-
Medical journals
- Career
Comparison of MRI findings of glioblastoma and gliosarcoma – can conventional MRI provide beneficial differences for diagnosis?
Authors: O. Kaya 1; O. Dilek 2; T. Koseci 3; T. Cil 3; C. Yilmaz 2; B. Gulek 2
Authors‘ workplace: Department of Radiology, Faculty of, Medicine, Cukurova University, Adana, Turkey 1; Department of Radiology, University, of Health Sciences, Adana City Training, and Research Hospital, Adana, Turkey 2; Department of Medical Oncology, University of Health Sciences, Adana, City Training and Research Hospital, Adana, Turkey 3
Published in: Cesk Slov Neurol N 2021; 84/117(3): 251-256
Category: Original Paper
doi: https://doi.org/10.48095/cccsnn2021251Overview
Aim: The imaging findings of glioblastoma (GBM) and gliosarcoma (GSM) are substantially similar. However, there may be some differences and the purpose of this study was to evaluate the differences in MRI findings between GBM and GSM. Material and methods: The contrast-enhanced MRI examinations of 15 GSM and 32 GBM cases that were followed up in January 2015–December 2019 were evaluated retrospectively. T1-WI, T2-WI and FLAIR signal properties; mass size and location; necrosis and peritumoural oedema; cystic component; and contrast-enhancement intensity and type were compared. The Kolmogorov-Smirnov test, independent-samples t-test, Mann-Whitney U test, chi-square test, and Fischer‘s exact test were used in the statistical analysis. P < 0.05 was considered significant. Results: The T2-WI pronounced hyperintensity and T1-WI isomild hyperintensity rates in the solid area were significantly higher in the GSM group. The contrast intensity did not differ significantly, but of the contrast-enhancement types, the homogeneous contrast ratio was slightly higher in the GSM group. In the group, necrosis width was significantly bigger and, the temporal localization rate was significantly higher, but no significant difference was found in terms of other anatomical locations. Conclusion: GSM and GBM are high-grade tumours and pathological evaluation is needed for differential diagnosis. Our study found, however that many conventional MRI findings such as localization, width of necrosis and T2-WI hyperintensity may also contribute to the diagnostic approach.
Keywords:
gliosarcoma – glioblastoma – MRI – differences
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumour in adults. The median life expectancy is 14.6 months and the prognosis is poor [1]. Gliosarcoma (GSM) is a rarer brain tumour and has a worse prognosis, although clinical and radiological findings are similar to GBM [2]. MRI is used in diagnosis, treatment planning, treatment-response evaluation and follow-up for both GBM and GSM [1,3]. There are similar radiological features in GBM and GSM, and GSM can easily be misdiagnosed as GBM [1,4]. In addition to conventional contrast MRI, the use of such techniques as diffusion-weighted imaging (DWI) and spectroscopy also increases diagnostic power [5]. Nevertheless, definitive differential diagnosis of GBM and GSM still cannot be made radiologically [6]. MRI as a non-invasive method is valuable in diagnosis [1]. There are many imaging and clinical studies about GBM and radiological-imaging findings have been evaluated many times. On the other hand, such studies involving GSM are limited. In this study, we compared conventional MRI findings for GBM and GSM with the hypothesis that they may have contributed to differential diagnosis.
Material and methods
This retrospective study was conducted with MRI images of GSM and GBM patients who were followed up in January 2016 – December 2019. The pathologically confirmed 15 GSM and 32 GBM cases that were followed up and in which sufficient quality contrast-enhanced MRI images were examined before treatment were included in the study. MRI examinations were carried out with either a 16-channel 1.5 T (OPTIMA 360, GE Healthcare, Chicago, IL, USA) or a multichannel 3 T (Achieva, Philips, Amsterdam, Netherlands) apparatus. Comparative evaluation of MRI examinations was conducted simultaneously by two radiologists. The MRI images were evaluated based on spin echo T1-WI, turbo spin echo T2-WI and flair fluid attenuated inversion recovery (FLAIR) signal properties; mass size and location; necrosis and peritumoural oedema size; cystic component; and contrast-enhancement intensity and type. The measurements are indicated on the axial scan and by measuring the biggest diameter. Necrotic area, solid component, cystic area and peritumoural oedema were determined using images with and without contrast and measurements were made accordingly. Necrotic areas were determined considering the signal characteristics such as not showing contrast enhancement and not showing cystic features. The T2 and T1 signals of the lesions were specified as hypo-iso-hyperintense, not by comparison with respect to the gray or white matter from which they originated, but with respect to white matter in terms of standardization in comparison. Homogeneous or heterogeneous contrast enhancement terms were used depending on whether the contrast enhanced area showed similar signal properties within itself.
