-
Medical journals
- Career
Treatment of idiopathic choroidal neovascular membrane with ranibizumab - our experience
Authors: O. Chrapek; Z. Vostrovská; I. Šínová; B. Chrapková
Authors‘ workplace: Oční klinika FN a LF UP v Olomouci, I. P. Pavlova 6, Olomouc, 779 00, Přednosta: prof. MUDr. Jiří Řehák, CSc., FEBO
Published in: Čes. a slov. Oftal., 75, 2019, No. 1, p. 25-28
Category: Original Article
doi: https://doi.org/10.31348/2019/1/3Overview
Objective: To evaluate the anatomical and functional outcome of ranibizumab therapy in patients with idiopathic choroidal neovascularization (CNV).
File and Methodology: The group consists of 6 patients. Patients were older 18 years but they were under 50 years of age. The monitoring period lasted 12 months. We confirmed active idiopathic CNV in subfoveal position with fluorescein angiography (FAg) and optical coherence tomography (OCT). The activity of idiopathic CNV we demonstrated with leakage of dye by FAg examination. The presence of serous retinal pigment epithelium detachment and / or subretinal fluid and / or intraretinal edema in the form of intraretinal cysts demonstrated activity of CNV on OCT scans. A decrease of the visual acuity under 85 letters was observed at the ETDRS (Early Treatment Diabetic Retinopathy Study) visual acuity chart. After the initial administration of ranibizumab a pro re nata regimen was used. We indicated repeated injection of ranibizumab in patients with signs of activity of idiopathic CNV on OCT scans or by FAg. Also we indicated repeated injection of ranibizumab in patients with new loss of visual acuity on the ETDRS visual acuity chart connected with signs of activity of CNV on OCT scans or by FAg.
Results: On average, we observed the gain of +11 letters on the ETDRS visual acuity chart after 12 months of the follow-up period. On average we observed reduction of central macular thickness -233μm. At the 12th month of follow-up we observed in all patients of our group only inactive scar without exudation. No serous retinal pigment eithelium detachment, subretinal fluid or intraretinal cysts were observed. Only 3 injections of ranibizumab were administered on average to each patient during the 12 months of the follow-up period.
Conclusion: In our study, we observed the positive effect of ranibizumab on the course of idiopathic CNV. With ranibizumab treatment we achieved regression of CNV with resorption of macular edema in all patients of our group. With the disappearance of the activity of idiopathic CNV ranibizumab gives real hope to improve visual acuity.
Keywords:
idiopathic choroidal neovascular membrane – anti-VEGF treatment – ranibizumab
INTRODUCTION
Choroidal neovascularisation (CNV) is a formation of newly-generated capillaries which grow out of the choriocapillaris, penetrate through the Bruch's membrane, grow beneath the retinal pigment epithelium and later potentially into the subretinal space. In adult patients the formation of CNV is primarily upon a background of wet form age-related macular degeneration (ARMD) and high myopia. Of the rarer causes it is worth noting uveitis, central serous choroiretinoathy (CSCR), angioid streaks, traumas, tumours and macular dystrophy. CNV may also be indicated as idiopathic in the case that its origin cannot be linked with a manifest cause or clinically demonstrable predisposing abnormality [2, 4, 10, 13]. It is stated that 17% of CNV in patients aged younger than 50 years is idiopathic [3].
An important role in the pathogenesis of CNV is played by vascular endothelial growth factor (VEGF), the blockade of which is the current standard of treatment. In the Czech Republic it is possible to use two anti-VEGF preparations in order to block VEGF molecules: ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland) and aflibercept (Eylea; Bayer AG, Leverkusen, Germany). Ranibizumab has demonstrated its effectiveness and safety in clinical trials in the treatment of CNV linked with wet form ARMD, pathological myopia and CNV occurring regardless of etiology [1, 6, 11, 14, 15, 16].
In our study we evaluate the anatomical and functional result of therapy by ranibizumab in patients diagnosed with idiopathic CNV.
