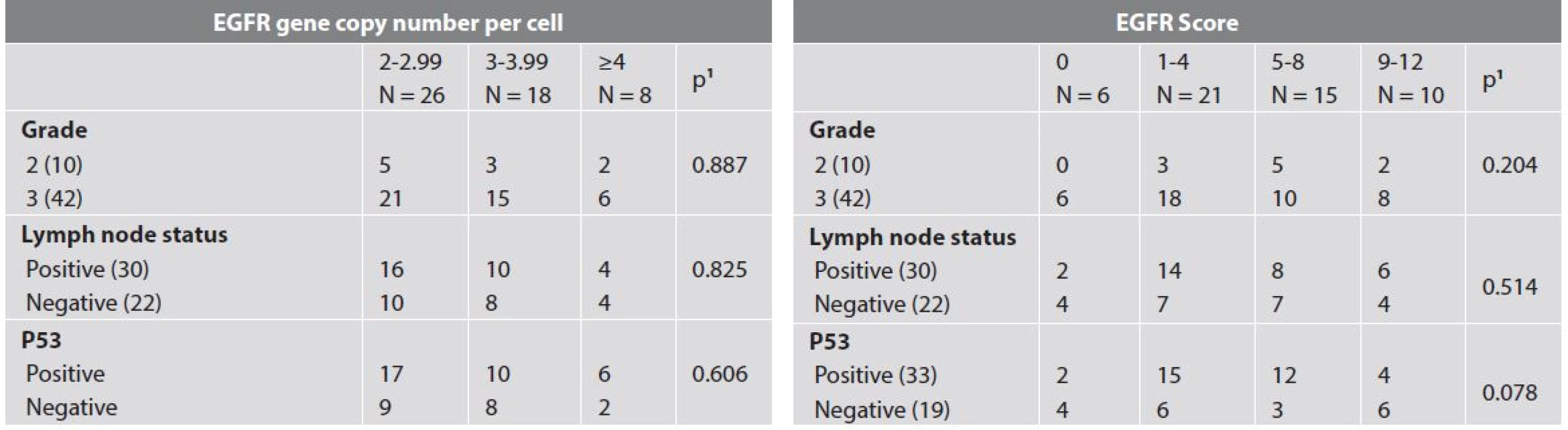
1 maximum likelihood chi-square test Fig. 2. Survival of all patients. A: Event-free survival of patients according to age. B: Event-free survival of patients according to tumor size. C: Event-free survival of patients according to N stage. 
Fig. 3. Strong diffuse IHC EGFR staining (EGFR score 12) (200x). 
Fig. 4. Weak to moderate incomplete IHC EGFR staining (EGFR score 4) (200x). 
Only one tumor showed EGFR gene amplification (G:C = 4.05) (Fig. 5). The rest of the tumors had normal G:C ratios. The EGFR amplified tumor was a stage IIA grade 3 ductal carcinoma NOS in a 42 year old woman. Eight tumors had ≥4 EGFR gene copies per cell. Details are shown in Table 7. None of the patients with these tumors developed distant metastasis. This finding however, was not statistically significant (p = 0.211). Interestingly, half (4/8) of the tumors with ≥4 EGFR gene copies per cell were non-ductal carcinomas; 2 apocrine carcinomas (Fig. 6), 1 atypical medullary carcinoma (Fig. 7) and 1 mucinous carcinoma (Fig. 8).
