-
Medical journals
- Career
The effect of erythropoietin on uterus inflammation during ischemia reperfusion injury in rats
Authors: C. Tsompos 1; C. Panoulis 2; K. Toutouzas 3; A. Triantafyllou 4; G. Zografos 3; A. Papalois 5
Authors‘ workplace: Department of Obstetrics & Gynecology, Messolonghi County Hospital, Etoloakarnania, Greece 1; Department of Obstetrics & Gynecology, Aretaieion Hospital, Athens University, Attiki, Greece 2; Department of Surgery, Ippokrateion General Hospital, Athens University, Attiki, Greece 3; Department of Biologic Chemistry, Athens University Medical School, Attiki, Greece 4; Exprerimental Research Center ELPEN Pharmaceuticals, S. A. Inc., Co., Pikermi, Attiki, Greece 5
Published in: Ceska Gynekol 2016; 81(5): 342-348
Overview
Objective:
This experiment evaluated the influence of erythropoietin (Epo) in an animal model of uterine ischemia reperfusion using the quoting established protocol.Design:
The effects of erythropoietin treatment were evaluated by mean uterus inflammation (UI) lesions. UI lesions were determined at the 60th reperfusion min (for groups A and C) and at the 120th reperfusion min (for groups B and D). Groups A and B received no drugs, whereas rats from groups C and D were administered with erythropoietin.Methods:
40 rats of mean mass 247.7 g were employed for the study.Results:
Epo administration non-significantly decreased the UI scores [without lesions] by 0.1 [-0.6244129 - 0.4244129] (p = 0.6294)). Reperfusion time kept non-significantly increased the UI scores by [without lesions] 0.15 [-0.60230385 - 0.50230385] (p = 0.5782). Together, Epo administration combined with reperfusion time non-significantly decreased the UI scores by [without lesions] 0.0727273 [-0.3886782 - 0.2432236] (p = 0.6439).Conclusions:
Epo administration whether it interacted or not with reperfusion time non-significantly short-term decreased the UI lesions scores. Perhaps, a longer study time than two hours or a higher Epo dose may provide more significant effects.Keywords:
ischemia, erythropoietin, uterus inflammation, reperfusionINTRODUCTION
Serious impacts may be induced on organs and adjacent tissues by ischemia and reperfusion (IR). Erythropoietin (Epo) administration in IR remains a challenge for many years. Despite the significant progress, several practical questions have not been clarified. They include: a) how potent Epo should be b) when should it be administered and c) at what optimal dose Epo should be administered. The promising effect of Epo has been tried in several IR studies. A meta-analysis of 25 published seric variables, coming from the same experimental setting, tried to provide a numeric evaluation of the Epo efficacy at the same endpoints (Table 1). Several publications tested similar molecules of growth factors to which the studied molecule Epo also belongs to. This experimental study tried to evaluate the effect of Epo in a rat setting of IR using mean uterus inflammation (UI) lesions.