METHOD
The cohort comprises retrospectively evaluated patients treated with ranibizumab for idiopathic CNV at the Department of Ophthalmology of the University Hospital and Faculty of Medicine, Palacký University in Olomouc in the period of 2012 – 2017. The patients were aged over 18 years and younger than 50 years, with active CNV in subfoveolar localisation demonstrated by fluorescein angiography (FAg) and optical coherence tomography (OCT). Infiltration of colouring into the zone of macular edema was considered a sign of activity of CNV on FAg examination. On OCT examination, signs of CNV activity were considered to be the presence of serous ablation of the retinal pigment epithelium (RPE) and/or subretinal fluid and/or intraretinal edema in the form of intraretinal cysts. On an ETDRS (Early Treatment Diabetic Retinopathy Study) chart a deterioration of visual acuity (VA) was observed to the level of < 85 letters (L). CNV was not connected with ARMD (age < 50 years) or pathological myopia (refractive error below -6 Dpt), and no polypoid choroidal vasculopathy or retinal angiomatous proliferation was demonstrated. Active diabetic retinopathy was excluded in the patients, and they did not suffer from ocular or periocular infection or intraocular inflammation. None of the patients had previously undergone laser treatment in the macular region, and none had undergone photodynamic therapy or pars plana vitrectomy. The patients had a negative anamnesis as regards CSCR, ocular trauma, ocular tumour, macular dystrophy and angioid streaks. In the past the patients had not received intravitreally applied anti-VEGF preparations, corticosteroids or depot implants. They did not suffer from a systemic inflammatory or infectious disease, other manifest cause or clinically demonstrable predisposing abnormality which could be linked with the occurrence of CNV.
The cohort comprised 6 patients (4 women and 2 men) with an average age of 38 years (median 41.5, standard deviation σ =8.9582), in which the youngest patients was aged 23 years upon the commencement of treatment, and the oldest 48 years. The right eye was treated 1x, the left eye 5x. The observation period was 12 months. We evaluated best corrected initial VA of all the patients on an ETDRS chart, and resulting VA after 12 months. By comparing initial and resulting visual acuity we evaluated improvement, stabilisation or deterioration of VA. A gain of > 5 letters on an ETDRS chart was evaluated as an improvement of VA, a loss of > 5 letters on an ETDRS chart was evaluated as a deterioration of VA. A change of visual acuity of +/-5 L on an ETDRS chart was evaluated as stabilisation of VA. We evaluated anatomical changes in the macula and macular thickness upon the commencement of therapy and after 12 months with the aid of OCT, in which an OCT instrument Cirrus (Zeiss, Jena), or Spectralis (Heidelberg) was used. The same OCT instrument was used on each patient upon commencement of treatment and at the end of the 12 month observation period to measure central macular thickness (CMT) and evaluate the degree of change thereto. CMT was defined as the average retinal thickness of the circular zone with a diameter of 1 mm around the centre of the foveola. The last evaluated parameter was the number of intravitreal injections administered to each individual patient in the cohort during the course of the 12 month observation period. After the initial application of ranibizumab, a pro re nata (PRN) regimen was applied, in which ranibizumab was reapplied upon signs of activity of CNV on OCT/FAg or upon a decrease of VA in connection with the activity of CNV on OCT/FAg.
