-
Medical journals
- Career
Triandrická monogynická tetraploidia ako výsledok DNA analýzy parciálnej moly hydatidózy
Authors: Lajos Gergely 1,2; Miroslav Korbeľ 2,3; Vanda Repiská 1,2; Ľudovít Danihel 2,4; J. Hutník 1; Liam McCullough 2,3; Petra Priščáková 1,2
Authors‘ workplace: Institute of Medical Biology, Genetics and Clinical Genetics, Faculty of Medicine, Comenius University Bratislava, Bratislava, Slovak Republic 1; Centre for Gestational Trophoblastic Disease of Slovak Republic, Bratislava, Slovak Republic 2; 1st Department of Gynaecology and Obstetrics, Faculty of Medicine, Comenius University Bratislava, Bratislava, Slovak Republic 3; Institute of Pathological Anatomy, Faculty of Medicine, Comenius University Bratislava, Bratislava, Slovak Republic 4
Published in: Ceska Gynekol 2023; 88(6): 446-449
Category: Case Report
Overview
Autori prezentujú prípad parciálnej hydatidóznej moly, kde DNA analýza (STR – genotypizácia krátkych tandemových opakovaní) poukázala na triandrickú monogynickú tetraploidnú kompozíciu genómu s gonozómovým komplementom XXXY. Uvedený genetický nález, ktorý je zriedkavý v porovnaní s typickými, diandrickými monogynickými triploidnými parciálnymi molami, klinicko-patologicky koreluje s parciálnou molou. Genetická analýza definitívne potvrdila podozrenie na parciálnu molu. Pre vylúčenie možnosti pôvodu molárnej gravidity ako zadržané produkty koncepcie po predchádzajúcej tehotnosti ukončenej interrupciou, boli porovnané STR profily molárnej gravidity a produktu predcházajúcej koncepcie. Genotypizácia krátkych tandemových opakovaní je užitočná molekulárno-genetická metóda v diferenciálnej diagnostike parciálnej hydatidóznej moly, kde sú klinicko-patologické nálezy často nejednoznačné.
Introduction
Partial hydatidiform moles (PHMs) typically have a triploid diandric monogynic genome as a result of dispermic fertilization (dispermic PHM) or duplication of the paternal pronucleus (monospermic PHM). Less frequently, in approximately 2–3% of the cases, a PHM has a tetraploid triandric monogynic genome composition as a result of trispermic fertilization (Fig. 1a) [1,2]. The ultrasonographic and histopathological/immunohistochemical appearance of PHMs is often ambiguous making it difficult to differentiate them from non-molar hydropic abortions. In these cases, genetic analysis is helpful in providing a definitive diagnosis. The ideal genetic diagnostic method is DNA analysis, specifically short tandem repeat (STR) genotyping. STR genotyping makes it possible to determine the parental origin of the sets of chromosomes by comparing STR allelic profiles of the product of conception and of the parents [3,4]. We present a case of a clinically suspected PHM, where DNA analysis revealed a rare triandric monogynic tetraploid genome composition confirming the diagnosis of PHM.
Case presentation
The 33-years-old, G3P1 patient was referred to the Centre for Gestational Trophoblastic Disease (Centre) of the Slovak Republic because of an ultrasonographically suspected partial hydatidiform mole (PHM). The patient underwent an elective pregnancy termination (at the 7th week of pregnancy) 19 weeks before the referral to the Centre. The patient was concerned that the current pregnancy represents retained products of conception after the elective pregnancy termination. The date of the last menstrual period in the current pregnancy was not reliable/accurate. A transvaginal ultrasonographic scan of the uterine cavity found non-homogenous masses resembling PHM with focally increased vascularisation. Furthermore, a possible irregular gestational sac corresponding to 7–8 gestational weeks was detected. There were no signs of an embryo. Vacuum evacuation of the uterine cavity was performed. One part of the tissue evacuated from the uterine cavity was fixed in formaldehyde for histopathologic analysis, while the other part was collected in normal saline for DNA analysis. Also, two millilitres of peripheral blood of the patient were collected in EDTA for DNA analysis. The sample in physiological solution/normal saline was immediately transported to the laboratory (the same work day) and dissected under a binocular loupe (Fig. 1c). Chorionic villi were collected, cleaned and stored at –20°C before DNA isolation.