1. The erythropoietin (Epo) influence (±SD) on the levels of some seric variables concerning reperfusion (rep) time (Tsompos et al, Tsompos et al) 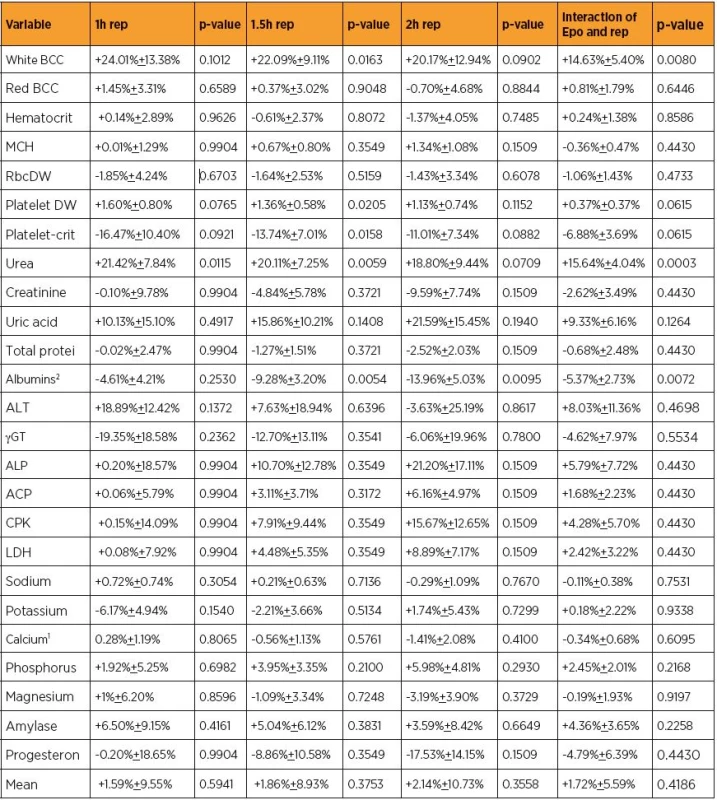
MATERIALS AND METHODS
Animal preparation
This basic experimental research was licensed by Veterinary Address of East Attiki Prefecture [No 3693l|/12-11 - 2010 & 14l|/10-1-2012 decisions]. All consumables, substances and equipment used, were a grant of ELPEN Pharmaceuticals Co. Inc. S.A. at Pikermi, Attiki. Accepted standards of human animal care were adopted for Albino female Wistar rats. Normal pre-experimental housing included 7 days ad libitum diet in laboratory. Post-experimental awakening and preservation of animals was not permitted even if euthanasia was needed. Rats were randomly delivered to the four groups by 10 rodent in each one, using following protocols of IR: 45 min ischemia followed by 60 min reperfusion (group A); 45 min ischemia followed by 120 min reperfusion (group B); 45 min ischemia followed by 60 min immediate Epo (Epoetin, Recombinant Human Erythropoetin Alfa, Janssen-Cilag, Beerse, Belgium) intravenous (IV) administration and reperfusion (group C); 45 min ischemia followed by 120 min immediate Epo IV administration and reperfusion (group D). The dose of Epo was 10 mg/Kg body mass of animals. Prenarcosis preceded of continuous intra-experimental general anesthesia; oxygen supply; electrocardiogram and acidometry providing [23, 24]. The protocol of IR was followed. Ischemia was induced by laparotomic clamping inferior aorta over renal arteries with forceps for 45 min. Reperfusion was established by removing the clamp and securing inferior aorta patency. Epo was administered starting reperfusion; through inferior vena cava catheter. The UI lesions were determined at 60th min of reperfusion (for A and C groups) and at 120th min of reperfusion (for B and D groups). Fourty female Wistar albino rats were used (mean mass 231.875 g, Standard Deviation [SD]: 36.59703 g], with minimum mass 165g and maximum mass 320 g. Rats‘ mass could be potentially a confusing factor, e.g. more obese rats to have more or less UI lesions scores. This assumption was also investigated. Also, detailed pathological study and grading of UI findings was performed by scores, this is: 0 lesions were not found, 1 mild lesions were found, 2 moderate lesions were found and 3 serious lesions were found [15]. The previous grading is transformed as follows: (0–0.499) without lesions, (0.5–1.499) mild lesions, (1.5–2.499) moderate lesions and (2.5–3) serious lesions damage, because the study concerns score ranges rather than point scores. UI scores were measured by 1st Department of Pathology at Department of Clinical - Laboratory studies in Faculty of Medicine of Athens University.
Model description of ischemia – reperfusion injury
Control groups
20 control rats (mean mass 252.5 g [SD: 39.31988 g]) experienced ischemia lasting 45 min followed by reperfusion.
Group A
Reperfusion lasted 60 min (n=10 controls rats) mean mass 243 g [SD: 45.77724 g], mean moderate UI score 1.5 [SD: 0.7071068] (Table 2).
