-
Medical journals
- Career
Gastric dysplasias.
A clinicopathological study of 35 cases
Authors: Alena Chlumská 1,2; Petr Mukenšnabl 1; Tomáš Waloschek 2; Michal Zámečník 3
Authors‘ workplace: Šikl`s Department of Pathology, Medical Faculty Hospital, Charles University, Pilsen, Czech Republic 1; Laboratory of Surgical Pathology, Pilsen, Czech Republic 2; Medicyt s. r. o., Department of Pathology, Trenčín, Slovak Republic 3
Published in: Čes.-slov. Patol., 49, 2013, No. 1, p. 35-38
Category: Original Article
Overview
Gastric epithelial dysplasia (GED) represents a recognized precursor lesion of gastric adenocarcinoma. GED types can be classified according to its morphology and patterns of mucin expression into adenomatous (intestinal), foveolar (gastric) and hybrid (mixed) types. We examined gastroscopic specimens with GED in 35 patients (21 men and 14 women, mean age 69.6 years). Adenomatous dysplasia was present in 17 patients (49 %), and was of low grade in 14 cases and high grade in 3 cases. Foveolar type dysplasia was found in 16 patients (46 %), and almost in one half of the cases it was high grade (in 7 cases, i.e. 46 %). In one woman, low grade foveolar dysplasia was found in polypoid mucosal prolapse of the gastric antrum. Hybrid dysplasia was found in only 2 cases (0.6 %), and in both of them this dysplasia was predominantly of foveolar type. One case was of low-grade and the second case was of a high-grade type. In our series GED was found mostly in the antrum. The findings in the adjacent mucosa usually included HP negative inactive chronic gastritis with intestinal metaplasia of both complete and incomplete types. In our series, foveolar type dysplasia was more frequent in comparison with previous studies. Our findings show that high grade dysplasia is more frequent in foveolar GD than in adenomatous GD, and this is in keeping with previous published findings.
Keywords:
stomach – dysplasia – immunohistochemistry – low and high grade lesions – intestinal metaplasia
Gastric epithelial dysplasia (GED) has been widely recognized as a precursor lesion for gastric adenocarcinoma. In the gastrointestinal tract, the dysplasia is synonymous with intraepithelial neoplasia, and implies architectural and cytological changes. GED frequently develops in the setting of chronic atrophic gastritis and intestinal metaplasia (1–5), although it may also occur in apparently normal gastric mucosa (1,6,7). Endoscopically, it can show polypoid, flat or slightly depressed growth patterns. Despite similar microscopic morphology found in all of these lesions, the polypoid or protruding lesions are commonly referred to as adenomas whereas the term dysplasia is used for flat or depressed types (5,7,8–11).
Recently, advances in mucin immunohistochemistry have led to efforts to classify GED according to their patterns of mucin expression. The majority of GED displays an intestinal phenotype, and they were labeled as adenomatous (intestinal) type that resembles colonic adenoma. Other variants include foveolar (gastric) type and hybrid (mixed) type (2,6–10,12); however, the hybrid (mixed) type dysplasia represents a less commonly used term. Adenomatous GED is composed of crowded, tubular glands lined by columnar cells with pseudo-stratified, pencillate hyperchromatic nuclei, and it expresses intestinal markers (CD10, MUC2), whereas gastric mucins (MUC5AC, MUC6) are negative. Foveolar variant shows cuboidal to columnar cells with pale-clear cytoplasm and hyperchromatic nuclei, and it predominantly produces MUC5AC. It is frequently of a high grade. The third type, hybrid dysplasia, is defined as a mixture of both adenomatous and foveolar dysplasia. Foveolar type morphology predominates usually in hybrid dysplasia (10). The second component in hybrid dysplasia should occupy at least 10 % of the cell population. The criteria for grading of GED have evolved over the past few decades. A two-tiered scheme that contains low grade and high grade was adopted, and it is now widely used in the classification of GED (5,10,13).
Here we present our experience with the diagnostics of gastric dysplasia in gastroscopic biopsies. We describe histological and immunohistochemical findings and biological differences in 35 cases of gastric dysplasia.
