-
Medical journals
- Career
Innovative strategies for treating retinal diseases
Authors: Z. Straňák 1*; B. Kousal 2,3*; T. Ardan 4; M. Veith 1
Authors‘ workplace: Oftalmologická klinika, 3. lékařská fakulta, Univerzita Karlova a Fakultní nemocnice Královské Vinohrady Přednosta: doc. MUDr. Pavel Studený, Ph. D., MHA 1; Oční klinika, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze Přednostka: prof. MUDr. Jarmila Heissigerová, Ph. D., MBA 2; Klinika dětského a dorostového lékařství, 1. lékařská fakulta, Univerzita Karlova a Všeobecná fakultní nemocnice v Praze. Přednosta: prof. MUDr. Tomáš Honzík, Ph. D. 3; Centrum PIGMOD, Ústav živočišné fyziologie a genetiky, Akademie věd České republiky. Ředitel centra: prof. MVDr. Jan Motlík, DrSc. 4
Published in: Čes. a slov. Oftal., 75, 2019, No. 6, p. 287-295
Category: Review Article
doi: https://doi.org/10.31348/2019/6/1Overview
Objective: The aim of this comprehensive paper is to acquaint the readers with innovative approaches in the treatment of retinal diseases, which could in the coming years to get into clinical practice. Retinal prostheses, retinal pigment epithelial (RPE) transplantation, gene therapy and optogenetics will be described in this paper.
Methodology: Describing the basic characteristics and mechanisms of different types of therapy and subsequently literary minireview clarifying the current state of knowledge in the area.
Results: Retinal prostheses, RPE transplantation, gene therapy and optogenetics offer yet unexplored possibilities and are considered as the future of treatment of retinal diseases where classical pharmacotherapy or surgical treatment are no longer sufficient. However, all these methods challenge not only in the innovative technical implementation itself, but also for the ethical, administrative and economic demands.
Conclusion: There will be certainly interesting development in the treatment of retinal diseases, but it is not possible to fully estimate which modality of treatment will be dominant in the future.
Keywords:
gene therapy – optogenetics – induced pluripotent cells – retinal prostheses
INTRODUCTION
The retina, due to its unique position and good accessibility for examination is a very popular target for innovative procedures, primarily of gene therapy, optogenetics, cell therapy and bionics. In the following text we shall present the principles of functioning of these therapeutic modalities and an overview of the current state of knowledge, using selected examples.
OBJECTIVE
To acquaint readers with innovative approaches in the treatment of retinal diseases. The article will describe retinal prostheses, transplantation of retinal pigment epithelium (RPE), gene therapy and optogenetics.
METHODS
Literary research on the given theme, with emphasis on the mechanisms of functioning of individual therapeutic modalities and ongoing clinical trials.
RESULTS
Cell therapy
Age related macular degeneration (ARMD), Stargardt’s disease or for example retinitis pigmentosa. Although these are different clinical pathologies, in all of these cases we find damage to the retinal pigment epithelium (RPE) [5]. This concerns a single layer localised on the Bruch’s membrane, which is responsible for maintaining homeostasis of the photoreceptors. Despite considerable advances in pharmacotherapy of retinal pathologies (e.g. vascular endothelial growth factor inhibitors), in many patients attempts have failed to stabilise the condition of patients with affliction of the RPE. As a result, with regard to the substantial advances in cell therapy, methods of RPE transplantation are coming to the forefront. In recent years several research centres have focused on these procedures, and their proof-of-concept evidence is generally accepted.
The first isolation of the RPE was conducted almost fourteen years ago [12], and so the fundamental characteristics of the cells are now well known. Unlike other retinal cells, in the case of the RPE synaptic connection is not required for it to perform its function, and as a result and due to the easy possibility of examining the cells, for example with the aid of optical coherence tomography (OCT), RPE transplantation has become an attractive target for cell therapy.
At the beginning of the study there was an attempt to perform an autologous transplantation (e.g. autologous RPE-choroidal graft or subretinal injection of a suspension of autologous RPE cells) or macular translocation. Despite the fact that some authors have described a partial improvement of visual functions [30], in clinical practice these procedures are not widespread due to the high incidence of adverse complications (haemorrhage, proliferative vitreoretinopathy, retinal detachment, distorsion of image in macular translocation, poor apicobasal orientation in suspensions of cells) [16,21,40].
Thanks to advances in surgical techniques and cellular biology, we now have the option of transplanting also other than autologous cells. In the literature we most often encounter human embryonic stem cells (hESC-RPE) [32] and human induced pluripotent stem cells (iPSC-RPE) [6]. We obtain embryonic cells after the fertilisation of an oocyte by sampling from a blastocyte, whereas we obtain induced pluripotent cells by reprogramming of a somatic cell, typically a fibroblast [36]. In 2012 the Nobel Prize was awarded for this discovery (Shinya Yamanaka, Sir John B. Gurdon). The resulting product of both methods is a pluripotent cell, which we can then direct toward development in the RPE. The main advantage upon use of iPSC-RPE is that it concerns an autologous source, and as a result it is not necessary to burden the patient with immune suppression. On the other hand, a disadvantage is the age of the cell (the source cell is the same age as the patient from whom it is taken), the same genetic makeup (persistence of any applicable genetic abnormalities) and the as yet uncertain teratogenic potential (lack of long-term data) [20]. The use of hESC-RPE is an advantage above all if it is desirable to transplant cells with a different genetic basis (genetically conditioned pathology, including mitochondrial), though naturally immune suppression is necessary. The main differences between iPSC and hESC are summarised by fig. 1 and table 1.
