-
Medical journals
- Career
Laparoscopic and robotic sacropexy: retrospective review of learning curve experiences and follow-up
Authors: R. Pilka; D. Gágyor; M. Študentová; D. Neubert; P. Dzvinčuk
Authors‘ workplace: Olomouc, Head Prof. MUDr. Radovan Pilka, Ph. D. ; Department of Obstetrics and Gynecology, Medical Faculty Palacký University and Faculty Hospital
Published in: Ceska Gynekol 2017; 82(4): 261-267
Overview
Objective:
To compare conventional laparoscopic (LSC) and robotic (RSC) sacrocolpopexy in the treatment of apical pelvic prolapse during robotic surgery „learning curve“. Operative characteristics, prolapse treatment outcomes, and postoperative results were assessed.Design:
Retrospective comparative study.Setting:
Department of Obstetrics and Gynecology, University Hospital and Palacky University, Olomouc.Methods:
We analyzed consecutive 51 patients treated with laparoscopic sacropexy and 13 women operated with robotic system. Data on patient age, body mass index (BMI), operation history, estimated blood loss, operation time, surgical outcomes (including pelvic organ prolapse quantification – POP-Q), and concomitant surgeries were retrospectively obtained from patient medical records. Subjective outcomes were measured through PGI-I and PISQ-IR questionaires when available at last follow up (n = 26).Results:
In both groups all procedures were performed correctly without conversion. The mean operative time was longer in robotic group: 212 (128–394) min, as compared to 164 (80–342) in the laparoscopic group. Blood loss was lower for the robotic 52 (10–200) ml compared to laparoscopic group 58 (10–350) ml. Differences in operative time and blood loss were not statistically significant. Differences between LSC and RSC groups in postoperative results were not statistically significant. Learning curve for robotic sacrocolpopexy was shorter than for laparoscopic procedure in case of experienced laparoscopic surgeons. No recurrences occurred during follow-up. Most patients were satisfied with surgical results.Conclusion:
The present study demonstrated that RSC may be comparable in surgical safety and efficacy. The decision regarding the best surgical approach has to be individualised according to the characteristics of the patient and their preferences as well as the local clinical setting and the surgical expertise of physicians.Keywords:
apical pelvic prolapse, robotic surgery, sacropexy, laparoscopyINTRODUCTION
Pelvic organ prolapse (POP) is a common, troublesome, and costly problem in women. with prevalence of 3–6% or up to 50% when based upon symptoms and vaginal examination respectively [2]. The apical prolapse has always been considered the most complex of the defects of the pelvic floor, for both the difficulty of the surgical corrective technique and for the high post-surgical recurrence rate. Historically, abdominal sacrocolpopexy since its introduction in 1962 has been considered the criterion standard for the repair of POP [9]. However, this approach has some shortcomings related to the use of laparotomy, such as a high morbidity rate and long hospital stay. In an effort, to overcome these concerns, laparoscopic sacrocolpopexy (LSC) was introduced in 1994 by Nezhat, integrating the success rates of open conventional surgery with the advantages of minimally invasive approach [12]. Today, the LSC can be considered the standard treatment for apical prolapse. However, LSC is technically more chalenging because it requires surgical skills to perform suturing with knot tying, the learning curve required to achieve expertise in this procedure has been for many limiting and operative time for LSC increased when compared to open surgery.
Robotic sacrocolpopexy (RSC) was first described by Di Marco in 2004 and since its introduction RSC has emerged as a possible alternative to a conventional laparoscopic technique [7]. Possible advantages of robot include the 3D-HD vision system, the use of a dedicated console and instruments with great flexibility and precision of the movements, which allows the surgeon being minimally invasive and having shorter learning curve. However, despite the fact that RSC has been rapidly incorporated into clinical practice, some questions regarding the efficacy, cost, training, and adoption when comparing LSC and RSC still need to be answered [6, 16, 18].