RESULTS
Classic CNV was diagnosed in all patients in the cohort, in which it did not exceed the size of two surfaces of the disc in any of the patients.
During the first 3 months of treatment, during reapplications and at the end of the 12 month observation period, VA and CMT are expressed together with the numbers of intravitreal applications of ranibizumab in the individual patients in the cohort. Table 1.
1. VA and CMT during the first 3 months of treatment, during reapplications and at the end of the 12 month observation period, expressed together with the numbers of intravitreal applications of ranibizumab in the individual patients in the cohort. 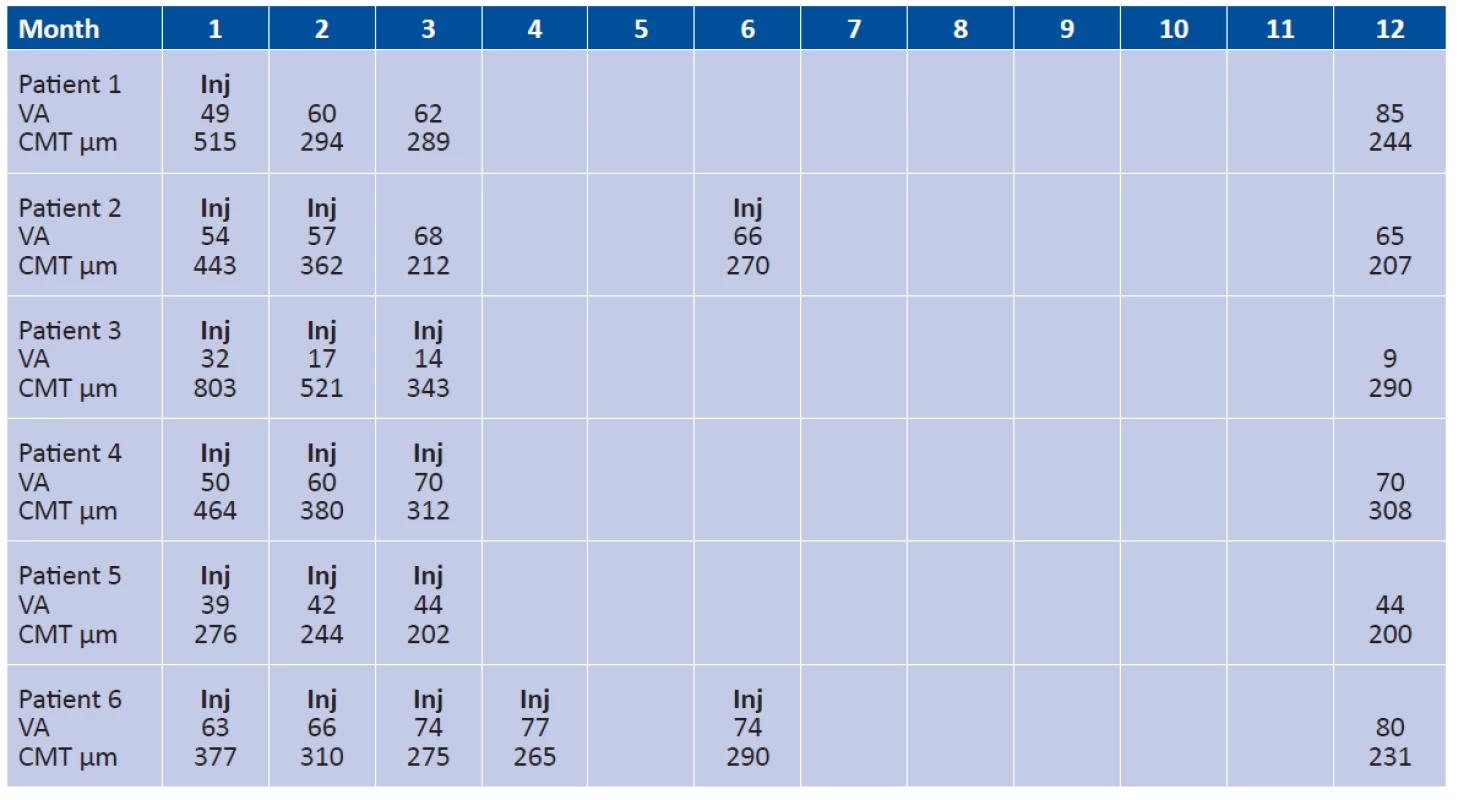
Average initial VA in the patients in the cohort was 48 L of ETDRS chart (median 49 L, σ = 10.0235), average resulting VA in the patients in the cohort was 59 L of ETDRS chart (median 67 L, σ =25.8161).
After 12 months of treatment, we observed an average gain of +11 L of ETDRS chart (median 14 L, σ =17.9536) in the patients in the cohort. In 4 patients in the cohort we observed an improvement of VA with an average gain of +21 L of ETDRS chart (median 18 L, σ =9.2466), in 1 patient a stabilisation of VA with a gain of +5 L of ETDRS chart and in 1 patient a deterioration of VA with a loss of -23 L of ETDRS chart.
The average initial CMT of the patients in the cohort was 480 µm (median 453.5µm, σ =162.9945), average resulting CMT of the patients in the cohort was 247 µm (median 237.5µm, σ = 40.0901). CMT improved in all the patients. On average we observed a reduction of CMT by -233µm (median 196µm, σ =140.2379).
In the 12th month of observation, an inactive submacular scar without exudation, thus without the presence of serous ablation of the RPE, subretinal fluid or intraretinal edema in the form of intraretinal cysts was detected in all of the patients on OCT.
During the course of the 12 months of treatment, each patient was applied an average of 3 injections of ranibizumab (median 3, σ =1.1547). Only one patient in the cohort was applied a single injection of ranibizumab at the beginning of treatment. In one patient the treatment was commenced with two injections of ranibizumab at an interval of one month, and after 6 months one reapplication of ranibizumab had to be repeated due to reactivation of CNV. In three patients in the cohort the treatment was commenced with three consecutive injections at an interval of one month, and during the course of the entire further observation period no further reapplication was required. In one patient the treatment was commenced by 4 consecutive applications of ranibizumab, and one further injection of ranibizumab was applied due to reactivation of CNV in the 6th month of observation. It is possible to notice that in all patients in the cohort, the applications of ranibizumab were performed during the course of the first 6 months of the observation period.