2. Weight and uterus inflammation (UI) score mean levels and Std. Dev. of groups 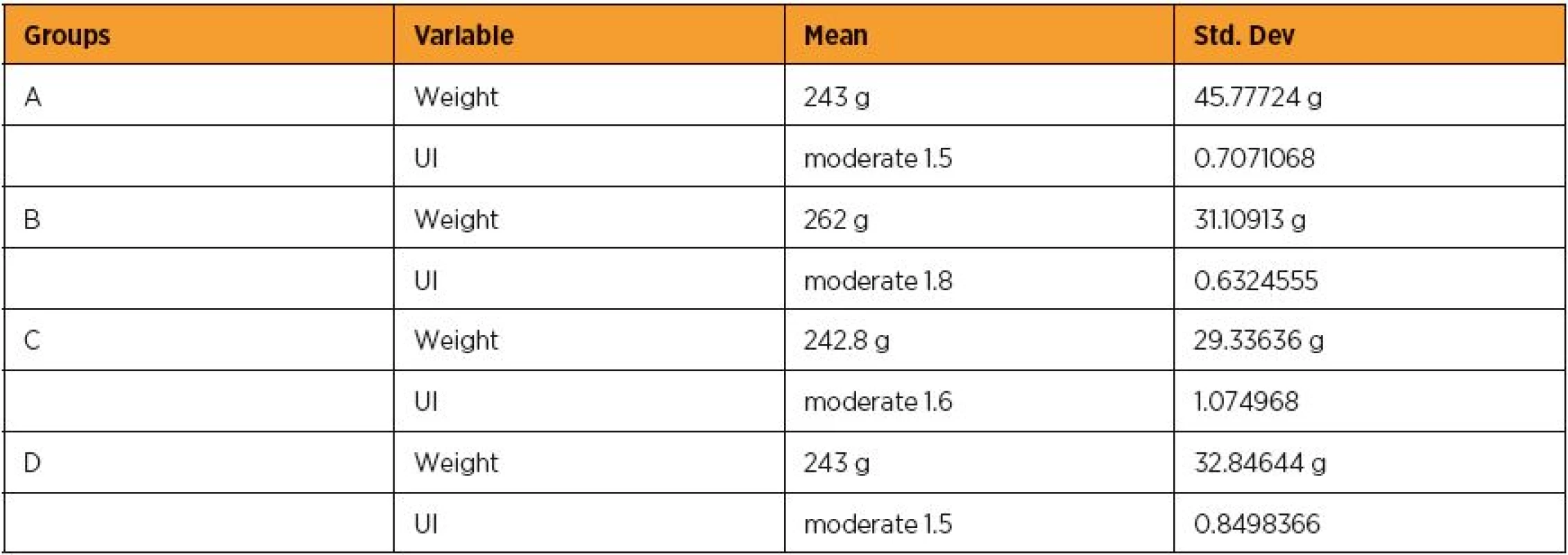
Group B
Reperfusion lasted 120 min (n=10 controls rats) mean mass 262 g [SD: 31.10913 g], mean moderate UI score 1.8 [SD: 0.6324555] (Table 2).
Erythropoietin group
20 Epo rats (mean mass 242.9 g [SD: 30.3105 g]) experienced ischemia lasting 45 min followed by reperfusion on the commencement of which 10 mg Epol|/kg body weight were IV administered.
Group C
Reperfusion lasted 60 min (n=10 Epo rats) mean mass 242.8 g [SD: 29.33636 g], mean moderate UI score 1.6 [SD: 1.074968] (Table 2).
Group D
Reperfusion lasted 120 min (n=10 Epo rats) mean mass 243 g [SD: 32.84644 g], mean moderate UI score 1.5 [SD: 0.8498366] (table 2).
Statistical analysis
Every mass group was compared with each other by statistical standard t-test. Along, every UI lesions scores group was compared with each other by statistical Wilcoxon signed-rank test (Table 3). Any probable significant difference among UI scores, was investigated whether was due to potent significant mass correlation. The application of generalized linear models (glm) with dependant variable the UI scores was followed. The 3 independent variables were the Epo or no drug administration, the reperfusion time and both variables in combination. The statistical analysis was performed by Stata 6.0 software [Stata 6.0, StataCorp LP, Texas, USA].