Although a few studies on sacrocolpopexy have been performed in Czech republic, data on laparoscopic versus robotic sacrocolpopexy in Czech patients have not been reported to date [11]. Therefore, the aim of this study was to compare LSC and RSC at one institution during learning curve and to examine the surgical outcomes and safety of these procedures in patients with apical prolapse.
MATERIAL AND METHODS
This retrospective study was conducted on patients with symptomatic stage II or greater uterine/vaginal vault prolapse who underwent laparoscopic or robotic sacropexy between October 2006 and January 2017 at University Hospital Palacky University Olomouc. We analyzed consecutive 51 patients treated with laparoscopic sacropexy and 13 women operated with robotic system. All procedures have been done by the same team experienced in laparoscopic gynecologic surgery with appropriate training and mentoring in robotic surgery.
Data on patient age, body mass index (BMI), operation history, estimated blood loss, operation time, surgical outcomes, and perioperative complications were retrospectively obtained from patient medical records. Each patient underwent a standard preoperative protocol with the collection of demographic data, careful gynecological and uro-gynecological evaluation with determination of functional symptoms (presence or absence of urinary stress incontinence), classification of the degree of severity of the prolapse using the POP-Q system. Operative time (OT) was defined as the time from initial incision to closure in both laparoscopic and robotic group. A standardized physical examination including pelvic organ prolapse quantification (POP-Q) and subjective outcomes measured through patient satisfaction by the PGI-I and PISQ-IR questionaires were performed when available at last follow up (n = 26). The retrospective nature of the present study precluded a standardized follow-up protocol in all patients. Typically, patients are seen roughly six weeks after surgery for postoperative evaluation, and then followed on an as needed basis (through office visits and written or telephone correspondence).
Robotic approach
All robotic procedures were performed using DaVinci S Surgical System (Intuitive Surgical Inc., Sunnyvale, California, USA). Patients were placed in a dorsal lithotomy and steep Trendelenburg position. A Foley catheter was placed, pneumoperitoneum was created, and 5 ports were placed (one 12-mm trocar for the camera port above the umbilicus, three 8-mm trocars for the robotic arms, and one 12-mm trocar for the assistant for suction, irrigation, retraction of tissues and mesh placement). The colon can be reflected laterally using a bowel retraction suture on the left abdominal wall as described by Burgess and Elliott [3]. The sacral promontory was then exposed with care taken to avoid the middle sacral artery. A vaginal retractor was used to push up the vaginal wall anteriorly, and then, the peritoneum of the uterovesical junction was dissected. For bladder dissection we identified the balloon of the Foley catheter. The posterior peritoneum was opened and the front surface of the promontory dissected to expose the anterior longitudinal ligament. A peritoneal dissection between the posterior uterus and vagina was performed to carry out peritoneal tunneling at the lateral level of the broad ligament opening. Then we placed a non-absorbable polypropylene Y mesh. Both branches of the mesh were attached to the anterior and posterior vagina surfaces with prolene. Then the prosthesis was passed through the avascular portion of the broad ligament. Then the long branch of the mesh was fixed to the sacral promontory with one or two long-term absorbable sutures. Concomitant surgeries, including hysterectomy (total or supracervical), posterior repair, and retropubic synthetic midurethral slings were performed when indicated.
Laparoscopic approach
In these procedures, we placed a 10-mm trocar at the umbilicus for the camera and in surapubic region and two other 5-mm trocars at each lower quadrant of the abdomen. The surgeon was on the left side of the patient and the first assistant on the right side. The surgical technique was the same used in robotic approach.
Statistic analysis
Mean and standard deviations were computed for continuous data. The Wilcoxon rank sum test was used to examine the association between changes before and after surgical treatment. The Mann-Whitney U-test was used to evaluate differences in surgical outcomes between laparoscopic and robotic group results. Statistical analyses were carried out using the IBM SPSS Statistics 23. All statistical tests were two-sided, with a p-value < 0.05 considered statistically significant.
RESULTS
The demographic and anamnestic characteristics of the two groups are described in Table 1.