1. Basic differences between embryonic stem cells and induced pluripotent stem cells (19) 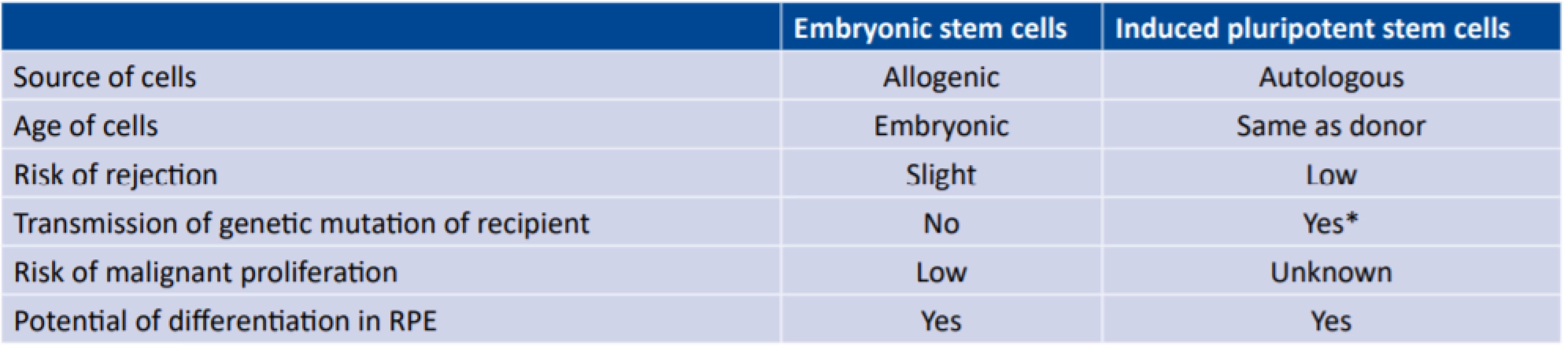
* in the case of autologous transplantation of induced pluripotent stem cells RPE – retinal pigment epithelium 1. Basic differences between cells of retinal pigment epithelium (RPE) from embryonic stem cells and induced pluripotent stem cells. Whereas we obtain induced pluripotent stem cells from somatic cells, typically mesenchymal cells from the skin of the donor (in the case of autologous transplantation the donor is also the recipient), we take embryonic cells from a blastocyte after fertilisation of the oocyte. Then we cultivate the pluripotent cells with the aid of appropriate growth mediators in a direction toward the RPE. 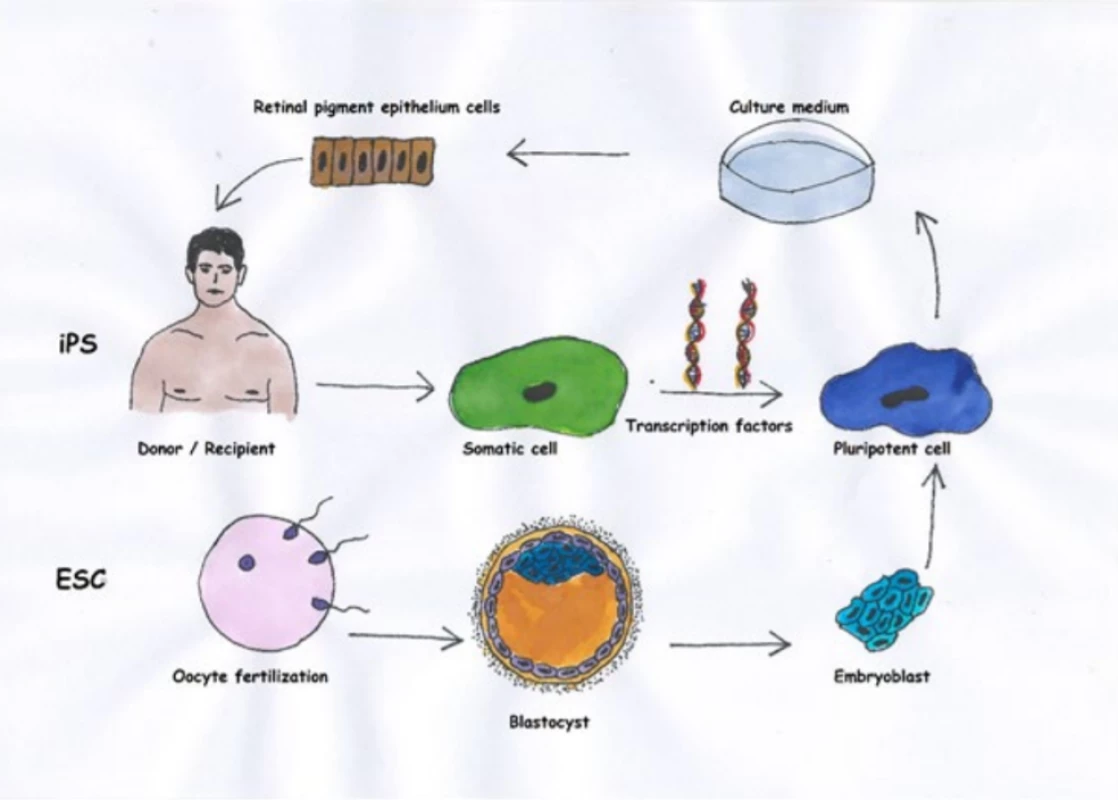
A second critical moment in cell therapy of the retina is surgical technique. Today there are three main directions in research: 1. subretinal injection of cellular suspension, 2. subretinal implantation of cell plate in form of epithelium, 3. subretinal injection of cell plate on supporting carrier (Fig. 2). Whereas injection of a cellular suspension is technically relatively simple, it is not possible to estimate entirely accurately whether the implanted cells will have the correct apicobasal orientation, and whether they will be capable of forming a functional monolayer with sufficient transepithelial resistance (TER, which indicates the resistance measured between the apical and basal side of the epithelium, in which the value is generally considered an indicator of the integrity of the epithelium). A role is played in this process among other factors by the Bruch’s membrane, which is often pathologically altered in affected patients [37]. Subretinal implantation of a cell plate with or without a supporting carrier requires a demanding surgical technique, which however is balanced by correct apicobasal orientation and in vitro measurable TER, the values of which may help us in advance to determine the quality of the implanted cells. If we implant without a supporting carrier, the cell plate may roll up perioperatively and the cells peel off. If we use a supporting carrier, handling is easier [21], but it is necessary to select a sufficiently porous carrier so as not to form a barrier to the diffusion of nutrients and waste substances.