The histopathological profile highly suggested PHM. Avascular, oedematous hydropic chorionic villi with a variable degree of trophoblast hyperplasia were detected. Positivity of p57 in the cytotrophoblast excluded a complete hydatidiform mole.
DNA was isolated from chorionic villi and peripheral blood using DNeasy Blood & Tissue Kit (QIAGEN). STR genotyping was performed with a quantitative fluorescence polymerase chain reaction (QF-PCR) technique using the commercial kit GenePrint 10 System. Comparing the STR alleles of the chorionic villi and of the patient, a triandric monogynic tetraploid composition of the genome of the products of conception was detected confirming the diagnosis of PHM (Fig. 2). According to the Amelogenin locus (sex determining marker), the gonosomal complement of the products of conception was XXXY (Fig. 2). Archived paraffin blocks from the previous electively terminated pregnancy were analysed to exclude the presence of PHM. DNA was isolated from chorionic villi using the QIAamp DNA FFPE Tissue Kit (QIAGEN) and STR genotyping was performed. Allelic profiles of PHM, the patient and the sample from the elective pregnancy termination were compared. STR analysis of the terminated pregnancy identified a monoandric monogynic diploid (biparental-physiologic) genome composition and a gonosomal complement XX, which is clearly distinct from the allelic profile of PHM. These results proved that PHM represents a new pregnancy after an elective pregnancy termination. Histopathological re-evaluation of the electively terminated pregnancy also did not reveal any signs of a hydatidiform mole.
Image 1. Mechanism of fertilisation and morphology of the presented tetraploid partial hydatidiform mole (Created with BioRender.com). a) Fertilisation of the ovum by three sperms.b) Zygote containing three paternal and one maternal sets of chromosomes.c) Dissected – cleaned chorionic villi before DNA isolation under binocular loupe showing marked hydrops and absence of vascularisation (Photographed at Institute of Medical Biology, Genetics and Clinical Genetics, Faculty of Medicine, Comenius University Bratislava). 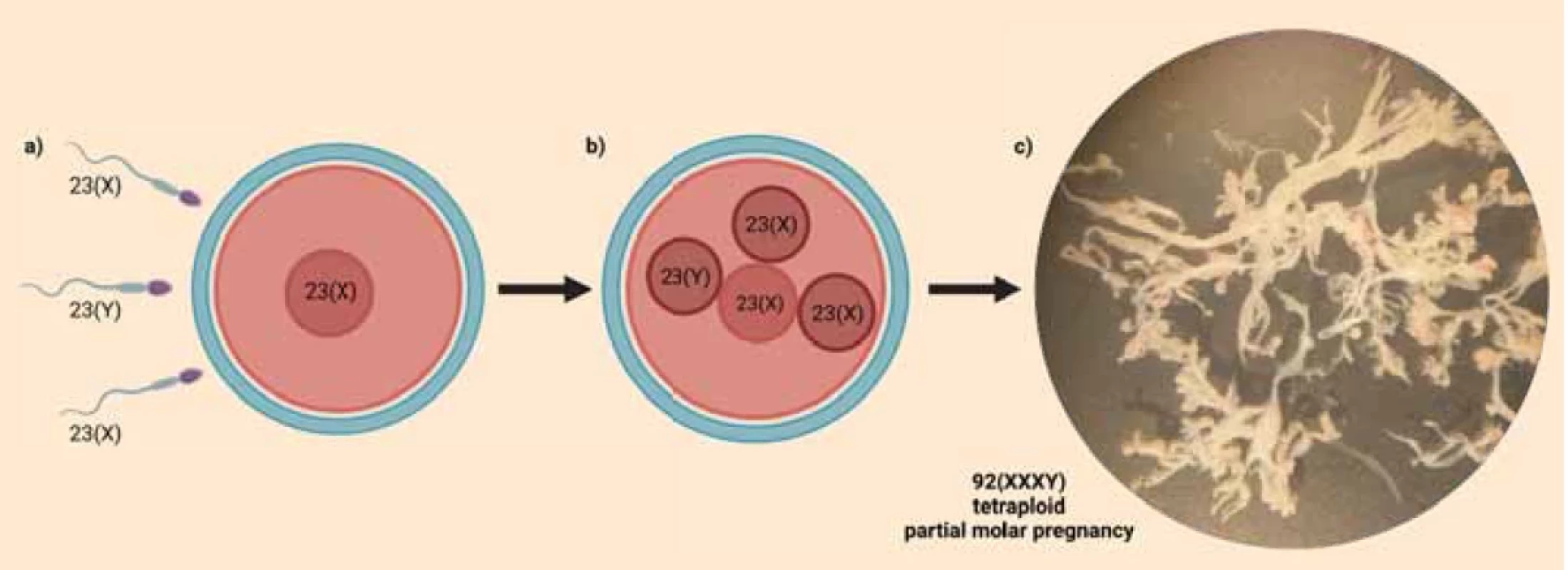