1. Baseline characteristics 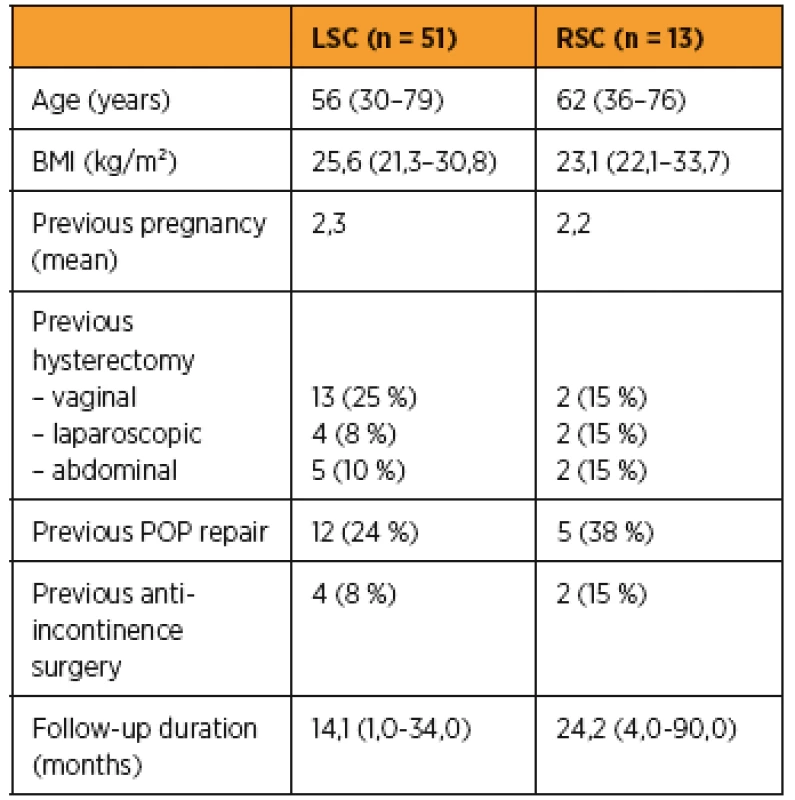
BMI – body mass index, POP – pelvic organ prolapse, LSC – laparoscopic sacrocolpopexy, RSC – robotic sacrocolpopexy 3For laparoscopic sacropexy group the mean age was 56 years (range 30–79), mean BMI was 25,6 (21,3–30,8), no woman was nulliparous; 43% of them had previous hysterectomy and 24% previous POP repair. Four patients (8%) had previously undergone midurethral sling surgery. In the RSC group the mean age was 62 years (range 36–76); mean BMI was 23,1 (22,1–33,7), no woman was nulliparous; 46% of them had previous hysterectomy and 38% previous POP repair. Two patients (15%) had previously undergone midurethral sling surgery.
A comparison of surgical outcome is presented in Table 2. In both groups all procedures were performed correctly without conversion. The mean operative time was longer in robotic group: 212 (128–394) min compared to 164 (80–342) in the laparoscopic group. Blood loss was lower for the robotic 52 (10–200) ml compared to laparoscopic group 58 (10–350) ml. Differences in operative time and blood loss were not statistically significant (Fig 1). The mean follow up duration was 14,1 (1,0–34,0) months and 24,2 (4,0–90,0) months in LSC and RSC groups respectively. Treatment results were assessed in 19 patients from LSC and 6 patients from RSC group who were examined at follow-up and who completed the PGI-I and PISQ-IR questionaires.