3. Statistical significance of mean values difference for groups (DG) after statistical standard t test application for weight and Wilcoxon signed-rank test for scores 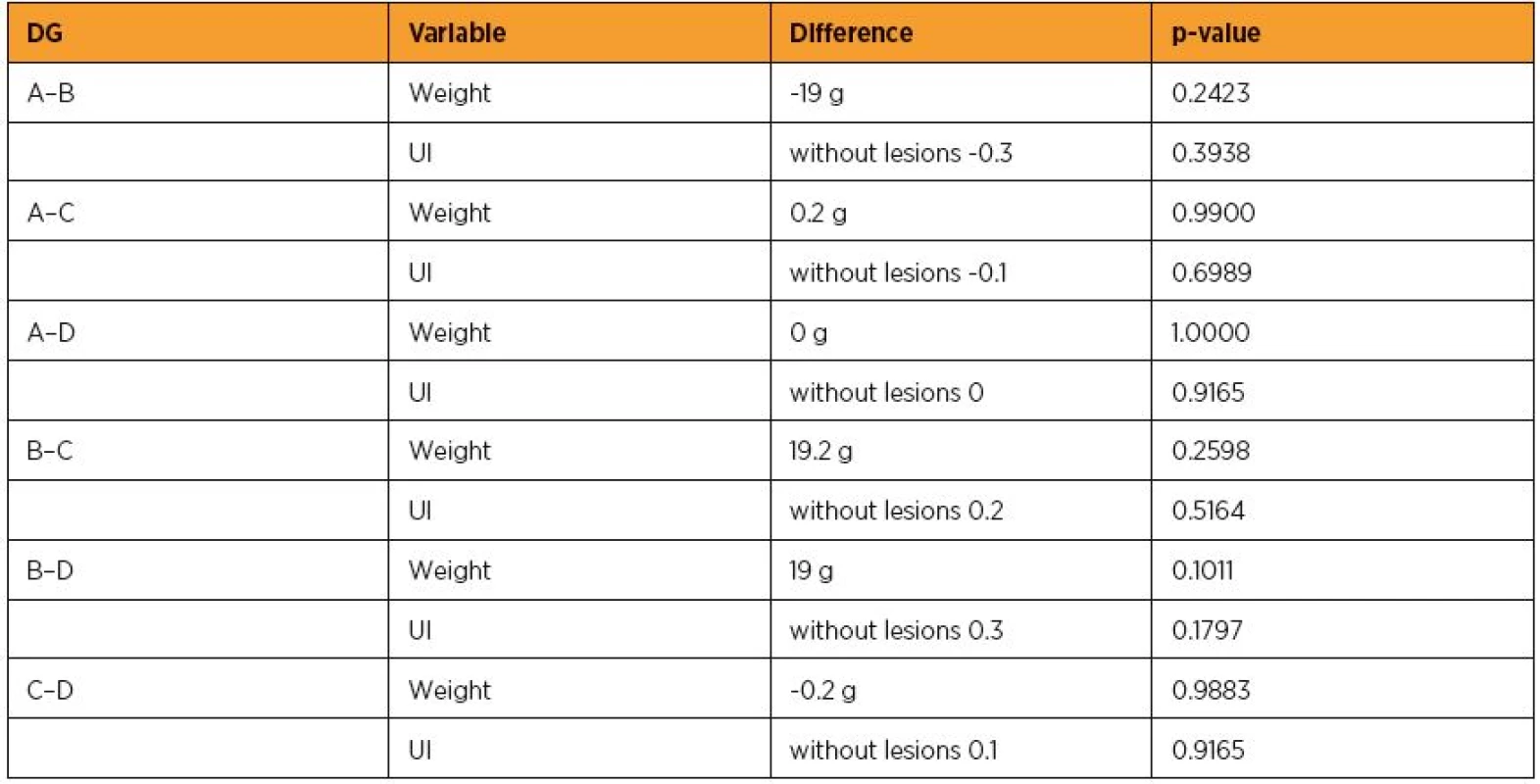
RESULTS
Epo administration possesed a trend to non-significantly decrease the UI scores by [without lesions] 0.1 [-0.6244129 – 0.4244129] (p=0.7016). This finding was crepant with the results of Wilcoxon signed-rank test (p=0.5573). Reperfusion time non-significantly increased the UI scores by [without lesions] 0.1 [-0.4244129 – 0.6244129] (p=0.7016), approximately in accordance with the Wilcoxon signed-rank test result [without lesions] 0.2 [-0.7801948 – 0.3801948] (p=0.4548). However, Epo administration and reperfusion time in combination non-significantly decreased the UI scores by [without lesions] 0.0727273 [-0.3886782 – 0.2432236] (p=0.6439). The above and table 3 are summed in tables 4 and 5. Considering the masses of the animals as independent variable at glm, a non significant association with UI lesions scores was revealed (p=0.6382), suggesting that further investigation was not needed.