2. Surgical technique of retinal pigment epithelium transplantation. Application of cellular suspension (A), self- -supporting cell plate (B), cell plate on artificial supporting carrier (C) 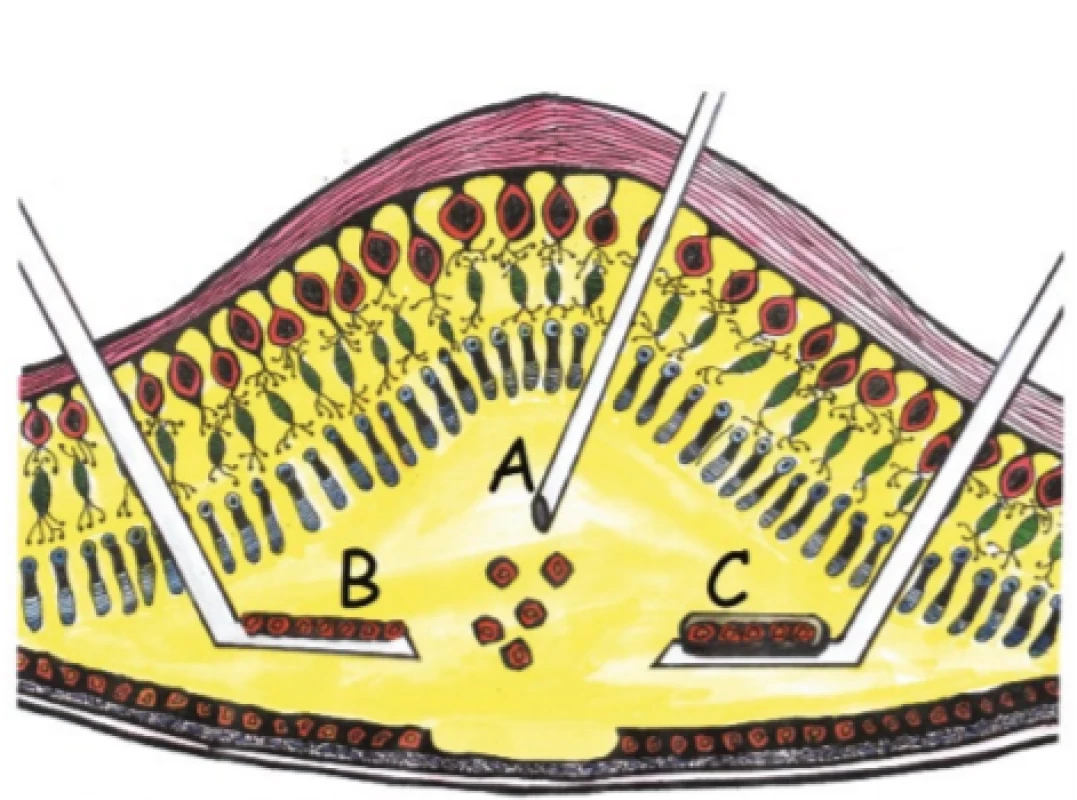
At present a number of clinical trials are currently under way, here we present one representative of each examined direction.
In 2016 Schwartz et al. published results of subretinal injection of hESC-RPE suspension into 18 eyes of 18 patients with dry form ARMD, Stargardt’s disease or myopic macular degeneration. Cells were injected into a locality selected in advance on the interface of a healthy and pathological retina. Although pigment clusters were perceptible on the retina following application, increased autofluorescence from the baseline condition, which is considered activity of RPE, was determined in only one case. Despite the fact that this was a study primarily focused on safety, a gain of 14 letters of ETDRS (Early Treatment Diabetic Retinopathy Study) optotypes was recorded in treated eyes in the 12th month in comparison with a gain of 1 letter in the untreated eyes. The frequency of adverse effects was relatively low (1 endophthalmitis, 1 vitritis). It was necessary to use general immune suppression [33].
One year later, a study from Japan [26] was presented, accompanied by considerable media interest, in which iPSC-RPE were used for transplantation. Originally fibroblasts were taken from two patients with advanced wet form ARMD, and subsequently iPSC-RPE were cultivated from them. However, implantation was performed only on one of the patients. The second patient did not undergo the procedure due to the large amount of genetic mutations in the cultivated cells and fears of subsequent formation of a teratoma. One year after implantation the cell plate was stable, but visual acuity was unchanged. Immune suppression was not necessary [26].
In 2018 a study was presented within the framework of the still ongoing London Project to Cure Blindness. This concerned phase I, with the use of hESC-RPE on a coated synthetic carrier [8]. This study presented the results of implantation of 2 eyes of 2 patients with extensive subretinal haemorrhage, in whom a subretinal carrier was implanted with monolayer hESC-RPE. In both cases there was an improvement of visual acuity (VA) by 29 and 21 letters of ETDRS optotypes respectively, however in one eye retinal detachment occurred, which required a further procedure, and in one patient decompensation of pre-existing diabetes mellitus occurred due to the influence of immune suppression.
As of today we do not have results available from larger clinical trials with the use of stem cells, nevertheless with regard to the initial results and large quantity of ongoing preclinical trials we can expect an advance in the coming years. With regard to surgical technique, the most advantageous appears to be a procedure with the use of a supporting carrier. Analogous experiments are taking place also in the Czech Republic, and following the phase of initial implantations [31], at the Institute of Animal Physiology and Genetics of Czech Academy of Sciences nanofibre carriers with dimensions of 2 x 5 mm [19] are being implanted, now also with iPSC-RPE or primary human RPE. The results shall be available within the following months.