2. Surgical outcomes 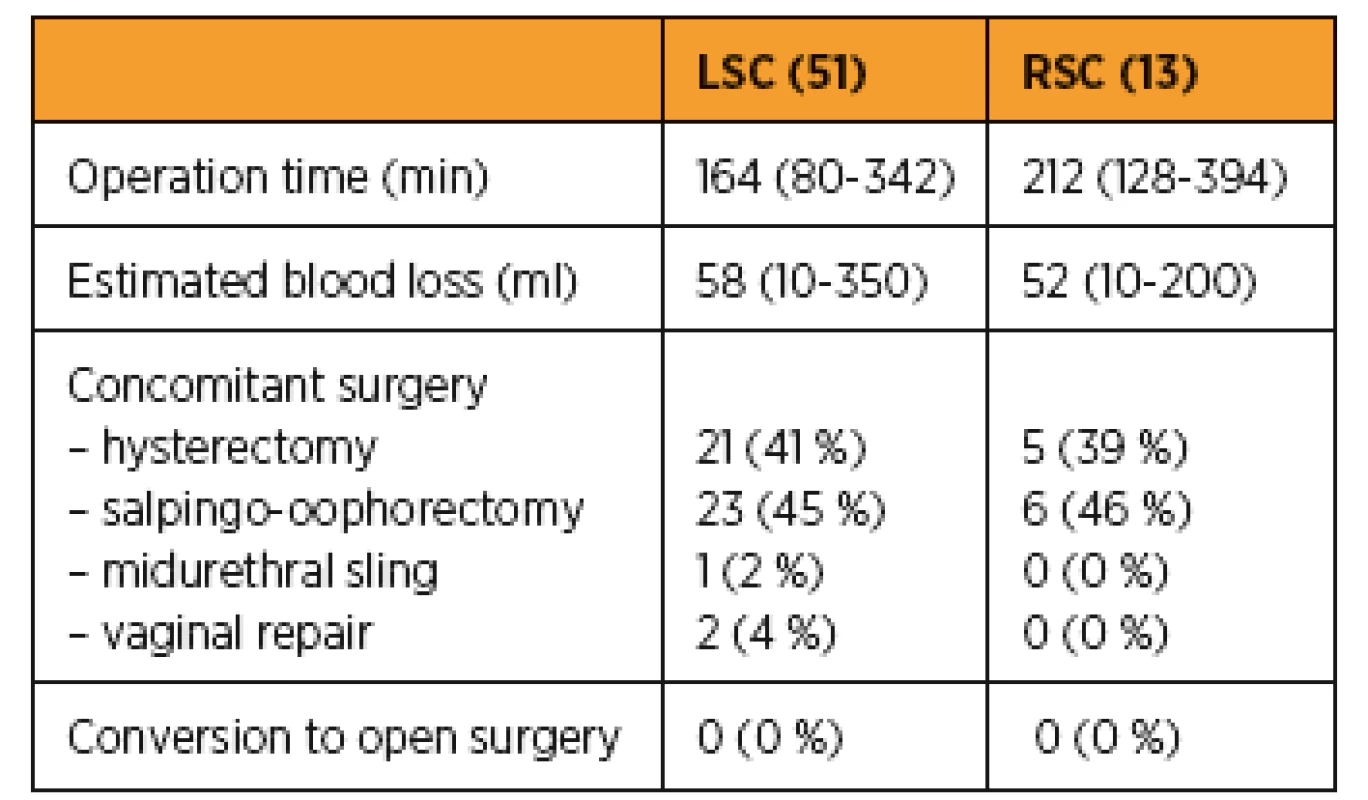
LSC – laparoscopic sacrocolpopexy, RSC – robotic sacrocolpopexy Fig. 1 Laparoscopic and robotic sacropexy, operative time of consecutive procedures 
As shown in Table 3 and 4, improvements in apical prolapse were assessed by POP-Q examination. In LSC group there were statistically significant improvements in anterior and posterior wall prolapse as well as apical prolapse (with the exception for Ap, all p-values < 0.05), (preoperative C point [mean ± SD (range); median], [1,79 ± 2,89 (-2–7); 1,5]; postoperative C point [-3,61 ± 3,49 (-7–6); -5]; P = 0.001). In RSC group there were statistically significant improvements in posterior wall as well as apical prolapse (preoperative C point [mean ± SD (range); median], [1,33 ± 3,76 (-5–6); 1,5]; postoperative C point -3,25 ± 1,47 (-5 to -1); -3,75]; p = 0.046) and trend in anterior wall prolapse improvement. Differences between LSC and RSC groups in postoperative results were not stastically significant, as shown in Tab 5. No recurrences occurred during follow-up. Results of the PGI-I showed that patients in the LSC as well as RSC groups were very satisfied (mean value 2 and 1,5 in LSC and RSC group respectively). However, one patient in LSC group reported that she was “very dissatisfied” with the treatment, although she had grade 0 apical prolapse at the final follow-up. She had presented with rectocele, which she preferred to treat conservatively. According to PISQ-IR results, 11 out of 19 patients (58%) in LSC group and 3 out of 6 patients (50%) in RSC group were sexually active during the follow-up.