Statistical analysis
Average, standard deviation, median, lowest, highest, frequency and ratio values were used as descriptive statistics of the data. The distribution of variables was measured by the Kolmogorov-Smirnov test. The independent - samples t-test and Mann-Whitney U test were used in the analysis of quantitative independent data. The chi-square test was used in the analysis of qualitative independent data and when chi-square test conditions were not provided, Fischer’s exact test was used. SPSS 26.0 software (IBM, Armonk, NY, USA) was also used for the analysis.
Results
Contrast-enhanced MRI images of 47 patients with a histopathological diagnosis (15 with GSM and 32 with GBM) were evaluated. The patients were 17–88 (median 57, average age 56.2 ± 16.3, SD = 11) years old. Eight of the GSM patients were males and seven were females, whereas 20 of the GBM patients were males and 12 were females (Tab. 1). Although the mean age of the GBM patients was about one decade lower than that of the GSM group, there was no statistically significant difference (Tab. 1). There was also no significant difference between the GSM and GBM patient groups in terms of sex (Tab. 1) (P > 0.05).
1. Age and sex characteristics of patients. 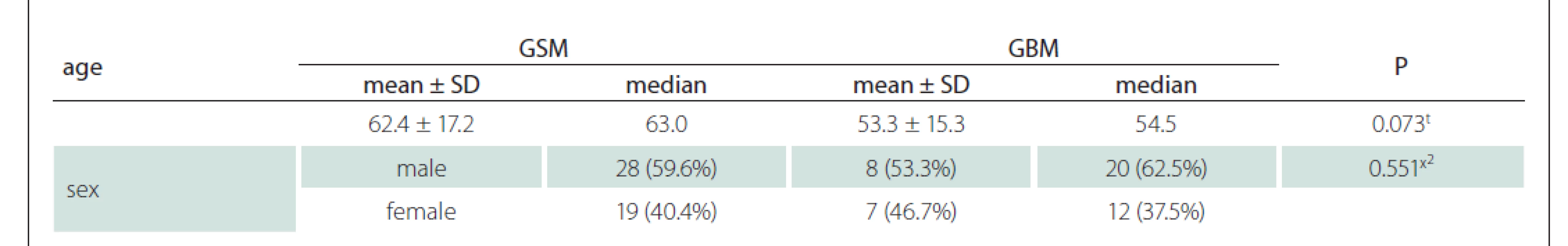
t t-test; x² chi-square test (Fischer test)
GBM – glioblastoma; GSM – gliosarcoma; SD – standart deviationUsing the MRI images (T1-WI, T2-WI and FLAIR signal properties) mass size and location, necrosis and peritumoural oedema size, cystic component, and contrast-enhancement intensity and type were evaluated. There was no significant difference between the GSM and GBM patient groups in terms of mass and oedema size along the axis of the cystic area (P > 0.05) (Tab. 2). There was significantly more necrosis in the GSM patient group (P < 0.05) (Tab. 2, Fig. 1). There was also a significantly higher rate of pronounced hyperintense T2-WI signal evaluation in the solid areas in the GSM group (P < 0.05) than in the GBM group (Fig. 2). On the other hand, there was also a significantly higher rate of isointense T2-WI signal in the solid areas in the GBM group (Fig. 3). Additionally, when the solid-component T1-WI signal was evaluated, the isointense rates were significantly higher (P < 0.05) in the GSM patient group (Fig. 2). On the other hand, the FLAIR and other T1-WI and T2-WI signal properties of the solid components did not differ significantly between the GSM and GBM patient groups (P > 0.05). There was also no significant difference between the groups in terms of contrast-enhancement intensity (P > 0.05). Additionally, the rates of peripheral and heterogeneous contrast-enhancement patterns observed in the GSM and GBM groups did not differ significantly (P > 0.05). Nonetheless, the rate of homogeneous contrast enhancement in the GSM patient group was significantly higher than in the GBM group (P < 0.05). The parietal, frontal and occipital localization rates did not differ significantly between the groups (P > 0.05), but the rate of temporal localization was significantly higher in the GSM patient group (P < 0.05). The data for all the compared features are provided in Tab. 3.