Retinal prostheses
These are appliances that are capable of converting a light signal entering the region of the eye into an electrical stimulus via a device placed on the retina. The typical target of stimulation is not the photoreceptors, which are mostly lacking due to the influence of the pathology, but some of the higher embedded cells such as the retinal bipolar cells (RBCs) and retinal ganglion cells (RGCs). The processing of the signal by the retina itself is thus partially impaired, in which the transmission of the signal from the photoreceptor to the bipolar cell and subsequently the ganglion cell is modified by a large amount of further factors such as e.g. amacrine or horizontal cells (i.e. processing). Retinal prostheses are mostly divided according to location into epiretinal, subretinal and suprachorideal (Fig. 3). Epiretinal prostheses are an advantage with regard to their easier surgical access and easy activation of the ganglion cells. On the other hand, a disadvantage is their entirely uncontrollable processing of the image by the retina, in which in addition to the ganglion cells themselves, the surrounding axons are also activated and the signal does not appear as a luminous spot [28]. Subretinal prostheses are the opposite of epiretinal prostheses. Here we must be prepared for a demanding surgical technique, nonetheless the remaining photoreceptors are activated, or alternatively the RBCs, and the signal therefore does not circumvent processing in the retina [22]. Suprachoroideal implants are used less frequently, primarily due to the surgical demand factor and greater distance of the electrodes from the retina. Today the most commonly presented implants are Argus II (Second Sight, Sylmar, USA), Alpha AMS (Retina Implant AG, Reutlingen, Germany) and Photovoltaic Retinal Implant (PRIMA) Bionic Vision System (Pixium vision, Paris, France).
3. Anatomical localisation of retinal prostheses. Reti- nal prostheses can be implanted epiretinally (A), subreti- nally (B) or suprachoroideally (C) 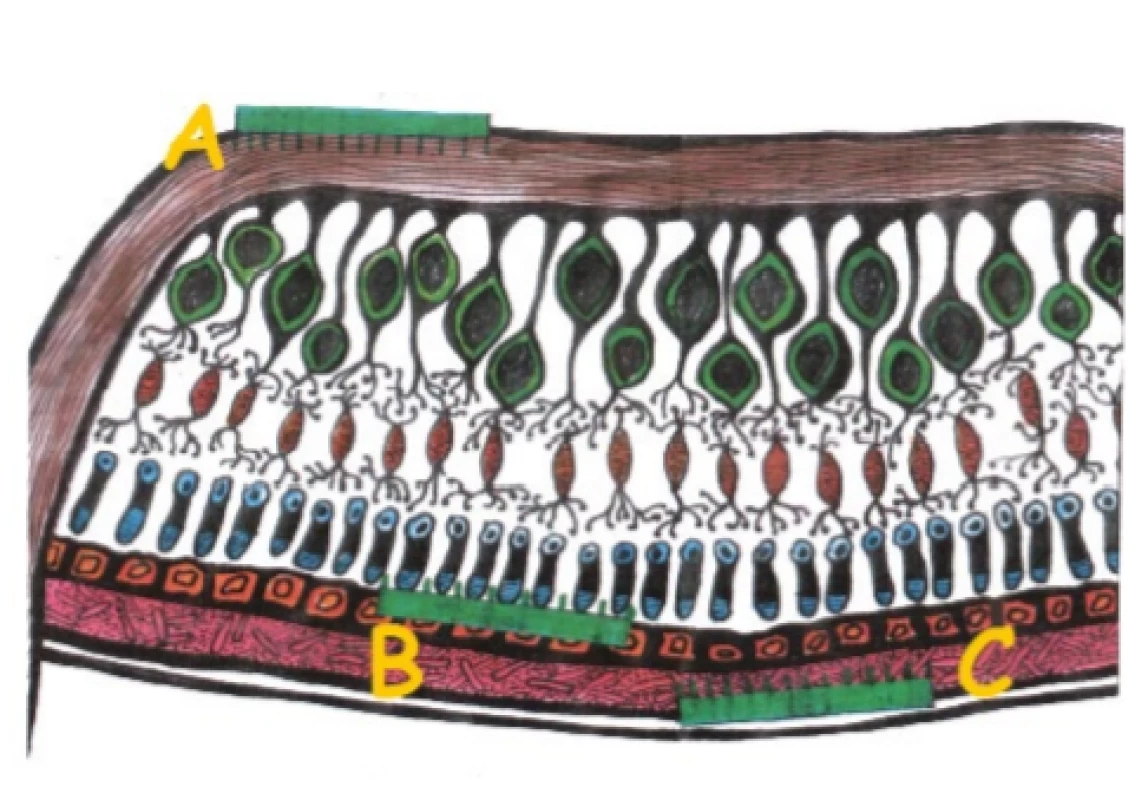
The most widespread implant is the Argus II, which has been implanted in more than 200 patients. This is an epiretinal implant with electrodes placed in six rows of ten. It is a camera attached to special glasses, generating a signal that is modulated in an external device. Subsequently the signal is transmitted by a radio frequency via a spool into a subconjunctivally implanted receiver, which is further transmitted transsclerally to the actual implant stimulating the retina [1]. The best documented visual acuity was only 20/1260, nonetheless the implant markedly improved the patient’s spatial orientation or for example searching for objects on a table [15]. The most common adverse effects include erosion or dehiscence of the conjunctiva, presumed endophthalmitis or hypotonia of the eye. Cases of perceive discrepancy between the fixed image from the camera and eye movement outside of direct gaze have also been reported.
The PRIMA system uses a different principle. Special glasses with a camera record the image, which is subsequently modulated and with the aid of a projector in the glasses sent in the form of infrared rays to a subretinally implanted wireless photovoltaic plate, which converts photovoltaic energy into electrical energy stimulating nearby neurons [22]. It is theoretically possible to obtain visual acuity of 20/200 with this implant. Three-year clinical trials were commenced in Europe and the USA (always five patients) in 2017 and 2018 respectively, and the results can be expected in 2021.
The last representative is Alpha AMS. This subretinal implant detects light and at the same time electrically stimulates primarily the bipolar cells. Energy is supplied by a transscleral cable, which leads subcutaneously behind the ear and is reminiscent of a cochlear implant. Here it is charged via a spool. The advantage of this implant is that it respects ocular movements. Average visual acuity with this implant is 20/1200, even if values of 20/550 have been recorded [11,35,42]. The Alpha AMS is no longer available, since it did not meet the commercial requirements of the manufacturer.