3. Measurement of pelvic organ prolapse by using pelvic organ prolapse quantification examination in LSC group (n = 19) 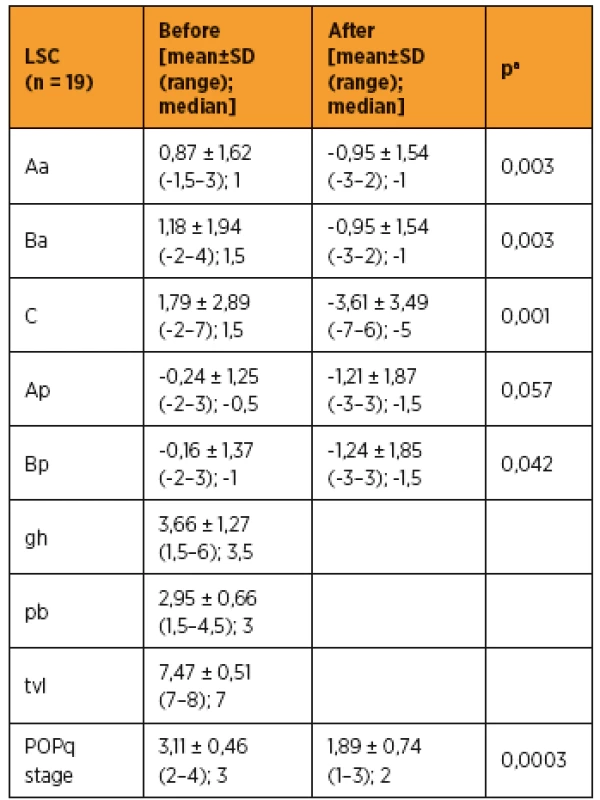
aWilcoxon test, LSC – laparoscopic sacrocolpopexy 4. Measurement of pelvic organ prolapse by using pelvic organ prolapse quantification examination in RSC group (n = 6) 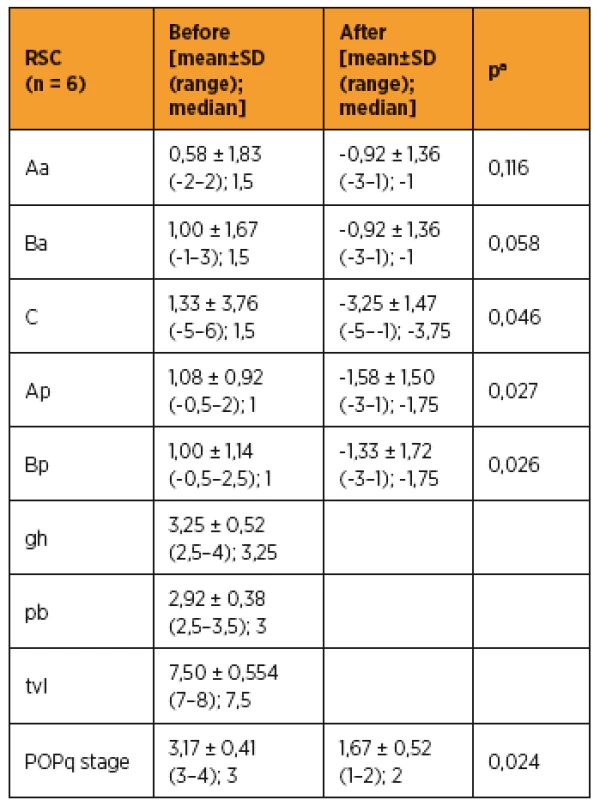
aWilcoxon test, RSC – robotic sacrocolpopexy 5. Differences between LSC and RSC groups in postoperative measurement of pelvic organ prolapse by using pelvic organ prolapse quantification examination 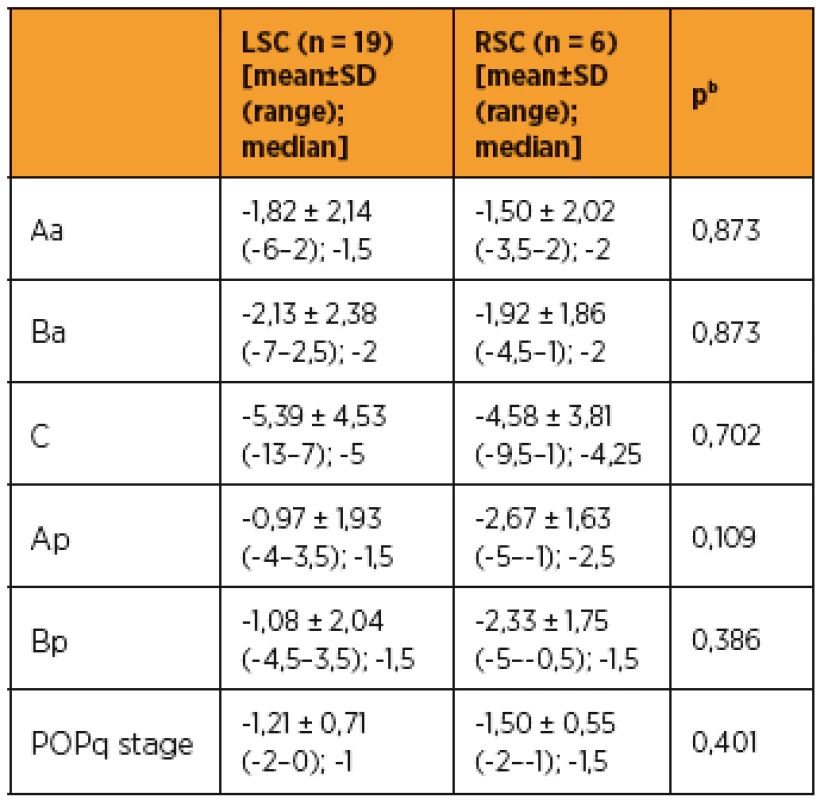
bMann-Whitney U-test DISCUSSION
We sought to compare the operative experiences for LSC and RSC, procedures newly introduced to the operating rooms at our academic institution and evaluate the learning curve for both types of surgeries. The present study demonstrated that RSC is an effective, reliable, and safe method for apical prolapse repair. This study showed that the mean time taken to perform surgery was longer in the robotic, although statistically not significant, but the anatomical and functional results were the same of a laparoscopic approach. Cucinella et al., in their case control study have seen significant differences (p < 0.001) in operative time which was longer in robotic group. This was explained by the relative lack of experience of the surgical team [5]. When OT (from incision to closure) were assessed in a systematic review and metaanalysis, three papers [1–7, 9, 11, 12, 15, 16, 18] found no significant differences between RSC and LSC, whereas in the other 3 [1, 4, 15]. OT were shorter with LSC (all p values < 0.001) [6]. At our institution both surgeons performing sacrocolpopexy had experience in advanced laparoscopic as well as robotic surgery, possibly resulting in similar operative times in LSC and RSC goups.