Materials and Methods
Our study includes gastroscopic biopsy specimens from 35 patients (21 men and 14 women, mean age 69.6 years) with GED. These 35 patients were followed for 1 – 36 months (mean 9 months). In 20 patients, the bioptic examination was repeated from 1 to 5 times. None of the patients had familial adenomatous polyposis or autoimmune gastritis.
All biopsies were processed routinely and stained with hematoxylin and eosin, PAS/alcian blue at pH 2.5, and silver impregnation for identification of H. pylori. Immunohistochemistry was performed using standard avidin – biotin complex peroxidase technique and included MUC5AC (Ventana), MUC2 (Ventana), CD10 (Ventana), p53 (Ventana), and Ki-67 (clone MIB-1, Ventana).
According to above-mentioned histological and immunohistochemical criteria, each lesion was classified as either adenomatous, foveolar or hybrid type, and each was graded as low or high grade dysplasia (2,6–10,12). The low grade dysplasia was diagnosed when only mild architectural abnormality was seen. It showed small glandular structures or tubules with little branching or irregularity. Cytologically, the atypical cells were enlarged, hyperchromatic and extended to the surface of the mucosa. High grade dysplasia contained neoplastic cells that are usually cuboidal rather than columnar. They showed a high nucleo-cytoplasmic ratio, prominent amphophilic nuclei, and numerous mitoses. High grade dysplasia shows more pronounced architectural disarray with prominent branching, budding, and in extreme cases, with a cribriform pattern. The intestinal metaplasia found in the vicinity of dysplasia was classified as either pure intestinal (i.e., complete) or mixed gastric-intestinal with the presence of foveolar type mucin (i.e., incomplete) (5,10,14,15).
Results
Among 37 patients with GED, adenomatous dysplasia was found in 17 cases (49 %). It was of a low grade in 14 cases (Fig. 1) and of a high grade in 3 patients (Fig. 2). Foveolar dysplasia was found in 16 patients (46 %), and it was of a low grade in 9 cases and of a high grade in 7 patients. Hybrid dysplasia was found in 2 cases (0.6 %), one case was low grade and the second was high grade. A number of particular types of dysplasia and their grading are shown in the summary table (Table 1). In both cases of hybrid dysplasia, the foveolar pattern predominated. Endoscopically, a polypous appearance was recorded in 6 adenomatous dysplasias and 3 foveolar dysplasias. In one case of an 82-ys-old woman, foveolar dysplasia was found in mucosal prolapse of non-inflammed antral mucosa (Fig. 3). GED was not multifocal in any of the cases.
1. Number and grading of the dysplasia types in the series. 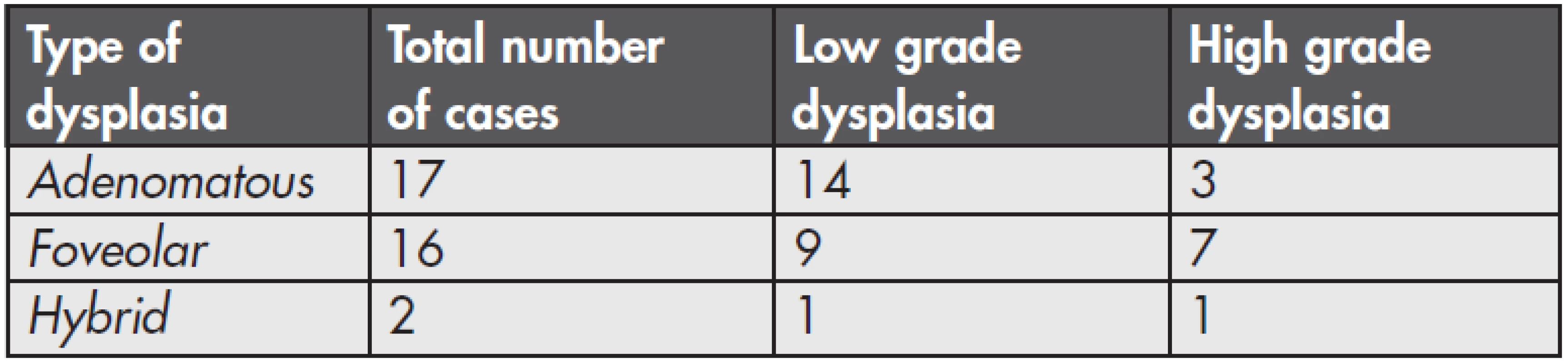
Fig. 1. Low grade adenomatous dysplasia with typical tubules resembling those of colonic adenoma (<b>A</b> and <b>B</b>). CD10 positivity along the apical surface of the cells (<b>C</b>), and negative immunostain for MUC5AC (<b>D</b>) (hematoxylin and eosin, ABC technique; original magnifications x100, x200, x100, x100). 