The main differences and specifications of retinal implants are summarised in table 2 [3].
2. Comparison of most commonly used retinal prostheses 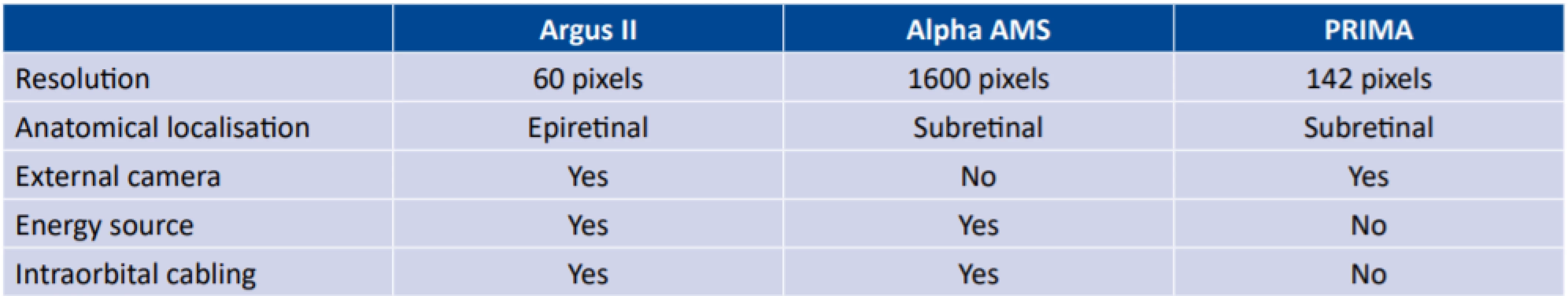
Gene therapy
The possibility of determining a diagnosis on the level of genes, and clinical testing of new targeted therapies has enabled the establishment of genetically focused clinical care in ophthalmology. Therapies based on knowledge of the genetic cause of the pathology represent an entirely new approach. With regard to its easy accessibility, small dimensions, immunological privilege, compartmentalisation and possibility of contralateral control, the eye is an ideal organ for clinical evaluation [9]. Gene therapy is a therapeutic procedure in which genetic material is introduced into the genome of cells, replacing or influencing the expression of the protein involved in the pathogenesis of a specific pathology. For the development and utilisation of gene therapy it is necessary to determine the gene responsible for the onset of the pathology and to know its function. If the cause of the pathology is a deficiency of a product of a mutated gene, it is sufficient to incorporate normal sequencing of the gene into the genome of the relevant cells (Fig. 4A), or to alleviate manifestations of the pathology by introducing a therapeutic gene (Fig. 4B). However, if an altered product of a mutated gene of the character of an aberrant protein has a pathological effect, it is necessary either to block the mutated gene (Fig. 4C) [2] or correct it (Fig. 4D) [9,38]. At the same time, treatment must not have negative impacts on the vitally important functions of the organism.
4. Strategies used in gene therapy [8,37] ![Strategies used in gene therapy [8,37]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/261890846f01659c6629766ad983416f.png)
Gene therapy can be performed either in vivo, in which the target cells are a part of the organism throughout the entire period of treatment, or in vitro, in which the target cells are removed from the body of the organism, and following the performance of therapy are returned to their original location [17]. A condition for success is the application of treatment within the “therapeutic window”, when irreversible damage to the tissue has not yet taken place.
Genetic information is transmitted into the target cells with the aid of carriers known as vectors. An ideal vector should penetrate into a large number of target cells and the expression of the introduced gene should take place for a sufficiently long period of time, in order accomplish the required therapeutic effect. In addition, the vector must not be toxic for the target cells or trigger adverse effects in the recipient such as viral infections or autoimmune reactions [25].
We divide vectors into viral and non-viral. Due to a range of disadvantages of physical and chemical vectors at present in clinical trials which are testing therapies for retinal pathologies in humans, viral vectors are used, in particular adenoviral and retroviral [25]. Genes in connection with the pathogenesis and reproduction of viruses are removed from viral particles. The genes are then replaced by an expression cassette prepared modification of DNA [9].
The risk of use of viral vectors consists in the fact that genetic information is inserted into the genome more or less at random. This may lead to a disturbance of the sequence of another gene with functional consequences. Another problem is the potential immunogenicity of viral vectors, causing inflammatory reactions [27]. Repeated application of therapeutics into the subretinal region also carries the risk of retinal damage or detachment [4]. Determination of the therapeutic dose and the amount of applied viral particles is also problematic.
In the treatment of retinal diseases, intravitreal and subretinal injections are most often used for the application of vectors. Although application into the vitreous body is less invasive for the retina, transmission of DNA takes place especially in the inner layers of the retina, thus in the Müller and ganglion cells. The membrana limitans interna and other retinal layers form a barrier to the penetration of vectors and pharmaceuticals into the deeper layers of the retina. Subretinal application is more suitable for the introduction of viral vectors into the layer of the photoreceptors and layer of RPE cells, in which the vector is injected into a vesicle between the aforementioned layers, and is thus in close contact with them [4].
Among the first cases proposed for gene therapy was Leber’s congenital amaurosis 2 (LCA2), mainly due to the early manifestation of the pathology, the relatively long preserved structure of the retina and the availability of animal models [24]. LCA2 is an autosomal recessive disease originating upon a background of mutations in the gene RPE65 (retinal pigment epithelium-specific 65 kDa protein) [13]. This gene is virtually exclusively exprimated in the RPE, where it contributes to the recycling of opsin and rhodopsin. Insufficient function or absence of RPE65 subsequently leads to a degeneration of photoreceptors [7]. After 2007 the gene RPE65 was clinically tested in gene therapy trials [2,14], which culminated in 2017 in the approval of the first gene therapy for clinical ophthalmological practice by the American Food and Drug Administration, and in 2018 by the European Medicines Agency [18].