Evaluation of the learning curve (Fig 1) for LSC and RSC operative times demonstrates that after first ten cases done either by laparoscopy or robot the OT times did not differ. The variability in individual OT reflected complexity of surgery as a function of concomitant procedures. Pulliam et al., observed dramatic flattening of the slope of the curve for robotic setup after 8 to 10 cases, whereas the laparoscopoic curve did not change over time, suggesting the RSC setup was a new proces [15].
The intraoperative blood loss in the robotic approach was lower than in laparoscopic group, although the difference was not statistically significant. Interestingly, the present blood loss estimates reflect the total blood loss for the entire operation inluding concomitant surgeries, and the estimates are not a true reflection of sacrocolpopexy as such. De Gouveia De Sa et al., in metaanalytic study found EBL to be significantly less with RSC. However, they report that sensitivity analyses do not support this result and it was noted that concomitant hysterectomies could potentially have an influence on these dissimilarities [6]. It is not possible to draw conclusions with the regard to this outcome with the evidence available thus far.
In the current study, one woman underwent midurethral sling surgery at the time of LSC, and eight out of all 63 (12,5%) patients with sacrocolpopexy negative preoperative stress testing had de novo stress incontinence. De novo stress incontinence after sacrocolpopexy remains controversial, although recent studies have reported an overall de novo stress incontinence rate of about 15%–30% in patients with negative preoperative stress testing [8, 10].
There was no difference between postoperative POP-Q values between groups. At the last follow up successful outcome measures based on POP-Q scores were reported in all patients. This may be attributable to the similar surgical techniques of sacrocolpopexy in both LSC and RSC groups. Similar surgical results reported Pulliama et al,. in their retrospective comparative study [15]. Paraiso et al. published a randomized controlled trial that included 78 women with stage 2–4 POP-Q for vaginal prolapse after hysterectomy, in order to compare robotic sacral colpopexy with laparoscopic technique [14]. They concluded that RSC is a reproducible technique, although safety and effectiveness have yet to be proven. These results correspond to systematic reviews where the recurrence rate also did not differ between groups [6, 18].
Using the validated PGI-I questionaire, the subjective outcomes of LSC and RSC were assessed. Most patients were satisfied with the operation, the mean results of PGI-I questionaires after surgery were ranked as „much better“ or „very much better“. Only 1 woman who was dissatisfied with the operation presented with rectocele which she preferred to treat conservatively. These coincide with the results of an RCT comparing robotic and laparoscopic hysterectomy and showing comparable postoperative quality of life [19].
CONCLUSION
The present study demonstrated that RSC may be comparable in surgical safety to LSC according to midterm follow-up data. Limitations of the current study include its retrospective nature and small size of groups. The learning curve of the RSC appears to be shorter than that of LSC, especially for experienced laparoscopic surgeons. Rather than replacing conventional laparoscopy, robot assisted laparoscopy can be advantageous for the purpose of mastering pure laparoscopic skills. The decision regarding the best surgical approach has to be individualised according to the characteristics of the patient and their preferences as well as the local clinical setting and the surgical expertise of physicians.
Podpořeno MZ ČR – RVO (FNOl, 00098892).
Prof. MUDr. Radovan Pilka, Ph.D.
Porodnicko-gynekologická klinika
FN a LF UP
I. P. Pavlova 6
779 00 Olomouc
e-mail: radovan.pilka@fnol.cz
Sources
1. Anger, JT., et al. Robotic compared with laparoscopic sacrocolpopexy: a randomized controlled trial. Obstet Gynecol, 2014, 123(1), p. 5–12.
2. Barber, MD., Maher, C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J, 2013, 24(11), p. 1783–1790.
3. Burgess, KL., Elliott, DS. Robotic/Laparoscopic prolapse repair and the role of hysteropexy: a urology perspective. Urol Clin North Am, 2012, 39(3), p. 349–360.
4. Chan, SS., et al. Laparoscopic sacrocolpopexy for the treatment of vaginal vault prolapse: with or without robotic assistance. Hong Kong Med J, 2011, 17(1), p. 54–60.