4. The alteration influence of erythropoietin in connection with reperfusion time p-values 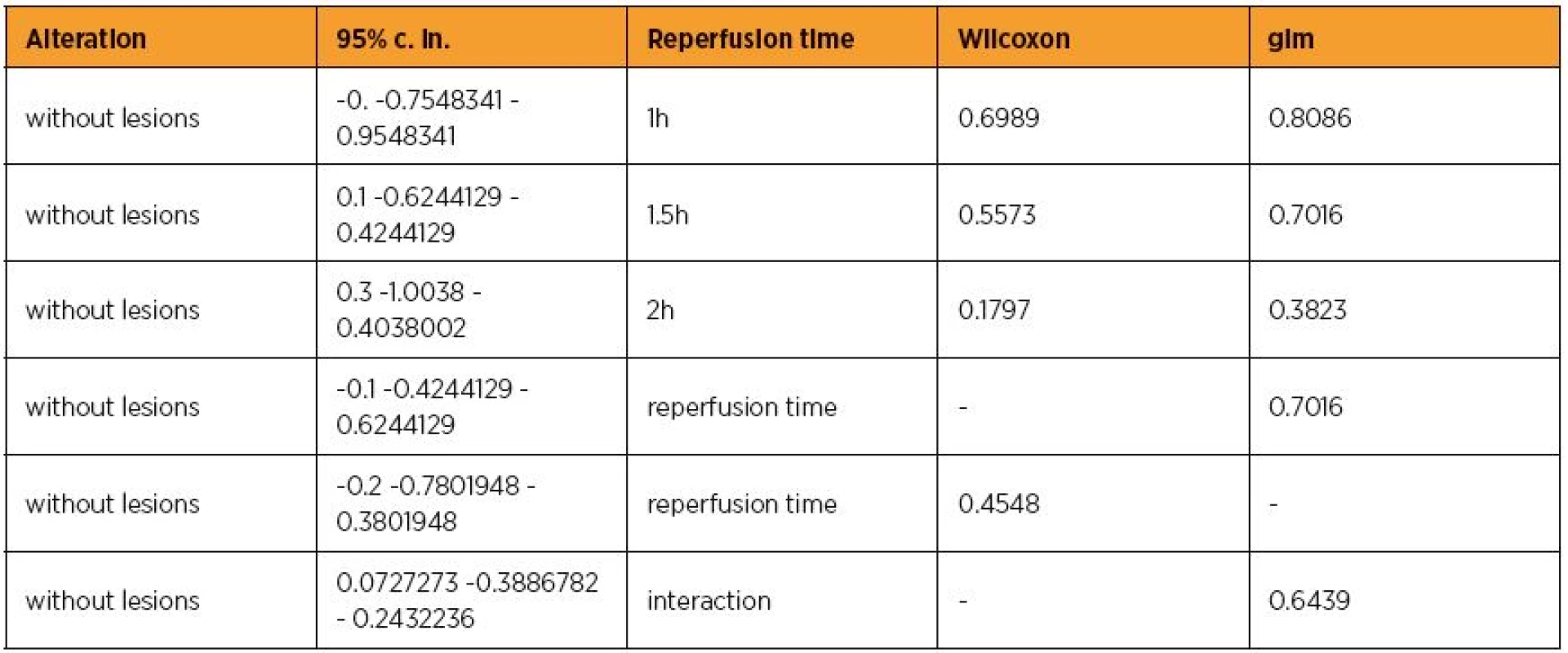
5. Synoptic presence of the alteration influence of erythropoietin in connection with reperfusion time 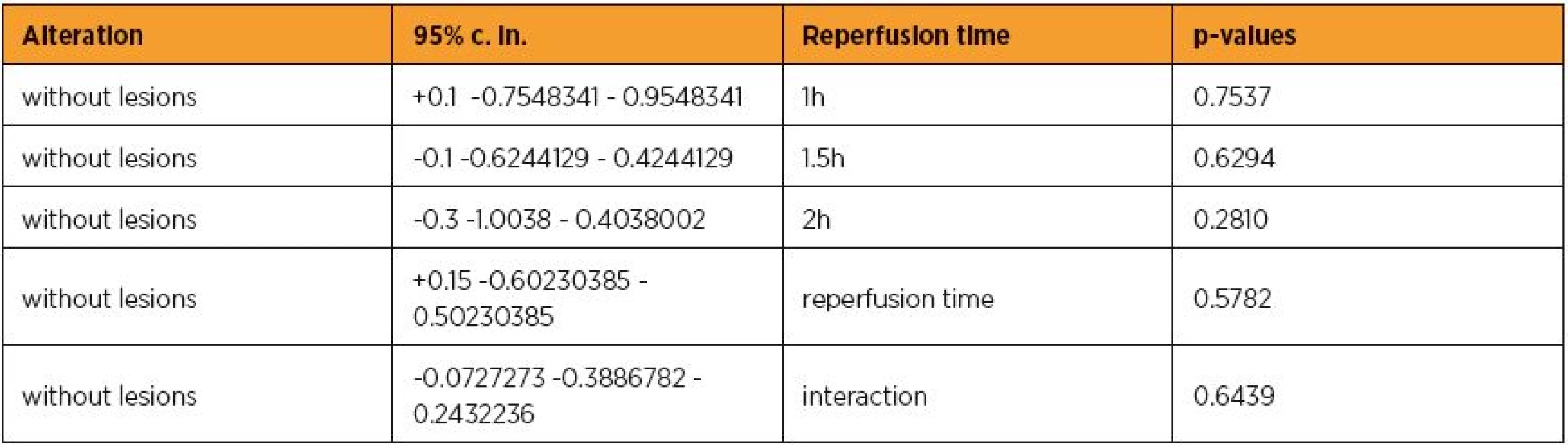
DISCUSSION
UI is part of a complex biological response of adjacent tissues to harmful stimuli, as pathogens, damaged cells, or irritants [8]. General chronic UI might lead the host to diseases, such as hay fever, rheumatoid arthritis and even cancer (as it happens e.g. on cholecystitis for gallbladder carcinoma). UI can be classified as either acute or chronic. Acute inflammation is the initial response to harmful stimuli and is achieved by the increased movement of plasma and leukocytes (especially granulocytes) from blood into the injured uterine tissues. Sequent biochemical events propagate and mature the inflammatory response, involving the local vascular system, the immune system, and various cells within the injured tissue. Prolonged chronic inflammation, leads to a progressive shift in the type of cells present at the site of inflammation and is characterized by simultaneous destruction and healing of uterine tissues. Acute inflammation is a short-term process, usually appearing within a few minutes or hours and ceasing upon the removal of the injurious stimulus [4, 16]. The five signs appear when acute inflammation occurs, whereas acute UI may not result in the full set [13, 18]. Pain happens only where the appropriate sensory nerve endings exist in the inflammed area-e.g., acute UI does not pain unless the inflammation access either the serosa or the myometrium, which have pain-sensitive nerve endings. Acute inflammation is initiated by cells already present in uterine tissues, mainly resident macrophages, histiocytes, and mastocytes. These cells present on their uterine surfaces certain receptors named pattern recognition receptors (PRRs). PRRs recognize molecules owned by pathogens but distinguishable from host molecules, collectively referred to as pathogen-associated molecular patterns (PAMPs). At the onset of inflammation, these cells undergo activation (one of their PRRs recognize a PAMP) and release inflammatory mediators responsible for the above mentioned clinical signs of inflammation.