In the Czech Republic complex diagnosis and research into genetically conditioned ocular pathologies is the focus of the Centre for Clinical Ocular Genetics at the 1st Faculty of Medicine, Charles University and General University Hospital in Prague.
Optogenetics
In the broader sense of the word, optogenetics is defined as a technique in which a gene coding a photosensitive protein is introduced with the aid of a vector into a neuron cell, as a result of which the target cell is subsequently stimulated. In ophthalmology, this typically concerns the insertion of a photoreceptive protein into one of the sub-populations of retinal cells [39], which primarily do not have a photoreceptive function.
In practice it is first of all necessary to find an appropriate photosensitive protein, which we subsequently insert into a suitable vector (most commonly adenoviral), and target at the correct cell. The final step is a corresponding “illumination” of the treated retina.
It is possible to use two types of opsin as a photoreceptive protein: type 1 (microbial) or type two (animal). The most commonly used is type 1, which has several subtypes, but in principle always concerns an ion channel which can be activated by light (e.g. channelrhodopsin, halorhodopsin, archeorhodopsin) [10]. After activation of the ion channel there is a change in the polarisation of the cell and subsequently a transmission of the signal further. Type 2 is a photosensitive protein coupled with a G-protein. After activation an intracellular signal cascade of chemical conversions takes place, with a resulting opening of the coupled cation channel. The main advantage of type 1 as against type 2 is the simpler molecule directly containing an ion channel, while the disadvantage is the frequently higher demands for light intensity necessary for a change of cell polarisation.
In the human retina we find more than one hundred different types of neuronal cells [41], which interreact to a certain degree and thereby ensure complex processing of the image before sending the signal further to the central nervous system by means of the axons of the ganglion cells. All attempts not to adhere to the natural procedure of photoreceptor-bipolar cell-ganglion cell therefore lead to a disruption of the retinal processing of the image, and thus to a reduction of its quality. As a result, our endeavour is always targeted at the outermost layers of the retina [34]. It is possible to use damaged photoreceptors which no longer have outer segments (here the preserved processing of the signal by the retina is an advantage), or upon circumventing this mechanism directly to use the bipolar or ganglion cells (Fig. 5).
5. Optogenetics targteted at retinal cells. Incorporation of photosensitive receptor, in this case channelrhodopsin, into the cell membrane of a bipolar cell 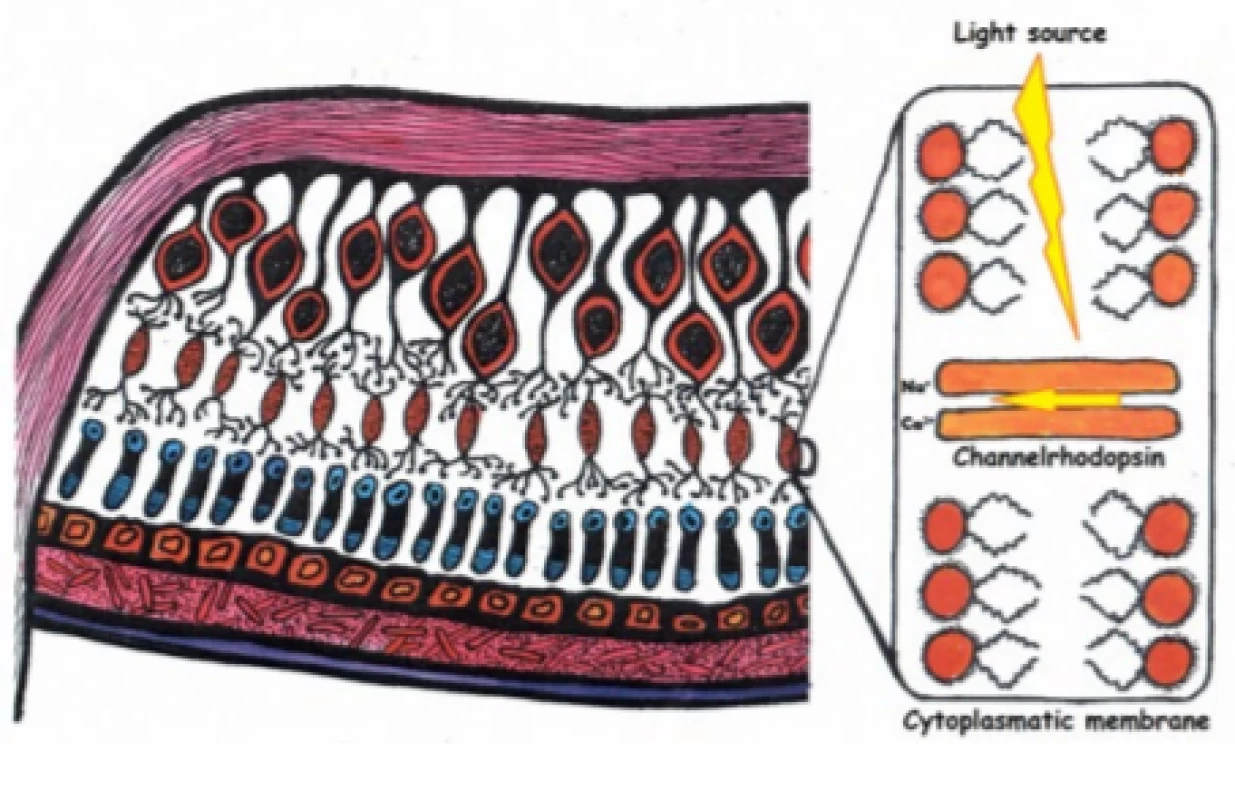
The final step is the trapping of the light by the treated cell. Various channels are sensitive to different wavelengths of light. It is necessary to bear this in mind with regard to the possibility of phototoxicity. For example, channelrhodopsin is most sensitive in short wavelengths, and like the majority of type 1 channels requires a larger number of photon hits. This, together with the low proportion of blue light especially under worsened light conditions and the lower permeability of these wavelengths through the optical environments of the eye [29] requires higher light intensity at these wavelengths for correct functioning. This can be attained only with the aid of an external light source, which may have an adverse influence, resulting in phototoxicity.