5. Cucinella, G., et al. Robotic versus laparoscopic sacrocolpopexy for apical prolapse: a case-control study. G Chir, 2016, 37(3), p. 113–117.
6. De Gouveia De Sa, M., et al. Robotic versus laparoscopic sacrocolpopexy for treatment of prolapse of the apical segment of the vagina: a systematic review and meta-analysis. Int Urogynecol J, 2016, 27(3), p. 355–366.
7. Di Marco, DS., et al. Robotic-assisted laparoscopic sacrocolpopexy for treatment of vaginal vault prolapse. Urology, 2004, 63(2), p. 373–376.
8. El Hamamsy, D., Fayyad, AM. New onset stress urinary incontinence following laparoscopic sacrocolpopexy and its relation to anatomical outcomes. Int Urogynecol J, 2015, 26(7), p. 1041–1045.
9. Lane, FE. Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol, 1962, 20, p. 72–77.
10. LeClaire, EL., et al. Is de novo stress incontinence after sacrocolpopexy related to anatomical changes and surgical approach? Int Urogynecol J, 2014, 25(9), p. 1201–1206.
11. Maskulikova, Z., et al. [Robotic sacrocolpopexy – two case reports and literature overview]. Ceska Gynekol, 2015, 80(5), p. 372–377.
12. Nezhat, CH., Nezhat, F., Nezhat, C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol, 1994, 84(5), p. 885–888.
13. Nosti, PA., et al. Outcomes of abdominal and minimally invasive sacrocolpopexy: a retrospective cohort study. Female Pelvic Med Reconstr Surg, 2014, 20(1), p. 33–37.
14. Paraiso, MF., et al. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol, 2011, 118(5), p. 1005–1013.
15. Pulliam, SJ., Weinstein, MM., Wakamatsu, MM. Minimally invasive apical sacropexy: a retrospective review of laparoscopic and robotic operating room experiences. Female Pelvic Med Reconstr Surg, 2012, 18(2), p. 122–126.
16. Reza, M., et al. Meta-analysis of observational studies on the safety and effectiveness of robotic gynaecological surgery. Br J Surg, 2010, 97(12), p. 1772–1783.
17. Sarlos, D., et al. Robotic compared with conventional laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol, 2012, 120(3), p. 604–611.
18. Serati, M., et al. Robot-assisted sacrocolpopexy for pelvic organ prolapse: a systematic review and meta-analysis of comparative studies. Eur Urol, 2014, 66(2), p. 303–318.
19. Tan-Kim, J., et al. Robotic-assisted and laparoscopic sacrocolpopexy: comparing operative times, costs and outcomes. Female Pelvic Med Reconstr Surg, 2011, 17(1), p. 44–49.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2017 Issue 4-
All articles in this issue
- Reconstruction of the posterior and central compartment with the Prolift Posterior: cohort study with a 5-year follow-up
- The results of five years follow-up prospective study of vaginal prolapse repaired by prolift total mesh surgery or sacrospinous fixation
- Fertility preserving methods in women with breast cancer before gonadotoxic therapy
- Current trends in gamete donation – psychosocial and ethical issues
- A case report of peritoneal tuberculosis: the diagnostic role of PET/CT and laparoscopy
- Catamenial pneumothorax – case reports and literature review
- Suburethral sling removal due to chronic pain
- Large hematoma – early postoperative complication after the transobturator tape procedure
- Tubo-ovarian abscess in the 39th week of pregnancy (case report)
- Awareness of the methods of primary and secondary childbirth trauma prevention among parturients
- Laparoscopic and robotic sacropexy: retrospective review of learning curve experiences and follow-up
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Catamenial pneumothorax – case reports and literature review
- Large hematoma – early postoperative complication after the transobturator tape procedure
- Suburethral sling removal due to chronic pain
- Laparoscopic and robotic sacropexy: retrospective review of learning curve experiences and follow-up
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

