-
Medical journals
- Career
Predictive Values of the Ultrasound Parameters, CA-125 and Risk of Malignancy Index in Patients with Ovarian Cancer
Authors: S. V. Antovska 1; N. Bashevska 2; N. Aleksioska 3
Authors‘ workplace: Department for Urogynaecology and Pelvic Floor Disorders, University Clinic for Gynaecology and Obstetrics, Medical Faculty, University Saint Cyril and Methodius, Skopje, Republic of Macedonia 1; Department of Histopathology and Clinical Cytology, Institute of Radiotherapy and Oncology, Medical Faculty, University Saint Cyril and Methodius, Skopje, Republic of Macedonia 2; University Clinic for Gynaecology and Obstetrics, Medical Faculty, University Saint Cyril and Methodius, Skopje, Republic of Macedonia 3
Published in: Klin Onkol 2011; 24(6): 435-442
Category: Original Articles
Overview
Backgrounds:
Assessment of predictive values for CA-125, ultrasound features (US) and risk of malignancy index (RMI) in ovarian malignancy.Material and Methods:
115 patients, divided into: 1) group-A (n = 41) – ovarian malignancy; group-B (n = 74) – benign ovarian tumor; 2) subgroup-CA(a) with low CA-125 (< 35 U/mL) (n = 64); subgroup-CA(b) with slightly elevated CA-125 (35–130 U/ml) (n = 26); subgroup-CA(c) with high CA-125 (> 130 U/ml) (n = 25).Results:
1) patients of group-A were older (p < 0.05); CA-125 < 35 U/ml predominated in group-B (p < 0.001); 2) CA-125 < 35 U/ml showed relatively high NPV, sensitivity and specificity (82.8%; 0732; 0.716, respectively). Our proposed graduation of CA-125 into three grades: a) < 35 U/mL; b) 35–130 U/mL; c) > 130 U/mL increased the specificity for both parameters: CA125 = 35–130 U/mL up to 0.811, and for CA-125 > 130 U/mL up to 0.905, and PPV for the latter parameter up to 72.0%; 3) US: a) highest sensitivity, as indicator for best distinguishing of diseased patients, showed: rugged margins and presence of septum/vegetations (0.878; 0.897, respectively); b) highest specificity, as indicator for best distinguishing of healthy patients: clear distinguish ability of tumor from surrounding tissue and absence of ascites (0.811; 0.932, respectively); c) presence of ascites had highest PPP (100%) i.e. it was the best malignancy predictor; 4) RMI showed only relatively high NPV for RMI ≤ 200 (76.8%); 4) additional analysis of RMI in correlation with proposed CA-125 gradation increased the predictive values of RMI: a) subgroup-CA(a): NPV and sensitivity for RMI ≤ 200 (81.6%; 0.818, respectively) and NPV for RMI > 200 (86.7%); b) subgroup-CA(b): specificity for RMI ≤ 200, as good indicator for distinguishing healthy patients (0.929); c) subgroup-CA(c): sensitivity for RMI > 200, as good indicator for distinguishing diseased patients (0.944).Conclusion:
CA-125 and US, as single criteria were not accurate. RMI is good indicator only in correlation with CA-125.Key words:
ovarian neoplasms – serum – tumor markers – CA-125 protein – ultrasonography – prognosisCondesation:
Serum-CA-125 and ultrasound findings as single criteria in malignancy diagnosis of ovarian tumors are not enough accurate. RMI increases the accuracy of the preoperative diagnosis.Backgrounds
Ovarian cancer is the most lethal gynecologic malignancy in adult women. The 5-year survival rate for stage III and IV is 31%, and for stage I is 95% [1]. Unfortunately, early diagnosis is difficult because the lack of specific symptoms in early disease due to inaccessible location of the ovaries. Common symptoms, such as abdominal bloating and early satiety, indicate more advanced disease, involving the upper abdomen and present in approximately 70% of patients at the time of diagnosis [2]. Because of the fact that currently available screening tests do not achieve high levels of sensitivity and specificity, screening is not recommended for the general population, but only for women at high risk (strongly family history of ovarian cancer and those with BRCA 1 or BRCA 2 mutations). Trans-vaginal ultrasound with its high resolution enables detailed scrutiny of ovarian lesions [3]. According to certain authors [4,5], the anechoic cysts with thin walls, without inner or outer nodularity, and associated with CA-125 levels within normal limits (< 35 U/ml) can be safely punctured. As some studies have proved [6], ovarian malignancy at its very early stage can be present in a small simple cyst or even in a normal-sized ovary. All subjects were given an explanation of the study and written informed consent was obtained.