Two ongoing studies are now registered in the international register of clinical trials https://clinicaltrials.gov.
Phase I/II, sponsored by the Allergan firm (NCT02556736), is designated for patients with advanced retinitis pigmentosa, by which a vector coding channelrhodopsin for the modulation of the retinal ganglion cells (substance RST-001) is injected intravitreally. At the time of writing of this article, recruitment of patients is still ongoing, with an envisaged total number of 21.
GenSight Biologics is currently recruiting patients for a study with substance GS030-DP (NCT03326336), which is a modified adenoviral vector for modified channelrhodopsin, with sensitivity shifted more to the red spectrum [23], thus theoretically guaranteeing a better safety profile with regard to phototoxicity. The Pixium Vision company is also involved in the project (see PRIMA system above), and it appears that a combination of optogenetics and glasses augmenting reality (i.e. the natural image is supplemented by a digital layer which enables for example the accentuation of certain predefined objects) represents a promising future pathway for reinforcing the effect.
DISCUSSION
Despite the fact that gene therapy, optogenetics, cell therapy and bionic eye are matters rather of the more or less distant future and bring with them a large number of unsolved questions, such as financing, effectiveness, indication criteria, ethical problems or technological demand factor, a number of initial commercial successes have already been achieved. The approval of vortigene neparvovec-ryzl as the first preparation for causal treatment of genetically conditioned disease has opened up a path for further promising products. It is therefore possible to assume that similar pharmaceutical preparation will progressively appear on the market and that treatment of an ever greater number of clinical units will be enabled.
Another good example is the implant Argus II, since though its resolution of 6 x 10 electrodes may not seem especially overwhelming today is nevertheless considered a prime mover and a “proof of concept” in the field. At the same time it has been demonstrated to be technologically very reliable, with no complications caused by a malfunction of the instrument appearing in the first three years of clinical practice [15]. This has led to a further development of more advanced systems, which are now in the phase of clinical evaluation (NCT03344848).
Great hopes have been invested in cell therapy, which is however more difficult to grasp in comparison with “exact” electronic implants, and there is immense variability of results with regard to the different quality of implanted cells and potential complications during the course of highly demanding surgical procedures. Uncertainty still predominates with regard to the safety profile of the cells, and there are also fears of possible teratogenicity. Ethical questions are also unclear. The implantation techniques are not uniform, and large discussions are taking place concerning artificial carriers for RPE, wherein on one hand manipulation of the cells is markedly improved upon the use of a carrier, while on the other this represents an artificial barrier which prevents the diffusion of substances into and out of the retina. A certain way out of this problem could be the use of nanotechnologies in the production of a carrier. A further issue that is as yet unclear is whether or not to drain off the original RPE cells perioperatively, and here there is a complete lack of data.
Optogenetics appears to be an interesting method, nonetheless we are still waiting for the first concrete results. A disadvantage is low photosensitivity, and it appears that this method will have to be supplemented by an external light source or e.g. glasses enabling the sending of an augmented reality image to the retina also in a wave spectrum other than visible light, on the precondition that photosensitive channels are sensitive to such a wavelength.
CONCLUSION
In the coming years we can expect fascinating developments in the treatment of retinal diseases, in which the greatest challenge shall perhaps not be technologies but rather correct indication for the selection of one of the above-described methods, which shall in their final consequence be supplemented, and thus offer an alternative for patients who cannot be helped at present.
*The first and second authors contributed to the article in an equal extent.
Presented at the 19th congress of the Czech Young Ophthalmologists Society held in Železná Ruda, 21-22 June 2019.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
The study was supported by the grants SVV UJ 260367/2017, Czech Science Foundation 18-04393S and the National Sustainability Programme LO1609.
MUDr. Zbyněk Straňák
Oftalmologická klinika, 3. lékařská fakulta, UK a FN Královské Vinohrady
Šrobárova 50,
100 34 Praha 10
Received: 1. 10. 2019
Accepted: 6. 11. 2019
Available on-line: 20. 5. 2020
Sources
1. Ahuja, AK., Yeoh, J., Dorn, JD., et al.: Factors Affecting Perceptual Threshold in Argus II Retinal Prosthesis Subjects. Transl Vis Sci Technol, 2; 2013 : 1.
2. Bainbridge, JWB., Mehat, MS., Sundaram, V., et al.: Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med, 372; 2015 : 1887–1897.
3. Bloch, E., Luo, Y., da Cruz, L.: Advances in retinal prosthesis systems. Ther Adv Ophthalmol, 11; 2019 : 1–16.
4. Boye, SE., Boye, SL., Lewin, AS., et al.: A comprehensive review of retinal gene therapy. Mol Ther J Am Soc Gene Ther, 21; 2013 : 509–519.
5. Bressler, NM., Bressler, SB., Fine, SL.: Age-related macular degeneration. Surv Ophthalmol, 32; 1988 : 375–413.
6. Carr, A-JF., Smart, MJK., Ramsden, CM., et al.: Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci, 36; 2013 : 385–395.
7. Cideciyan, AV.: Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res, 29; 2010 : 398–427.
8. da Cruz, L., Fynes, K., Georgiadis, O., et al.: Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol, 36; 2018 : 328–337.
9. Dudakova, L., Kousal, B., Kolarova, H., et al.: Gene therapy for inherited retinal and opticnerve disorders: current knowledge. Cesk Slov Oftalmol. 72; 2016; 128–136.
10. Duebel, J., Marazova, K., Sahel, J-A.: Optogenetics. Curr Opin Ophthalmol, 26; 2015 : 226–232.
11. Edwards, TL., Cottriall, CL., Xue, K., et al.: Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa. Ophthalmology, 125; 2018 : 432–443.
12. Flood, MT., Gouras, P., Kjeldbye, H.: Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Invest Ophthalmol Vis Sci, 19; 1980 : 1309–1320.