2. Comparison of groups in terms of mass, necrosis, peritumoral oedema and cystic component size. 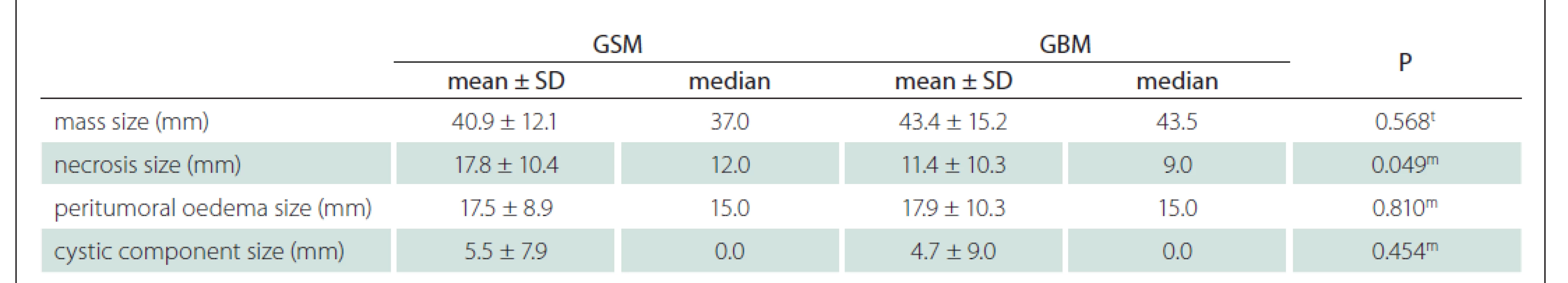
t t-test; m Mann-Whitney U test
GBM – glioblastoma; GSM – gliosarcoma; SD – standart deviationFig. 1. T2-weighted (A), T1-weighted (B) and contrast-enhanced T1-weighted (C) images of a 55-year-old male patient with gliosarcoma show width necrosis (white arrow head, A), minimal cystic area (black star, A), width oedema causing midline shift (white stars, A), haemor rhage (white arrow head, B), and peripheral contrast enhancement (white arrow head, C).
Obr. 1. Vážené snímky T2 (A), T1 (B) a postkontrastní T1 se zvýšeným kontrastem (C) 55letého pacienta mužského pohlaví s GSM ukazují šíři nekrózy (vršek bílé šipky, A), minimální cystickou oblast (černá hvězdička, A) s edémem způsobujícím přesun střední čáry (bílé hvězdičky, A), hemoragií (vršek bílé šipky, B) a periferní zvýšení kontrastu (vršek bílé šipky, C).
Fig. 2. T2-weighted (A), T1-weighted (B) and contrast-enhanced T1-weighted (C) images of a 78-year-old male patient with GSM show solid area hyperintensity on T2-WI (black star, A), isointensity on T1-WI (white arrow head, B) and homogeneous intense contrast enhancement (white arrow head, C).
Obr. 2. Vážené snímky T2 (A), T1 (B) a postkontrastní T1 (C) 78letého pacienta mužského pohlaví s GSM ukazují solidní oblast hyperintenzity na T2-váženém snímku (černá hvězdička, A), izointenzity na T1-váženém snímku (vršek bílé šipky, B) a homogenní intenzivní zvýšení kontrastu (vršek bílé šipky, C).
Fig. 3. T2-weighted (A), T1-weighted (B) and contrast-enhanced T1-weighted (C) images of a 62-year-old male patient with GBM show solid area isointensities on T2-WI (white stars, A), cystic area (black star, A), isointensities on T1-WI (white stars, B), contrast enhancement in solid areas (white arrow head, C) and cystic area with no enhancement (white star, C). Please note that there is no signifi cant necrosis in the contrast-enhanced image (C), it is only the cystic area that is not enhanced.
Obr. 3. Vážené snímky T2 (A), T1 (B) a postkontrastní T1 (C) 62letého pacienta mužského pohlaví s GBM ukazují solidní oblast izointenzit na T2-váženém snímku (bílé hvězdičky, A), cystickou oblast (černá hvězdička, A), izointenzity na T1-váženém snímku (bílé hvězdičky, B), zvýšení kontrastu v solidních oblastech (vršek bílé šipky, C) a cystickou oblast bez zvýšení kontrastu (bílá hvězdička, C). Je třeba poznamenat, že se v postkontrastním obrazu (C) nenachází žádná významná nekróza, jedná se pouze o cystickou oblast bez zvýšení kontrastu.