Obr. 1. Mechanizmus oplodnenia a morfológia prezentovanej tetraploidnej parciálnej hydatidóznej moly (Vytvorené pomocou BioRender.com).a) Oplodnenie vajíčka tromi spermiami.b) Zygota obsahujúca tri paternálne a jednu maternálnu sadu chromozómov.c) Vypreparované – očistené choriové klky pred izoláciou DNA pod binokulárnou lupou s výrazným hydropsom a absenciou vaskularizácie (Fotografované na Ústave lekárskej biológie, genetiky a klinickej genetiky Lekárskej fakulty Univerzity Komenského v Bratislave). Discussion
In some rare cases, like in our presented case, PHMs arise from a zygote containing three paternal and one maternal sets of chromosomes (Fig. 1b). The result of STR genotyping of a tetraploid PHM, proving the triandric monogynic tetraploid genome composition, was first published in 2012 by Murphy et al. [5]. Although STR genotyping is helpful in distinguishing between paternal and maternal alleles, when there are three paternal chromosome sets, it is not possible to determine whether they originate from three different sperms. This is because a healthy man has typically up to two different alleles for one STR marker. That’s why the exact mechanism of fertilisation in these cases was long unknown until Bynum et al. in 2020 published their study, where the Single-Nucleotide Polymorphism (SNP) array technique was used to define different paternal chromosomal contributions. They found out, that tetraploid PHMs arise when three different sperm fertilize a single, normal ovum [1].