13. Gu, SM., Thompson, DA., Srikumari, CR., et al.: Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet, 17; 1997 : 194–197.
14. Hauswirth, WW., Aleman, TS., Kaushal, S., et al.: Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther, 19; 2008 : 979–990.
15. Ho, AC., Humayun, MS., Dorn, JD., et al.: Long-Term Results from an Epiretinal Prosthesis to Restore Sight to the Blind. Ophthalmology, 122; 2015 : 1547–1554.
16. Chen, FK., Patel, PJ., Uppal, GS., et al.: Long-term outcomes following full macular translocation surgery in neovascular age-related macular degeneration. Br J Ophthalmol, 94; 2010 : 1337–1343.
17. Kaufmann, KB., Buning, H., Galy, A., et al.: Gene therapy on the move. EMBO Mol Med, 5; 2013 : 1642–1661.
18. Kousal, B., Ďuďáková, Ľ., Moravíková, J., et al.: Vzácná oční onemocnění v oftalmologické praxi. Oftalmol Praxi, 2018 : 7–11.
19. Kozak, I., Stranak, Z., Popelka, S., et al.: Safety and feasibility of new nanofiber subretinal delivery system with injector for RPE cell transplantation. Invest Ophthalmol Vis Sci, 59; 2018 : 5670–5670.
20. Kvanta, A., Grudzinska, MK.: Stem cell-based treatment in geographic atrophy: promises and pitfalls. Acta Ophthalmol (Copenh), 92; 2014 : 21–26.
21. Lee, E., MacLaren, RE.: Sources of retinal pigment epithelium (RPE) for replacement therapy. Br J Ophthalmol, 95; 2011 : 445–449.
22. Lorach, H., Goetz, G., Smith, R., et al.: Photovoltaic restoration of sight with high visual acuity. Nat Med, 21; 2015 : 476–482.
23. Mace, E., Caplette, R., Marre, O., et al.: Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV Restores ON and OFF visual responses in blind mice. Mol Ther J Am Soc Gene Ther, 23; 2015 : 7–16.
24. Maguire, AM., Simonelli, F., Pierce, EA., et al.: Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med, 358; 2008 : 2240–2248.
25. Mali, S.: Delivery systems for gene therapy. Indian J Hum Genet, 19; 2013 : 3–8.
26. Mandai, M., Watanabe, A., Kurimoto, Y., et al.: Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med, 376; 2017 : 1038–1046.
27. Misra, S.: Human gene therapy: a brief overview of the genetic revolution. J Assoc Physicians India, 61; 2013 : 127–133.
28. Nanduri, D., Fine, I., Horsager, A., et al.: Frequency and amplitude modulation have different effects on the percepts elicited by retinal stimulation. Invest Ophthalmol Vis Sci, 53; 2012 : 205–214.
29. von Noorden, GK.: Application of basic research data to clinical amblyopia. Ophthalmology, 85; 1978 : 496–504.
30. Parolini, B., Di Salvatore, A., Pinackatt, SJ., et al.: Long-term results of autologous retinal pigment epithelium and choroid transplantation for the treatment of exudative and atrophic maculopathies. Retina, 2018.
31. Popelka, S., Studenovska, H., Abelova, L., et al.: A frame-supported ultrathin electrospun polymer membrane for transplantation of retinal pigment epithelial cells. Biomed Mater Bristol Engl, 10; 2015 : 045022.
32. Ramsden, CM., Powner, MB., Carr, A-JF., et al.: Stem cells in retinal regeneration: past, present and future. Dev Camb Engl, 140; 2013 : 2576–2585.
33. Schwartz, SD., Tan, G., Hosseini, H., et al.: Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci, 57; 2016: ORSFc1-9.
34. Simunovic, MP., Shen, W., Lin, JY., et al.: Optogenetic approaches to vision restoration. Exp Eye Res, 178; 2019 : 15–26.
35. Stingl, K., Bartz-Schmidt, KU., Besch, D., et al.: Arteficial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci, 280; 2013 : 1–8.
36. Takahashi, K., Yamanaka, S.: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126; 2006 : 663–676.
37. Tezel, TH., Kaplan, HJ., Del Priore, LV.: Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Invest Ophthalmol Vis Sci, 40; 1999 : 467–476.
38. Wang, D., Gao, G.: State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov Med, 18; 2014 : 151–161.
39. Yue, L., Weiland, JD., Roska, B., et al.: Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog Retin Eye Res, 53; 2016 : 21–47.
40. van Zeeburg, EJT., Maaijwee, KJM., Missotten, TOAR., et al.: A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am J Ophthalmol, 153; 2012 : 120-127.e2.
41. Zeng, H., Sanes, JR.: Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci, 18; 2017 : 530–546.
42. Zrenner, E., Bartz-Schmidt, KU., Benav, H., et al.: Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci, 278; 2011 : 1489–1497.
Děkuji Mgr. Štěpánce Elišce Straňákové za přípravu obrázků 1, 2, 3 a 5.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2019 Issue 6-
All articles in this issue
- 100 let od narození prof. MUDr. Heleny Lomíčkové, DrSc. 40 let oční kliniky dětí a dospělých v Motole
- OCENĚNÍ ČLS JEP
- CENA PREZIDENTA ČLK
- Innovative strategies for treating retinal diseases
- Assessment of the efficacy of photodynamic therapy in patients with chronic central serous chorioretinopathy
- Sensitivity and specificity in methods for examination of the eye astigmatism
- Evaluation of retinal light scattering, visual acuity, refraction and subjective satisfaction in patients after Acrysof IQ PanOptix intraocular lens implantation
- Eyelid edema as a first sign of lymphoma
- Ocular Symptoms of Rosacea
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Ocular Symptoms of Rosacea
- Eyelid edema as a first sign of lymphoma
- Assessment of the efficacy of photodynamic therapy in patients with chronic central serous chorioretinopathy
- Innovative strategies for treating retinal diseases
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