DISCUSSION
Idiopathic CNV is a disease affecting people aged younger than 50 years, and may cause a severe loss of sight. The precise etiopathogenesis remains unclear, with speculation concerning abnormalities on the level of the choroid such as focal choroiditis, choroidal ischemia, abnormalities of the choroidal capillaries, or impaired functional activity of circulating haematopoietic stem cells [3, 7, 9, 12].
In a cohort of 39 idiopathic CNV, Houfa confirmed the benefit of ranibizumab for the visual acuity of the treated eyes. Upon commencement of treatment and in the 12th month of observation, average best corrected VA was 0.67 ± 0.45 of the logarithm of minimal angle of resolution (logMAR) and 0.16 ± 0.21 logMAR respectively, in which this difference was evaluated as statistically significant (P < 0.001) [7].
In the MINERVA clinical trial, evaluating the effectiveness and safety of ranibizumab in CNV occurring regardless of etiology but not connected with wet form ARMD and pathological myopia, a statistically significantly better effect of ranibizumab was demonstrated in comparison with a placebo. The patients treated with ranibizumab already had an average gain of +9.5 L of ETDRS chart in the 2nd month of treatment, in comparison with patients receiving placebo therapy, who on average lost -0.4 L of ETDRS chart. Idiopathic CNV was one of the etiological CNV groups in which the difference in the gain of letters in the 2nd month of treatment between the group treated with ranibizumab and a placebo was >10 L of ETDRS chart. In the 12th month an average gain of +11 L of ETDRS chart was recorded in the patients treated with ranibizumab in the MINERVA trial [11].
In our small cohort also we observed a positive influence of ranibizumab on VA in the treated patients, who after 12 months of therapy gained an average of +11 L of ETDRS chart.
In the referred cohorts, a positive impact of the treatment on CMT is also mentioned together with the positive influence of ranibizumab on VAT. Houfa demonstrated a reduction of macular thickness in a cohort of 39 patients with idiopathic CNV, in which initial average macular thickness of 354.05 ± 86.52 μm was reduced after 12 months of treatment to the value of 219.15 ± 19.11 μm, and this change was evaluated as statistically significant (P < 0.001) [7]. In the MINERVA clinical trial, patients treated with ranibizumab had initial average CMT of 392.5 µm (SD 145,20), while after 12 months of therapy with ranibizumab there was a reduction of CMT on average by 102.7µm [11].
In our cohort also we observed a reduction of CMT in all patients, on average by -233 µm. After 12 months, signs of activity of CNV disappeared in all patients, with a regression of intraretinal edema, subretinal fluid and serous ablation of the RPE. Nevertheless, in only 4 patients (66%) was this anatomical success accompanied by a functional improvement and increase of VA. In one patient, despite the anatomical success of treatment, VA remained unchanged and in one patient it actually deteriorated. We see the cause in a combination of atrophic and degenerative changes of the macula as a consequence of the patient having suffered a disease with fibrotic restructuring of originally active CNV, with probable damage to the photoreceptors of the fovea upon originally subfoveolar localisation of CNV.
Clinical trials evaluating the effectiveness and safety of ranibizumab in the treatment of CNV caused by the wet form of ARMD state a relatively high requirement for reapplication in order to attain a therapeutic effect. In the PrONTO trial, in which patients with wet form ARMD were treated in a PRN regimen, the average number of injections of ranibizumab was 5.6 in the first 12 months of treatment [5].
In 12 months of treating a cohort of 39 patients with idiopathic CNV, Houfa required an average of 2.1 ± 1.1 injections [7] in order to attain a therapeutic effect. Chuangfeng Fan applied ranibizumab to 44 patients with idiopathic CNV. Over the course of 12 months of treatment, for patients in whom the period of duration of the complains was shorter than 3 months he required an average of 1.22 ± 1.01 injections, and for patients with a duration of complaints of 3 – 6 months 2.53 ± 1.76 injections [8]. In our cohort, over the course of 12 months of treatment we applied an average of 3 injections of ranibizumab with a very good anatomical and functional effect.
CONCLUSION
In our cohort we observed a positive influence of ranibizumab on the course of idiopathic CNV. Ranibizumab achieved a regression of CNV in all patients, with a disappearance of macular edema. With the regression of activity of CNV, ranibizumab provides realistic hope for an improvement of VA. Based on our experience and that of other authors [7, 8] it appears that idiopathic CNV responds to ranibizumab better than CNV in patients with wet form ARMD, and consequently a lower number of reapplications of ranibizumab is required in order to attain a therapeutic effect. However, a larger prospective study shall be necessary in order to confirm this fact.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
Received by the Editorial Department on: 30 July 2018
Accepted for printing on: 3 January 2019
doc. MUDr. Oldřich Chrapek, PhD.