Immunohistochemically, Ki-67 (MIB1) reactivity was seen in the whole thickness of the dysplastic lesions, and it was more pronounced in high grade dysplasias (Fig. 2D). In all low grade cases p53 positivity was low, contrasting with stronger positivity seen in high grade dysplasias. This p53 expression was unrelated to the type of dysplasia.
Fig. 2. High grade adenomatous dysplasia (<b>A</b>). Positivity for CD10 (<b>B</b>), negativity for MUC5AC (<b>C</b>), and numerous cells positive for Ki-67 (<b>D</b>) (hematoxylin and eosin, ABC technique; original magnification x160). 
Fig. 3. Low grade foveolar dysplasia in the polypoid mucosal prolapse (<b>A</b>). Visible goblet cells represent intestinal metaplasia which is often adjacent to the foveolar dysplasia. The lesion is negative for CD10 (<b>B</b>) whereas MUC5AC is positive (<b>C</b>) (hematoxylin and eosin, ABC technique; original magnification x100). 
GED was found in the antrum in 24 patients and in the corpus in 3 cases. In remaining cases the location was impossible to determine, because no adjacent mucosa was present in these specimens. In specimens with adjacent mucosa, HP negative inactive chronic gastritis was found in all 27 cases. In 22 cases intestinal metaplasia in foveolas was found. Intestinal metaplasia was complete in 10 cases, incomplete in 7 cases, and “mixed” complete/incomplete in 5 cases. The type of intestinal metaplasia appeared to lack any association with a specific type of dysplasia.
Discussion
Gastric dysplasia represents a well recognized precursor lesion for the development of gastric cancer, and it is also an indicator of an increased risk of synchronous adenocarcinoma elsewhere in the stomach (5,10,13,16). The majority of GEDs display an intestinal phenotype referred to as adenomatous dysplasia that is considered the precursor of intestinal type adenocarcinoma (2,3,5,11). The less common histological variant, a foveolar type, is more often of a high grade and it has been shown to be more commonly associated with poorly differentiated adenocarcinoma of the intestinal type (9, 13).
In our series of 35 GEDs, the dysplasia was of the adenomatous type in 17 cases (49 %). It was polypoid in only 6 cases, and it was of a low grade in the majority of the cases (14 cases). The foveolar dysplasia was found in 16 patients (46 %), and almost in half of these cases (7 cases) the dysplasia was of a high grade. The hybrid dysplasia was found in only 2 patients (0.6 %). In both of these cases, the foveolar pattern was prevailing over the intestinal one, and the grade was low in one and high in the other case.
A proportion of particular types of GED seen in our series differs from previously published findings (6, 8, 10, 13), because we found the foveolar type more frequently and the hybrid type less often. This difference is difficult to explain. We think that it can be caused by the relatively low number of cases in our series.
Regarding the grade of GED, our findings confirm that the high grade is more frequent in foveolar than in adenomatous dysplasias (10). Repeated examinations and biopsies showed the same type of dysplasia in 11 cases. This persistence of dysplasia can be explained by its incomplete removal during the previous examination. In 3 patients with high grade dysplasia, poorly differentiated intestinal type adenocarcinoma was diagnosed during the first to third months. This carcinoma was probably present already at the time of initial biopsies, but it was missed (2,16).
Regarding the localization, all dysplasias were more prevalent in the gastric antrum, as described previously (1,2,7,17).
The surrounding gastric mucosa revealed chronic inactive gastritis with complete or incomplete intestinal metaplasia of the foveolar epithelium. Both types of intestinal metaplasia were found in both types of dysplasia, and it appears that there is no association between the types of dysplasia and the intestinal metaplasia, which is in keeping with published data (7). Only in one study of Park et al. (10), a more frequent (but statistically insignificant) occurrence of complete intestinal metaplasia in the vicinity of adenomatous dysplasia was found.