3. Comparison of groups in terms of T1-WI, T2-WI and FLAIR signal properties, contrast enhancement intensity and type, location of mass. 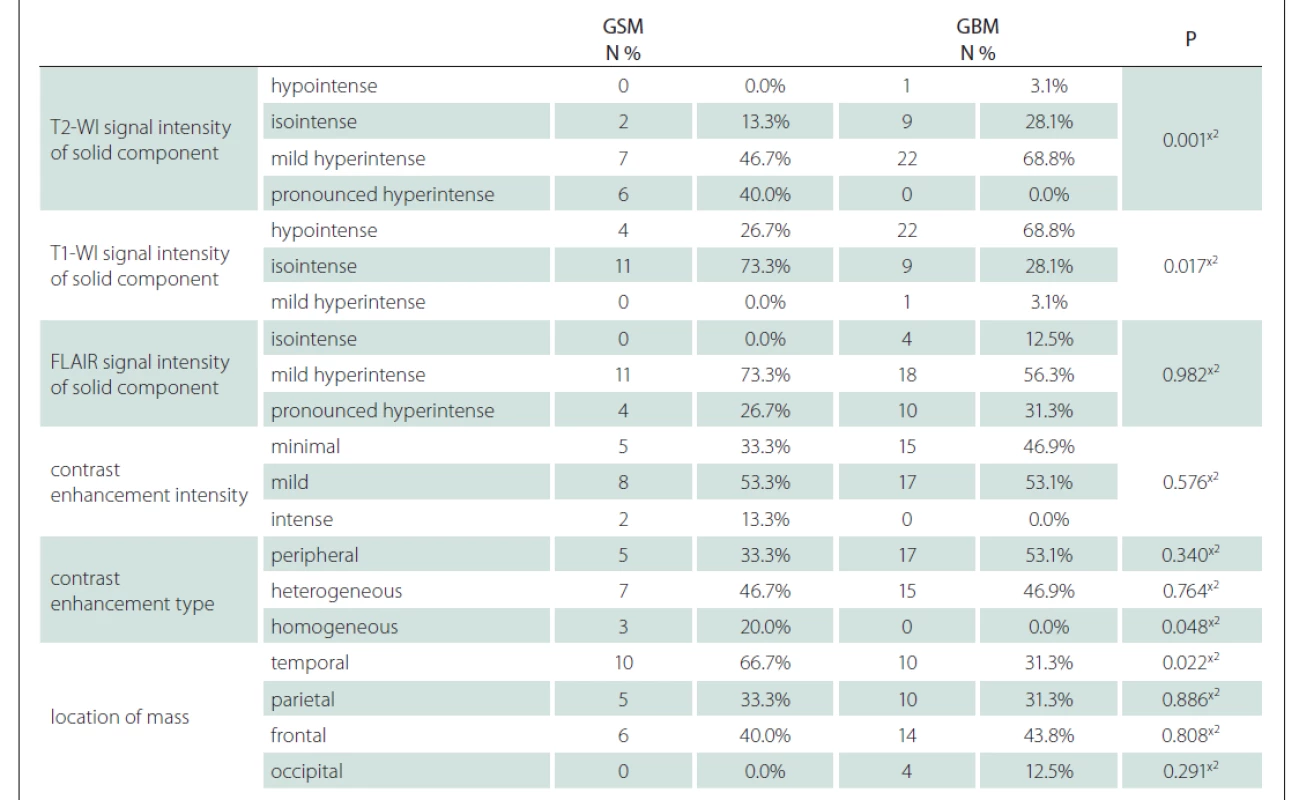
x² Chi-square test (Fischer test)
FLAIR – fl uid attenuated inversion recovery; GBM – glioblastoma; GSM – gliosarcoma; N – number; T1-WI – T1-weighted image;
T2-WI – T2-weighted imageDiscussion
Although GSM was previously defined as a rare variant of GBM with sarcomatous content [7], in the last update it was classified under the title isocitrate dehydrogenase wildtype glioblastoma [8]. Variable-imaging findings in GBM and GSM and also in many high-grade glial tumours may be similar [4]. This, therefore makes differential diagnosis difficult, but it has been reported in recent studies that some imaging findings may assist in differential diagnosis [2].