7. Clinico-pathologic characteristics of tumors with ≥4 EGFR gene copies per cell. 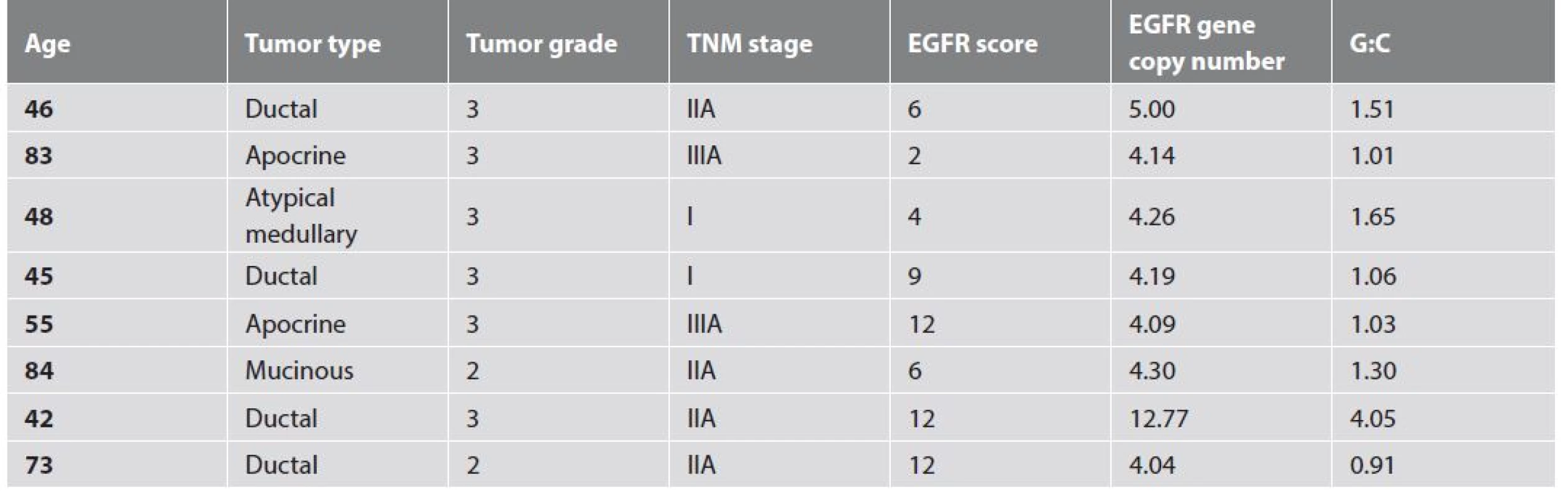
Fig. 5. EGFR gene amplification in triple negative breast carcinoma (dual in situ hybridization) (400x). 
Fig. 6. Apocrine triple negative breast carcinoma (hematoxylin and eosin) (400x). 
Fig. 7. Atypical medullary triple negative breast carcinoma (hematoxylin and eosin) (200x). 
Fig. 8. Mucinous triple negative breast carcinoma (hematoxylin and eosin) (200x). 
Discussion
Triple negative breast carcinoma is a phenotypically and genetically heterogeneous group of tumors with varied patterns of clinical behavior and response to therapy (7,8). The morphological features associated with triple negative invasive ductal carcinoma NOS include marked cellular pleomorphism, lack of tubule formation, scant stromal content, pushing borders of invasion, lymphoplasmacytic inflammatory infiltrate and central acellularity (3,9,10). There are also certain rare morphological subtypes of breast cancer with specific histological features and predictable clinical behavior that are associated with triple negativity. Examples include adenoid cystic carcinoma and medullary carcinoma, both of which are associated with favorable outcome (11-13). Metaplastic carcinomas on the other hand are frequently triple negative and often associated with poor prognosis (14). Despite the fact that TNBC is clearly a group of distinct entities, it is usually considered as one disease from the clinical point of view and is managed as such. TNBC continues to be associated with aggressive behavior and a poor outcome.
EGFR belongs to the family of transmembrane receptors with intrinsic protein tyrosine kinase activity (15). It is a receptor for multiple ligands and its activation leads to a wide range of biological responses, from apoptosis and migration to proliferation and dedifferentiation (15). A wide variety of solid tumors show EGFR gene amplification and/or protein over-expression (16). For colorectal, gastric and non-small cell lung carcinomas increased expression is associated with an advanced stage and unfavorable prognosis (16). EGFR expression has been described in 14 - 43 % of all breast carcinomas (17-19) and in up to 60 % of TNBCs (20). EGFR is thus an attractive candidate for targeted biological therapy provided a suitable method for patient selection can be devised.