Mediators such as released bradykinin increase the sensitivity to pain (hyperalgesia, dolor) [25]. The extravasated neutrophils migrate along a chemotactic gradient created by the local cells to reach the site of injury. The loss of function (functio laesa) is perhaps the result of a neurological reflex in response to pain. Furthermore, along several acellular biochemical cascade systems consisting of preformed plasma proteins act to initiate and establish the inflammatory response. These include the complement, coagulation and fibrinolysis systems activated by necrosis, e.g. a burn, trauma or bacteria [10]. Specific patterns of acute or chronic UI are seen arising in uterus, such as when surface inflammation occurs on endothelium or at medulla: granulomatous inflammation, serous inflammation, ulcerative inflammation, but mainly fibrinous or purulent inflammation. Fibrinous inflammation results when much fibrin passed through the blood vessels. If an appropriate procoagulative stimulus exists, such as cancer cells, a fibrinous exudate is deposited. This is commonly seen in serous surfaces, where the conversion of fibrinous exudate into a scar can occur between serous membranes [12]. The deposit sometimes forms adhesions. During UI, uterine synechiae can be formed. During inflammation of the perimetrium, pelvic adhesions can be formed. Purulent inflammation resulting in large amount of pus. It consists of neutrophils, dead cells, and fluid. Infection by pyogenic bacteria such as staphylococci is characterized by this kind of inflammation. Large, localised collections of pus enclosed by surrounding tissues are called abscesses. During neglected vaginal tampons, streptococcal toxic shock syndrome can be formed [3]. Inflammatory diseases are a large group of disorders that underlie a vast variety of gynecologic pathology. Non-immune diseases with etiological origins in inflammatory processes may include cancer and local ischemic disease. Examples of disorders associated with UI include: interstitial cystitis, autoimmune diseases, vasculitis, autoinflammatory diseases, transplant rejection, rheumatoid arthritis, celiac disease, glomerulonephritis, reperfusion injury, hypersensitivities and pelvic inflammatory disease.