STR genotyping is a helpful tool in the differential diagnosis of PHM, where the ultrasonographic and histopathologic profile is often not specific enough to provide a definitive diagnosis [6,7]. According to our experiences, the ideal material for DNA analysis of PHM are fresh-frozen unfixed chorionic villi (because formalin fixation leads to DNA fragmentation and decreases the chances for successful analysis). In our case, STR genotyping was essential to confirm the diagnosis of PHM, and to prove the origin of molar pregnancy [8].
Image 2. Results of STR genotyping of the partial mole.Loci proving the presence of three paternal (nonmaternal) alleles (♂) and one maternal allele (♀) in the partial hydatidiform mole, which points to a triandric monogynic tetraploid genome composition. The parental origin of the alleles was determined by comparison with STR genotyping results of the blood of the patient. Amelogenin (AMEL) locus proves the presence of three X and one Y chromosomes in the partial mole. 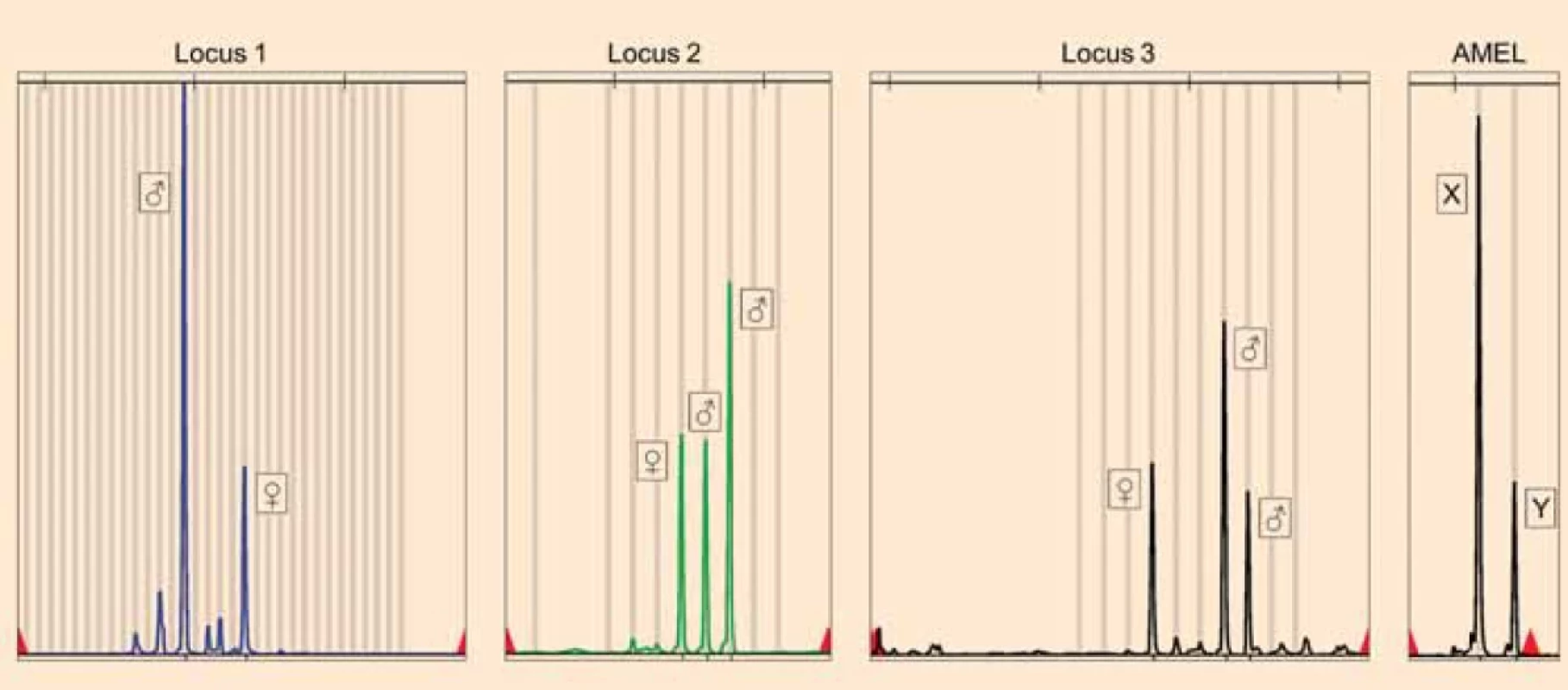
Obr. 2. Výsledky STR genotypizácie parciálnej moly. Lokusy dokazujúce prítomnosť troch paternálnych (nonmaternálnych) alel (♂) a jednej maternálnej alely (♀) v parciálnej hydatidóznej mole, čo poukazuje na triandrickú monogynickú tetraploidnú kompozíciu genómu. Parentálny pôvod alel bol určený porovnaním s výsledkami STR genotypizácie krvi pacientky. Amelogenínový (AMEL) lokus dokazuje prítomnosť troch chromozómov X a jedného chromozómu Y v parciálnej mole. Conclusion
STR genotyping represents a helpful tool with the potential of confirming or excluding the diagnosis of PHM. The rare finding of a triandric monogynic tetraploid genome composition with this method in cases of suspected PHM confirms the diagnosis of PHM and indicates the need for adequate follow up. The presented case highlights the importance of centralized management of patients with gestational trophoblastic disease to ensure the active cooperation of gynaecologists, pathologists and geneticists familiar with this group of diseases. The ideal way to collect bio logical material for yielding high quality DNA for STR genotyping is storing the bio psies in normal saline right after evacuation of the uterine cavity.