In our study, no significant difference was found between the size (long axis) averages between the GSM and GBM tumour groups. In a study with the same number of GSM cases [9], the mean mass size (long axis) was approximately 1 cm more than in our group. In a GBM study in which 79 patients were evaluated [1], the maximum mean tumour diameter was found to be 4.89 ± 1.75 cm, and this value is close to our study group’s mean tumour diameter. Peritumoural oedema size did not differ significantly between the groups. Although there are other factors that affect oedema width, it has been reported that it is related to the size of the contrast-enhanced solid part of the tumour [10]. In our study, no statistically significant difference was found between the groups in terms of oedema width, as well as in terms of mass diameter and cystic and solid component sizes, and these can be regarded as compatible findings.
When the T1-WI signals of the solid component were evaluated, the isointensity ratio was higher in the GSM group. The T1-WI signals of all cases were hypointense and they isopointed in both groups, however, with the exception of only one GBM patient. It is stated in the literature that T1-WI signals are expected to be hypo-isointense, and this is compatible with our findings [2]. In the evaluation of the T2-WI signals of solid components, there was significantly more pronounced hyperintensity detected in the GSM group than the GBM group. In a recent study [11], it was emphasized that T2-WI signals are more isointense, but the small number of cases in that study may have affected the results. On the other hand, in a study with a large number of GSM cases [4], the T2-WI signal intensities of components other than necrotic-cystic areas were more similar to those of cerebrospinal fluid, which coincides with our results. In the same study, it was stated that contrast enhancement of solid components was concentrated and done peripherally. In another study [12], 37 GBM cases were evaluated and peripheral enhancement was frequently observed. In our study, although the peripheral enhancement rate was higher in the GSM group, the homogeneous enhancement pattern was significantly higher in the GSM group due to a slightly lower rate among the GBM group.
As a result of a high metabolism in both types of tumour, a lack of adenosine triphosphate ensues, and necrosis development due to related mechanisms has been reported frequently [13,14]. On the other hand, both in our study and the study of Yi X et al [2], internal necrosis was widely reported in GSM cases, and the rate of necrosis was higher in GSM cases to the point of there being no comparison with GBM. GBM and GSM have a similar treatment response and median survival time, but GSM has been reported to have a slightly worse prognosis when compared to GBM [2,14]. It is em - immunohistochemiphasized in molecular studies that necrosis is among the properties that lead to a poor prognosis and directly contribute to high malignant features [14–16].
Our GBM cases were mostly located in the frontal lobe, whereas our GSM cases were mostly located in the temporal lobe. In statistical terms, only the GSM temporal-lobe localization was significantly higher. Similarly, the temporal-lobe localization of GSM was found to be significantly higher in the study of Yi X et al [2]. In a study conducted by Fukuda A et al [11], GSM were usually located in the frontal or temporal lobe. In a study in which the clinical responses of both tumours were evaluated using a large number of cases [17], GSM were most commonly located in the temporal lobe and GBM were most common in the frontal lobe, similar to our study.
The limitations of our study include the low number of cases, especially GSM cases; the lack of susceptibility weighted imaging (SWI) sequences or CT images, which can be used to evaluate bleeding; and no interobserver evaluation. SWI and DWI sequences were obtained in MRI examinations of some patients. However, no comparison was made for those sequences, as they were not available in all examinations.
In conclusion, GSM and GBM are both high-grade tumours, and pathological evaluation is needed for definitive differential diagnosis. Some conventional MRI findings, such as localization, size of necrosis and T2-WI hyperintensity may contribute to the diagnostic approach. On the other hand, there is a need for studies with radiopathological correlations involving a greater number of cases and additional sequence evaluation and texture analysis.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). Approval for the study was obtained from the ethics committee of Adana City Training and Research Hospital (approval dated 02.01.2018 and numbered 147).
Financial support
The authors received no financial support for the research, authorship and/ or publication of this article.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Omer Kaya, MD
Department of Radiology Faculty of Medicine Cukurova University 01240 Adana Turkey
e-mail: dr.omerkaya@gmail.com
Accepted for review: 22. 10. 2020
Accepted for print: 21. 4. 2021
Sources
1. Molina D, Pérez-Beteta J, Luque B et al. Tumour heterogeneity in glioblastoma assessed by MRI texture analysis: a potential marker of survival. Br J Radiol 2016; 89(1064): 20160242. doi: 10.1259/ bjr.20160242.
2. Yi X, Cao H, Tang H et al. Gliosarcoma: a clinical and radiological analysis of 48 cases. Eur Radiol 2019; 29(1): 429–438. doi: 10.1007/ s00330-018-5398-y.
3. Liao W, Liu Y, Wang X et al. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol 2009; 50(2): 217–225. doi: 10.1080/ 02841850802616752.