Pelvic cancer pain was classified at visceral, neuropathic and somatic according to the cause [17]. Visceral pain is the result of smooth muscles spasms of hallow viscus; distortion of solid organs capsule; inflammation; chemical irritation; traction or twisting of mesentery, ischemia or necrosis and encroachment of pelvis and presacral tumors.
The inflammatory response is terminated when no longer needed to prevent unnecessary „bystander“ damage to uterine tissues. Failure to do so results in chronic UI and cellular destruction. Resolution of inflammation varies in different tissues. Mechanisms that serve to terminate inflammation include: short half-life of inflammatory mediators in vivo, production and release of transforming growth factor (TGF)β, interleukin 10 (IL-10), lipoxins, from macrophages, interleukin 1 receptor antagonist or the soluble tumor necrosis factor receptor (TNFR), downregulation of pro-inflammatory molecules such as leukotrienes and receptor activity by high concentrations of ligands, apoptosis of pro-inflammatory cells,desensitization of receptors, increased survival of cells in regions of inflammation due to their interaction with the extracellular matrix (ECM) cleavage of chemokines by matrix metalloproteinases (MMPs) might lead to production of anti-inflammatory factors, production of resolvins, protectins or maresins [1, 2, 5, 6, 9, 11, 14, 19, 20, 22, 24].
Ω-3 polyunsaturated fatty acids, resolvins and protectins critically shorten the period of neutrophil infiltration by initiating apoptosis. Consequently, apoptotic neutrophils undergo phagocytosis by macrophages, leading to neutrophil clearance and release of anti-inflammatory and reparative cytokines such as transforming growth factor-β1. The anti-inflammatory phase finishes with the departure of macrophages through the lymphatics [21]. The outcome is individualized in any circumstance up to the layer in which the injury has occurred and the injurious agent that caused it. The possible outcomes to UI are: resolution, fibrosis, abscess formation or chronic inflammation. However, assuming some anti inflammatory capacity of Epo, whether it prevents both arachidonic acid-induced and iron-dependent lipid peroxidation, its administration will significantly decrease the UI lesions. Thus, inflammation is associated with Epo in different tissues. Epo as member of the cytokine superfamily has significant homology with mediators of growth and inflammation [7].
Results from studies have shown that Epo and its receptor are widely expressed in embryonic, fetal and adult tissues, including the lung, central nervous system, gonads, gut, pancreas, kidney, muscle (eg, smooth, skeletal, and heart), retina and uterus.
CONCLUSION
Epo administration whether it interacted or not with reperfusion time non-significantly short-term decreased the UI lesions scores. Perhaps, a longer study time than 2 hours or a higher Epo dose, may provide more significant effects.
Acknowledgment
This study was funded by Scholarship by the Experimental Research Center ELPEN Pharmaceuticals (E.R.C.E), Athens, Greece. The research facilities for this project were provided by the aforementioned institution.
Tsompos Constantinos, Consultant B
Department of Obstetrics & Gynecology
Mesologi County Hospital
Nafpaktou street
Mesologi 30200
Etoloakarnania
Greece
e-mail: Tsomposconstantinos@gmail.com
Sources
1. Ashcroft, GS. Bidirectional regulation of macrophage function by TGF-β. Microbes Infect, 1999, 1 (15), p. 1275–1282.
2. Ashcroft, GS., Gillian S., Yang Xiao, et al. Mice lacking smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol, 1999, 1 (5), p. 260–266.
3. Braun, M., Kietzmann, M. Ischemia reperfusion injury in the isolated hemoperfused bovine uterus--a model for the investigation of anti-inflammatory substances? ALTEX, 2004, 21, Suppl. 3, p. 49–56.
4. Cotran, RS., Kumar, V., Collins, T. Robbins pathologic basis of disease. 1998 Philadelphia: W.B. Saunders Company.
5. Dudley, DJ. Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol, 1999 Jan, 180(1 Pt 3), p. S251–256.
6. Eming, SA., Krieg, T., Davidson, JM. Inflammation in wound repair: molecular and cellular mechanisms. J Investigative Dermatol, 2007, 127(3), p. 514–525.
7. Erbayraktar, S., Yilmaz, O., Gökmen, N., et al. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep, 2003, 2(6), p. 465–470.