Department of Ophthalmology, University Hospital and Faculty of Medicine, Palacký University Olomouc, I.P. Pavlova 6, Olomouc, 779 00
Sources
1. Brown, D.M., Michels, M., Kaiser, P.K. et al.: Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology, 116, 2009; 1 : 57–65.e5.
2. Carneiro, A.M., Silva, R.M., Veludo, M.J. et al.: Ranibizumab treatment for choroidal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica, 225, 2011; 2 : 81–88.
3. Cohen, S.Y., Laroche, A., Leguen, Y. et al.: Etiology of choroidal neovascularization in young patients. Ophthalmology, 103,1996; 8 : 1241-1244.
4. Derosa, J.T., Yannuzzi, L.A., Marmor, M. et al.: Risk factors for choroidal neovascularization in young patients: a case-control study. Doc Ophthalmol, 91,1995; 3 : 207–222.
5. Fung, A.E., Lalwani, G.A., Rosenfeld, P.J. et al.: An Optical Coherence Tomography-Guided, Variable Dosing Regimen with Intravitreal Ranibizumab (Lucentis) for Neovascular Age-related Macular Degeneration. Am J Ophthalmol, 143, 2007; 4 : 566-583.
6. Holz, F.G., Amoaku, W., Donate, J. et al.: Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age related macular degeneration: the SUSTAIN study. Ophthalmology, 118, 2011; 4 : 663–671.
7. Houfa, Y., Xiaoyun, F., Jian, M. et al.: Idiopathic Choroidal Neovascularization: Intraocular Inflammatory Cytokines and the Effect of Intravitreal Rinibizumab Treatment. Scientific Reports, 6, 2016, Article number: 31880. Doi:10.1038/srep31880., Dostupné na: https://www.nature.com/articles/srep31880
8. Chuanfeng, F., Qiang, J., Yu, W. et al.: Clinical Efficacy of Intravitreal Ranibizumab in Early and Mid-Idiopathic Choroidal Neovascularization. Journal of Ophthalmology, Volume 2014, Article ID 382702, Dostupné na http://dx.doi.org/10.1155/2014/382702.
9. Inagaki, M., Harada, T., Kiribuchi, T. et al.: Subfoveal choroidal neovascularization in uveitid. Ophthalmologica, 2010, 1996; 4 : 229 – 233.
10. Klein, R., Peto, T., Bird, A., et al.: The epidemiology of age-related macular degeneration. Am J Ophthalmol, 137, 2004; 3 : 486 – 495.
11. Lai, T.Y.Y., Staurenghi, G., Lanzetta, P. et al.: Efficacy and safety of ranibizumab for the treatment of choroval neovascularization due to uncommon cause. Twelve-Month Results of the Minerva Study. Retina, 0, 2017; 1-14.
12. Sasahara, M., Otani, A., Yodoi, Y. et al.: Circulating hematopoietic stem cells in patients with idiopathic choroidal neovascularization. Invest Ophthalmol Vis Sci, 50, 2009; 4 : 1575–1579.
13. Spaide, R.F.: Choroidal neovascularization in younger patients. Curr Opin Ophthalmol, 10, 1999; 3 : 177–181.
14. Tufail, A., Narendran, N., Patel, P.J. et al.: Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology, 120, 2013; 9 : 1944–1945.e1.
15. Tufail, A., Patel, P.J., Sivaprasad, S. et al.: Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye (Lond), 27, 2013; 6 : 709–715.
16. Wolf, S., Balciuniene, V.J., Laganovska, G. et al.: RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology, 121, 2014; 3 : 682–692.e2.
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2019 Issue 1-
All articles in this issue
- Usage of shear wave elastography for diagnosis of changes of oculomotor muscles in endocrine orbitopathy
- Treatment of idiopathic choroidal neovascular membrane with ranibizumab - our experience
- RADIATION MACULOPATHY AFTER ONE-DAY SESSION STEREOTACTIC RADIOSURGERY IN PATIENTS WITH CILIARY BODY AND CHOROIDAL MELANOMA
- Effective Tissue Layer Separation of Donor Cornea for DMEK by Fluid Injection Between Descemet
- Primary orbital teratoma – case study
- Late postoperative capsular bag distension syndrome
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Late postoperative capsular bag distension syndrome
- Primary orbital teratoma – case study
- Treatment of idiopathic choroidal neovascular membrane with ranibizumab - our experience
- RADIATION MACULOPATHY AFTER ONE-DAY SESSION STEREOTACTIC RADIOSURGERY IN PATIENTS WITH CILIARY BODY AND CHOROIDAL MELANOMA
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