4. Sampaio L, Linhares P, Fonseca J. Detailed magnetic resonance imaging features of a case series of primary gliosarcoma. Neuroradiol J 2017; 30(6): 546–553. doi: 10.1177/ 1971400917715879.
5. Price SJ, Young AM, Scotton WJ et al. Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J Magn Reson Imaging 2016; 43(2): 487–494. doi: 10.1002/ jmri.24996.
6. Peckham ME, Osborn AG, Palmer CA et al. Gliosarcoma: neuroimaging and immunohistochemiphasized cal findings. J Neuroimaging 2019; 29(1): 126–132. doi: 10.1111/ jon.12565.
7. Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114(2): 97–109. doi: 10.1007/ s00401-007-0243-4.
8. Louis DN, Perry A, Reifenberger G et al. The 2016 WHO classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131(6): 803–820. doi: 10.1007/ s00401-016-1545-1.
9. Han L, Zhang X, Qiu S et al. Magnetic resonance imaging of primary cerebral gliosarcoma: a report of 15 cases. Acta Radiol 2008; 49(9): 1058–1067. doi: 10.1080/ 02841 850802314796.
10. Blystad I, Warntjes JBM, Smedby Ö et al. Quantitative MRI for analysis of peritumoral edema in malignant gliomas. PLoS One 2017; 12(5): e0177135. doi: 10.1371/ journal.pone.0177135.
11. Fukuda A, Queiroz LS, Reis F. Gliosarcomas: magnetic resonance imaging findings. Arq Neuro-Psiquiatr 2020; 78(2): 112–120. doi: 10.1590/ 0004-282X20190158.
12. Eidel O, Burth S, Neumann JO et al. Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: a correlation with histopathology. PLoS One 2017; 12(1): e0169292. doi: 10.1371/ journal.pone.0169292.
13. Barker FG, Davis RL, Chang SM et al. Necrosis as a prognostic factor in glioblastoma multiforme. Cancer 1996; 77(6): 1161–1166. doi: 10.1002/ (sici)1097 - 0142(19960315)77 : 6<1161::aid-cncr24>3.0.co;2-z.
14. Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol Ther 2009; 8(19): 1791–1797. doi: 10.4161/ cbt.8.19.9762.
15. Pierallini A, Bonamini M, Pantano P et al. Radiological assessment of necrosis in glioblastoma: variability and prognostic value. Neuroradiology 1998; 40(3): 150–153. doi: 10.1007/ s002340050556.
16. Smith SF, Simpson JM, Brewer JA et al. The presence of necrosis and/ or microvascular proliferation does not influence survival of patients with anaplastic oligodendroglial tumours: review of 98 patients. J Neurooncol 2006; 80(1): 75–82. doi: 10.1007/ s11060-006 - 9158-5.
17. Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro Oncol 2009 : 11(2): 183–191. doi: 10.1215/ 15228517-2008-076.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2021 Issue 3-
All articles in this issue
- Ventilation treatment in patients with amyotrophic lateral sclerosis
- Developmental dysphasia – functional and structural correlations
- Proteomics of cerebrospinal fluid in pediatric oncology patients
- Surgical treatment possibilities of drug-resistant Ménière‘s disease
- Malignant prolactinoma – can a long-term survival be affected?
- Surgery for idiopatic glossopharyngeal neuralgia – case report and overview of therapeutic modalities
- Ethylenglycol poisoning
- Guidelines on intravenous thrombolysis in the treatment of acute cerebral infarction – 2021 version
- Aktualita z kongresu AAN 2021
- Comparison of MRI findings of glioblastoma and gliosarcoma – can conventional MRI provide beneficial differences for diagnosis?
- Selective expanded polytetrafluorethylene patch angioplasty vs. primary closure after carotid endarterectomy – a 13-year experience
- Age-dependent specifics of epidural hematoma
- Multifocal, metaphyseal osteonecrosis of knee due to pulse steroid treatment after cessation of fingolimod treatment in a 19-week-pregnant patient
- Polymorphous low-grade neuroepithelial tumor of the young
- Treatment of a large supraclinoid aneurysm via clipping with a prophylactic low-flow bypass
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Developmental dysphasia – functional and structural correlations
- Guidelines on intravenous thrombolysis in the treatment of acute cerebral infarction – 2021 version
- Ethylenglycol poisoning
- Surgical treatment possibilities of drug-resistant Ménière‘s disease
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