8. Ferrero-Miliani, L., Nielsen, OH., Andersen, PS., et al. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol, 2007, 147 (2), 061127015327006.
9. Greenhalgh, DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol, 1998, 30 (9), p. 1019–1030.
10. Hutton, LC., Castillo-Melendez, M., Walker, DW. Uteroplacental inflammation results in blood brain barrier breakdown, increased activated caspase 3 and lipid peroxidation in the late gestation ovine fetal cerebellum. Dev Neurosci, 2007, 29(4-5), p. 341–354.
11. Jiang, D., Jiurong, F., Juan, Y., et al. Regulation of lung injury and repair by toll-like receptors and hyaluronan. 2005, Nat Med, 11(11), p. 1173–1179.
12. Kannan, S., Saadani-Makki, F., Muzik, O., et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med, 2007, 48(6), p. 946–954.
13. Li, J., LaMarca, B., Reckelhoff, JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol, 2012, 303(1), p.H1–8.
14. McQuibban, GA., Tam, EM., McCulloch, CA., et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science, 2000, 289(5482), p. 1202–1206.
15. Osmanağaoğlu, MA., Kesim, M., Yuluğ, E., et al. Ovarian-protective effects of clotrimazole on ovarian ischemia/reperfusion injury in a rat ovarian-torsion model. Gynecol Obstet Invest, 2012, 74(2), p.,125–130.
16. Parakrama, C., Clive, R., Taylor. Part A. General Pathology, Section II. The host response to injury, Chapter 3. The acute inflammatory response, sub-section cardinal clinical signs. Concise Pathology. 3. ed. New York, McGraw-Hill, 2005.
17. Rigor, BM, Sr. Pelvic cancer pain. J Surg Oncol, 2000, 75(4), p. 280–300.
18. Ruth, W. A massage therapist guide to pathology 2009 (4th ed.). Philadelphia, PA and Baltimore, MD: Wolters Kluwer.
19. Sato, Y., Ohshima, T., Kondo, T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun, 1999, 265 (1), p. 194–199.
20. Serhan, CN. Controlling the resolution of acute inflammation: a new genus of dual anti-inflammatory and proresolving mediators. J Periodontol, 2008, 79(8 Suppl.), p. 1520–1526.
21. Serhan, CN., Savill, J., Savill, P. Resolution of inflammation: the beginning programs the end. Nat Immunol, 2005, 6(12), p. 1191–1197.
22. Teder, P., Jiang, D., Liang, J., et al. Resolution of lung inflammation by CD44. Science, 2002, 296(5565), p. 155–158.
23. Tsompos, C., Panoulis, C., Toutouzas, K., et al. The effect of erythropoietin on calcium levels during hypoxia reoxygenation injury in rats. Geriatric Care, 2016, 2,5722, p. 12–16.
24. Tsompos, C., Panoulis, C., Toutouzas, K., et al. The effect of erythropoietin on albumins levels during hypoxia reoxygenation injury in rats. Res J Pharmacol Toxicol, 2015, 1(3), p. 42–45.
24. Werner, F., Feinberg, MW., Sibinga, NE., et al. Transforming growth factor-β1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem, 2000, 275(47), p. 36653–36658.
25. Wranning, CA., Dahm-Kähler, P., Mölne, J., et al. Transplantation of the uterus in the sheep: oxidative stress and reperfusion injury after short-time cold storage. Fertil Steril, 2008, 90(3), p. 817–826.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicine
Article was published inCzech Gynaecology

2016 Issue 5-
All articles in this issue
- Ultrasound comparison of adjustable single-incision (Ajust) and transobturator tapes to assess post-operative fixation and function and clinical efficacy
- Histopathological changes in placental tissue with connection to chosen clinical cases in obstetrics
- Sexual morbidity of cervical carcinoma survivors
- Postpartum blues – a Czech adaptation of the Maternity Blues Questionnaire
- Current knowledge about HPV infection
- Peripheral precocious puberty
- Nonculture techniques of microorganisms determination in amniotic fluid in patients with preterm prelabor rupture of membranes
- Acute myocardial infarction complicated pregnancy of patient after kindey transplantation and knee osteosarcoma
- Acute uterine inversion after delivery
- The effect of erythropoietin on uterus inflammation during ischemia reperfusion injury in rats
- Czech Gynaecology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Peripheral precocious puberty
- Current knowledge about HPV infection
- Sexual morbidity of cervical carcinoma survivors
- Acute uterine inversion after delivery
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