Though 88.5 % of our sample showed EGFR expression, amplification of the gene was seen in only one case. Our finding of rare EGFR gene amplification in TNBC was also reported by Nakajima et al in their study of 84 TNBCs (21). None of their tumors showed EGFR amplification. In addition to IHC and in situ hybridization, they also performed EGFR gene mutation analysis. No EGFR gene activating mutations were found. Jacot et al also found no EGFR-activating mutations in their group of 229 TNBCs (22). Lv et al on the other hand observed a higher incidence of EGFR gene amplification in their set of 139 unselected breast carcinomas; positivity in 33.1 % of all cases (18). They did however also report a low rate of EGFR mutations (in 1.4 % of all cases) and concluded that EGFR mutations should not be used in trials testing anti-EGFR therapy in breast cancer.
In our sample of TNBCs, neither IHC EGFR positivity nor increased gene copy number was associated with a worse outcome; not even in the only amplified case. In fact, we observed a favorable clinical course in all patients with tumors that had high EGFR gene copy numbers (≥ 4 copies per cell). Despite the limitations set by the small size and retrospective nature of our study, the results are highly suggestive; high EGFR copy number, at least in a certain sub-set of TNBCs, could be a marker for a good prognosis. Inhibiting EGFR in such tumors could potentially have deleterious effects.
One of the characteristics of an ideal target for targeted therapy, as noted by Pritchard, is an association with poor outcome (23). The fact we did not observe an association between EGFR expression and a worse outcome makes us question the suitability of EGFR inhibition for the therapy of all TNBCs. After a phase II trial testing cetuximab in patients with metastatic TNBC Carey et al concluded that ‘therapy targeting growth factor pathways in this subtype (TNBC) may require a far better understanding of the pathways maintaining EGFR activity’ (6). Our findings put us in agreement with this statement.
Tumor morphology may play a role in the identification of tumors likely to harbor clinically significant EGFR changes. Four of the 8 TNBCs in our sample that showed elevated EGFR copy numbers were ‘special’ histological types of breast cancer. None of the 6 special type carcinomas we observed had less than 3 EGFR gene copies per cell.
Reis-Filho et al also showed a link between breast tumor morphology and EGFR changes. They observed frequent overexpression of EGFR and EGFR gene amplification in metaplastic breast carcinomas and suggested that some patients with metaplastic breast carcinomas might benefit from EGFR targeted therapy (24). This suggests that morphology is an important manifestation of breast tumor biology and thus could play a significant role in patient selection for various types of targeted therapy. Similar associations are observed in lung carcinomas. Up to 80 % of non-small cell lung carcinomas are reported to overexpress EGFR (25). In the past, clinical trials testing the efficacy of anti-EGFR therapy in unselected non-small cell lung carcinoma patients also proved unsuccessful in a vast majority of cases. This led to studies that showed that the tumors which responded to anti-EGFR therapy were mostly adenocarcinomas with somatic mutations in the EGFR TK domain (26).
The challenge remains to identify a specific subtype of TNBC that is likely to harbor predictive EGFR changes. Further studies with much larger sample sizes, with a focus on special histological subtypes, are essential in understanding the role EGFR plays in TNBC biology. Morphological, IHC and in-situ hybridization studies, mutation analysis and clinical follow up data ought to be assessed simultaneously in order to provide a clearer picture of how EGFR changes influence TNBC.
Acknowledgments
This study was financially supported by the PRVOUK P37/11 program and LM2010004 project.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
Correspondence address:
Folakemi Sobande, MD
The Fingerland Department of Pathology
Faculty Hospital, Sokolská 581
50005 Hradec Králové, Czech Republic
tel.: +420774152896
e-mail: folakemis@hotmail.co.uk
Sources
1. Ueno N, Zhang D. Targeting EGFR in triple negative breast cancer. J Cancer 2011; 2 : 324-328.
2. Amos K, Adamo B, Anders C. Triple-negative breast cancer: An update on neoadjuvant clinical trials. Int J Breast Cancer 2012; 2012 : 385978.
3. Ryden L, Jirstrom K, Haglund M, Stal O, Ferno M. Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Treat 2010; 120(2): 491-498.
4. Bouchalova K, Cizkova M, Cwiertka K, Trojanec R, Hajduch M. Triple negative breast cancer - current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and c-MYC gene assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2009; 153(1): 13-17.
5. Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor in breast cancer. Breast Cancer Res Treat. 2012; 136(2): 331-345.
6. Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012; 30(21): 2615-2623.