ORCID authors
L. Gergely 0000-0003-3407-7914
M. Korbeľ 0000-0002-5773-2371
V. Repiská 0000-0003-1907-8468
L. McCullough 0009-0002-9237-7472
P. Priščáková 0000-0001-7287-3837
Submitted/Doručené: 13. 7. 2023
Accepted/Prijaté: 28. 7. 2023Lajos Gergely, MD
Institute of Medical Biology
Genetics and Clinical Genetics
Faculty of Medicine
Comenius University Bratislava
Sasinkova 4
811 08 Bratislava
Slovak Republic
lajos.gergely@fmed.uniba.sk
Sources
1. Bynum J, Batista D, Xian R et al. Tetraploid partial hydatidiform moles: molecular genotyping and determination of parental contributions. J Mol Dia gn 2020; 22 (1): 90–100. doi: 10.1016/j.jmoldx.2019.09.006.
2. Xing D, Adams E, Huang J et al. Refined dia g – nosis of hydatidiform moles with p57 immunohistochemistry and molecular genotyping: updated analysis of a prospective series of 2217 cases. Mod Pathol 2021; 34 (5): 961–982. doi: 10.1038/s41379-020-00691-9.
3. Heřman J, Rob L, Robová H et al. Histopathological and clinical features of molar pregnancy. Ceska Gynekol 2019; 84 (6): 418–424.
4. Meng Y, Yang X, Yin H. Application of short tandem repeat (STR) genotyping in partial hydatidiform mole. Am J Transl Res 2023; 15 (5): 3731–3738.
5. Murphy KM, Descipio C, Wagenfuehr J et al. Tetraploid partial hydatidiform mole: a case report and review of the literature. Int J Gynecol Pathol 2012; 31 (1): 73–79. doi: 10.1097/PGP.0b 013e31822555b3.
6. Fisher RA, Maher GJ. Genetics of gestational trophoblastic disease. Best Pract Res Clin Obstet Gynaecol 2021; 74 : 29–41. doi: 10.1016/ j.bpobgyn.2021.01.004.
7. Zavadil M, Feyereisl J, Krofta L et al. New dia g nostic approach to different hydatidiform mole types, hydropic abortions and relevant clinical management. Ceska Gynekol 2009; 74 (3): 177–182.
8. Gergely L, Gbelcová H, Repiská V et al. Importance of the genetics in the diagnostics of hydatidiform mole. Ceska Gynekol 2020; 85 (4): 275–281.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2023 Issue 6-
All articles in this issue
- Je rozdíl mezi akutní appendicitou u těhotných a netěhotných žen?
- Jsou sérový neutrofilní delta index a systémový zánětlivý index užitečné jako prediktivní parametry preeklampsie a HELLP syndromu?
- Umělé ukončení těhotenství farmakologickou metodou (UUT-F) v I. trimestru – význam stanovení lidského choriového gonadotropinu a ultrazvukového vyšetření při diagnostice těhotenství a hodnocení výsledku UUT-F
- Indikace a výsledky ukončení těhotenství před 24. týdnem těhotenství – série případů
- Nový pohľad na diagnostiku a liečbu idiopatickej granulomatóznej mastitídy
- Ojedinělý případ živého abdominálního těhotenství u ženy s následnou graviditou v děloze
- Triandrická monogynická tetraploidia ako výsledok DNA analýzy parciálnej moly hydatidózy
- Výsledok pacientky s Herlyn-Werner-Wunderlichovým syndrómom liečenej balónikovou septostómiou – pred- a pooperačný ultrazvukový nález
- Metody a techniky ochrany reprodukce u pacientek s endometriózou
- Asistovaná aktivace oocytů
- Vliv stavu pánevního dna na výsledek operačního řešení sestupu pánevních orgánů
- Preeklampsie a diabetes mellitus
- Diagnostika komplikovaných gynekologických zánětů pomocí výpočetní tomografie – zkušenosti jednoho centra
- Emergentní hysterektomie pro spektrum placenta accreta – některé další techniky
- Diagnostika a léčba endometriózy: Doporučený postup Sekce pro léčbu endometriózy ČGPS ČLS JEP
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Diagnostika a léčba endometriózy: Doporučený postup Sekce pro léčbu endometriózy ČGPS ČLS JEP
- Preeklampsie a diabetes mellitus
- Asistovaná aktivace oocytů
- Je rozdíl mezi akutní appendicitou u těhotných a netěhotných žen?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