In one case, we observed low grade foveolar dysplasia in antral mucosal prolapse polyp without inflammatory changes. To our best knowledge, dysplasia in gastric mucosal prolapse polyp has not been described so far.
In the differential diagnosis of GED, it is our experience that difficulties frequently arise in the distinction between the mild dysplasia and the non-dysplastic regenerative changes, and between the severe dysplasia and the intramucosal carcinoma. Gastric dysplasia shows full-thickness mucosal involvement extending from the base of the mucosa to the surface, whereas regenerative non-dysplastic changes show some zonal distribution as atypical appearing nuclei are usually seen in the deep areas of the foveolas. However, Agoston et al. recently described so-called pit dysplasia in the mucosa adjacent to carcinoma (18). This dysplasia remains limited to the bases of the pit epithelium, and therefore it resembles regenerative changes. However, it shows traditional architectural and cytological features of dysplasia, such as pit distortion, branching and dilatation, cribriforming, nuclear enlargement and hyperchromasia, irregular nuclear membranes, increased nucleus-to-cytoplasmic ratio, clumped chromatin pattern, prominent nucleoli, atypical mitoses, mucin depletion, loss of cell polarity, and nuclear stratification (4,18).
The most objective and discriminatory criterion for separating low and high grade dysplasia is the loss of nuclear polarity in severe dysplasia. However, this criterion is useful for the adenomatous GED, but is not applicable for the foveolar dysplasia. Most important features for diagnosis of foveolar dysplasia are nuclear size, prominent nucleoli and back-to-back crowded glandular architecture (14). To increase diagnostic accuracy, biomarkers such as Ki-67 (MIB-1) and p53 were used. Like in other studies (19,20) we found diffuse Ki-67 expression as a potential marker of dysplasia, with its strong activity in high grade dysplasias. Moreover, p53 protein expression is also a potential marker of dysplasia and neoplastic progression, but its over-expression is neither sensitive nor specific because not all p53 mutations result in p53 protein accumulation (19,20). In our cases, strong p53 expression was seen in almost all cases of high grade dysplasia of both main types. In low grade lesions, p53 expression was usually low. We did not find higher p53 expression in adenomatous dysplasia, in contrast with the results of Abraham et al. (6,8).
A diagnosis of gastric dysplasia indicates an increased risk of progression to gastric cancer. However, the prediction of the lesion’s evolution is difficult to determine in individual cases. Prognosis differs between the low grade and the high grade dysplasias. The low grade dysplasia has been shown to regress in 40–50 % of the cases, to persist in 20–30 % and the progression to high grade dysplasia has been seen in 0% to 25 % of the cases (9,17). High grade dysplasia has been noted to persist in about 14 – 58 % of the patients and to progress to adenocarcinoma in 60 % to 85 % of the cases (7,9,11,16,17).
It has been hypothesized that there might be different genetic alterations in foveolar-type and adenomatous-type dysplasias to account for this divergent biologic behavior (21). Abraham et al. (6,8) investigated alterations in APC, beta-catenin, k-ras and MSI in both types of dysplasia, but no statistically significant differences in a particular genetic alteration were found. However, that series was limited and therefore additional studies are needed for establishing the genetic events accounting for clinicopathologic differences between the main types of dysplasia.
Our results showed, in keeping with results of published studies, that current classification distinguishing foveolar and adenomatous dysplasia and two-tiered grading system are meaningful and helpful for management of patients with gastric dysplasia. It permits the determination of the risk of cancer in the patients. Our results also indicate that adenomatous and foveolar dysplasias are different phenotypically, and that they differ regarding the grade of dysplasia and the risk of cancer development.
Correspondence address:
Alena Chlumska, MD, CSc
Biopticka lab.
Mikulasske nam. 4
32600 Pilsen, Czech Republic
e-mail: chlumska@medima.cz
phone: +420-737-220-403
Sources
1. Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut 1999; 45(5): 784–790.