7. Foulkes W, Smith I, Reis-Filho J. Triple-negative breast cancer. N Engl J Med 2010; 363(20): 1938-1948.
8. Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat 2011; 125(3): 627-636.
9. Cakir A, Gonul II, Uluoglu O. A comprehensive morphological study for basal-like breast carcinomas with comparison to non basal-like carcinomas. Diagnostic Pathology 2012; 7 : 145.
10. Bhargava R, Beriwal S, McManus K, Dabbs D. CK5 is more sensitive than Ck5/6 in identifying the ‘basal-like’ phenotype of breast cancer. Anatomic Pathology 2008; 130(5): 724-730.
11. Page D. Special types of invasive breast cancer, with clinical implications. Am J Surg Pathol 2003; 27(6): 832-835.
12. Law Y, Quek S, Tan P, Wong S. Adenoid cystic carcinoma of the breast. Singapore Med J 2009; 50(1): e5-e11.
13. Muslimani AA, Ahluwalia MS, Clark CT, Daw HA. Primary adenoid cystic carcinoma of the breast: case report and review of the literature. Int Semin Surg Oncol 2006; 3 : 17.
14. Jung S, Kim H, Nam B, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat 2010; 120(3): 627-637.
15. Wells A. EGF receptor. Int J Biochem Cell Biol 1999; 31(6): 637-643.
16. Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 2008; 52(6): 738-746.
17. Shawarby M, Al-Tamimi D, Ahmed A. Very low prevalence of epidermal growth factor receptor (EGFR) protein expression and gene amplification in Saudi breast cancer patients. Diagnostic Pathology 2011; 6 : 57.
18. Lv N, Xie X, Ge Q, et al. Epidermal growth factor receptor in breast carcinoma: association between gene copy number and mutations. Diagnostic Pathology 2011; 6 : 118.
19. Martinazzi M, Crivelli F, Zampatti C, Martinazzi S. Epidermal growth factor receptor immunohistochemistry in different histological types of infiltrating breast carcinoma. J Clin Pathol 1993; 46(11): 1009-1110.
20. Teng Y, Tan W, Thike A, et al. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast Cancer Research 2011; 13(2): R35.
21. Nakajima H, Ishikawa Y, Furuya M, et al. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer. Breast Cancer 2012; 21(1): 66-74.
22. Jacot W, Lopez-Crapez E, Thezenas S, et al. Lack of EGFR-activating mutations in European patients with triple-negative breast cancer could emphasize geographic and ethnic variations in breast cancer mutation profiles. Breast Cancer Research 2011; 13(6): R133.
23. Pritchard KI. Endocrine therapy: is the first generation of targeted drugs the last? J Intern Med. 2013; 274(2): 144-152.
24. Reis-Filho JS, Milanezi F, Carvalho S, et al. Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Research 2005; 7(6): R1028-R1035.
25. Silvestri GA, Rivera MP. Targeted therapy for the treatment of advanced non-small cell lung cancer. A review of the epidermal growth factor receptor antagonists. Chest 2005; 128(6): 3975-3984.
26. Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Modern Pathology 2008; 21: S16-S22.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2015 Issue 2-
All articles in this issue
- Molecular pathology of endometrial carcinoma – a review
- Practical comments on examination of placenta in the second and third trimester of gravidity
- Unusual lung finding of massive alveolar filling with foamy macrophages in congenital epidermolysis bullosa after amnion fluid aspiration in 15-day-old newborn without any clinical signs of respiratory impairment
- Microvascular density in lymphomas - evaluation methods and clinical impact
- EGFR in triple negative breast carcinoma: significance of protein expression and high gene copy number
- Celiac disease-like enteropathy due to antihypertensive therapy with the angiotensin-II receptor type 1 inhibitor eprosartan
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Practical comments on examination of placenta in the second and third trimester of gravidity
- Molecular pathology of endometrial carcinoma – a review
- EGFR in triple negative breast carcinoma: significance of protein expression and high gene copy number
- Unusual lung finding of massive alveolar filling with foamy macrophages in congenital epidermolysis bullosa after amnion fluid aspiration in 15-day-old newborn without any clinical signs of respiratory impairment
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