2. Misdraji J, Lauwers GY. Gastric epithelial dysplasia. Semin Diagn Pathol 2002; 19(1): 20–30.
3. Rugge M, Farinati F, Baffa R, et al. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Gastroenterology 1994; 107(5): 1288 –1296.
4. Shin N, Jo HJ, Kim WK, et al. Gastric pit dysplasia in adjacent gastric mucosa in 414 gastric cancers: prevalence and characteristics. Am J Surg Pathol 2011; 35(7): 1021–1029.
5. Bosman FT. World Health Organization; International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed., Lyon, IARC Press, 2010.
6. Abraham SC, Montgomery EA, Singh VK, et al. Gastric adenomas. Intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol 2002; 26(10): 1276–1285.
7. Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol 2003; 36(Suppl. 1): S37–S43.
8. Abraham SC, Park SJ, Lee JH, et al. Genetic alterations in gastric adenomas of intestinal and foveolar phenotypes. Mod Pathol 2003; 16(8): 786–795.
9. Alfaro EE, Lauwers GY. Early gastric neoplasia: diagnosis and implications. Adv Anat Pathol 2011; 18(4): 268–280.
10. Park DY, Srivastava A, Kim GH, et al. Adenomatous and foveolar gastric dysplasia: distinct patterns of mucin expression and background intestinal metaplasia. Am J Surg Pathol 2008; 32(4): 524–533.
11. Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol 2000; 24(2): 167–176.
12. Chlumská A, Mukenšnabl P, Waloschek T, Zámečník M. Dysplázie žaludku. Kongresové noviny, No 2, p. 5, 32nd Meeting of Czech and Slovak Gastroenterologist, Nov 3–5, 2011, Brno, Czech Republic.
13. Srivastava A, Lauwers GY. Gastric epithelial dysplasia: the Western perspective. Dig Liver Dis 2008; 40(8): 641–649.
14. Mahajan D, Bennett AE, Liu X, et al. Grading of gastric foveolar-type dysplasia in Barrett’s esophagus. Mod Pathol 2010; 23(1): 1–11.
15. Khor TS, Alfaro EE, Ooi EMM, et al. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett esophagus? Am J Surg Pathol 2012; 36(3): 331–342.
16. Farinati F, Rugge M, Di Mario F, et al. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients. A prospective study. Endoscopy 1993; 25(4): 261–264.
17. Lauwers GY. Gastric dysplasia: diagnosis and significance. Pathol Case Rev 2002; 7(1): 27–34.
18. Agoston T, Lauwers GY, Odze RD. Evidence that dysplasia begins in the bases of the pits in the pathogenesis of gastric cancer. Gastroenterology 2009; 136: A460(S1).
19. Moyes LH, Going JJ, et al. Still waiting for predictive biomarkers in Barrett’s oesophagus. J Clin Pathol 2011; 64(9): 742–750.
20. Wang WCh, Wu TT, Chandan VS, et al. Ki-67 and ProExC are useful immunohistochemical markers in esophageal squamous intraepithelial neoplasia. Hum Pathol 2011; 42(10): 1430–1437.
21. Kushima R, Müller W, Stolte M, Borchard F. Differential p53 protein expression in stomach adenomas of gastric and intestinal phenotypes: possible sequences of p53 alteration in stomach carcinogenesis. Virchows Arch 1996; 428(4-5): 223–227.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2013 Issue 1-
All articles in this issue
- The testing strategy for detection of biologically relevant infection of human papillomavirus in head and neck tumors for routine pathological analysis
- Autophagic vacuolar myopathies: what we have learned from the differential diagnosis of vacuoles in muscle biopsy
-
Nanopathology as a new scientific discipline
Minireview - Czech eponyms in pathology
-
Gastric dysplasias.
A clinicopathological study of 35 cases - With the president of the ESP - Prof. Carneiro - about the 24th European Congress of Pathology in Prague 2012
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Czech eponyms in pathology
-
Gastric dysplasias.
A clinicopathological study of 35 cases - Autophagic vacuolar myopathies: what we have learned from the differential diagnosis of vacuoles in muscle biopsy
- The testing strategy for detection of biologically relevant infection of human papillomavirus in head and neck tumors for routine pathological analysis
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career