The quantitative evaluation of some tumor markers, such as: CEA, CA-125, CA-72-4 or even CA-19-9, since certain time has been considered as valid method which can indicates of the malignant potential of ovarian tumors. So, in 1994 Woolas et al [7] reported about a screening program of the ovarian cancer with CA-125 evaluation. CA-125 is a glycoprotein, which expression can be found in almost all cells of the coelomic epithelium. In 1% of healthy women, elevated values of this tumor marker can be found in the absence of some cancer process [8]. In about 80% of women with non-mucinous epithelial cancer the serum levels of CA-125 is higher than 35 U/ml, but it is not rare the situation when the CA-125 is into the normal levels in presence of ovarian cancer in earlier stages [9]. In a review of several studies only 44% of women with ovarian cancer stage I had increased levels of CA-125 [10]. This is the main disadvantage of this method, as well as its increase during some physiological or benign pelvic diseases, such as: pelvic endometriosis, pelvic inflammatory disease or first trimester pregnancy. The combination of the ultrasound evaluation and the estimation of the serum levels of CA-125 could be an effective method for screening of ovarian cancer. A simple algorithm called risk of malignancy index (RMI), reported by Jacobs et al [11] or RMI 1, which incorporated the serum CA-125 level, menopausal status and ultrasound morphological features gave sensitivity of 85.4% and specificity of 96.9%. Currently, the network guidelines recommend calculation of the RMI [12] as modified by Tingulstad et al [13] or RMI 2.
Material and Methods
Eligibility criteria
- the presence of ovarian tumor required surgical treatment
The setting, location and timing
- University Clinic of Gynecology and Obstetrics, Medical Faculty, Skopje, in the period from the 1st of January 2009 to the 1st of January 2010
Precise details of the interventions
The whole study group was consisted of patients with presence of ovarian tumor (n = 115). The experimental group: group A (n = 41) was consisted of patients with histological feature of ovarian malignancy, but control group: group B (n = 74) of patients with histological feature of benign ovarian tumor. In every group, the Serum CA-125 level, ultrasound evaluation and RMI were performed preoperatively. Additionally, the whole study group was divided into three subgroups regarding the serum CA-125 levels:
- subgroup CA(a) with low serum levels of CA-125 (< 35 U/mL) (n = 64);
- subgroup CA(b) with slightly elevated serum levels of CA-125 (35–130 U/ml) (n = 26);
- subgroup CA(c) with high serum levels of CA-125 (> 130 U/ml) (n = 25). The study was approved by the local research ethics committee (LREC) of the Association of Gynecologists and Obstetricians of Macedonia.
How sample size was determined
Every patient who had ovarian tumor required operative treatment, assessed for eligibility (n = 121). Six patients were excluded because they refused to be operated. So, 115 patients were randomised.
Aim of the work
- to estimate the predictive values of: Serum CA-125, ultrasound findings and RMI as a single criterion for malignancy;
- to estimate the predictive values for RMI in cases with mildly elevated Serum CA-125 (35–130 U/ml), levels, which are doubtful for malignancy;
- to establish the most accurate ultrasound parameter for malignancy;
- to estimate whether our proposed graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL can improve the accuracy of the parameters: CA-125 and RMI in prediction of ovarian malignancy.
Specific hypotheses
- serum CA-125, ultrasound findings and RMI as a single criterion are not enough reliable parameters for excluding ovarian malignancy;
- RMI > 200 in cases with mildly elevated Serum CA-125 (35–130 U/ml), is not enough reliable parameter for ovarian malignancy;
- presence of ascites is the most accurate ultrasound parameter for malignancy, but tumor size ≤ 6 cm and cystic structure for benign tumor nature;
- our proposed graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL could improve the accuracy of RMI in prediction of ovarian malignancy.
Clearly defined primary and secondary outcome measures
The preoperative evaluation:
- Demographic data;
- Ultrasound examination, regarding: size, tumor structure, distinguish ability of the tumor from the surrounding tissue, spread of the tumor, presence of the ascites, feature of margins, thickness of the capsule if the tumor was cystic, as well as presence of septum/papillary vegetation or quality of liquor into the cystic tumor;
- Preoperative value of Serum CA-125 with ECI (enhanced chemiluminiscence technique) with original CA-125 II tm kit (Johnson & Johnson);
-
The modified RMI according to Tingulstad et al [13] for each woman was calculated using the formula:
RMI = U × M × serum CA-125
Five ultrasound features suggestive of malignancy were sought to derive the ultrasound score (U): multilocular feature, presence of solid elements, bilateral appearance, presence of ascites, evidence of metastases. An ultrasound score (U) of 1 was given if none or one of these features was present, and a score of 3 was given if two or more of these features were detected. A menopausal score (M) of 1 or 3 was given to pre - and postmenopausal women, respectively. Referral to the Northern Gynaecological Oncology Centre [14] the value RMI > 200 was considered as sign of malignancy.
Histopathological examinations: The operative specimens were fixed in 10% neutral buffered formalin for 24 to 48 hours and routinely processed in paraffin wax. They were examined by light microscopy by the same pathologist, who was not informed of the patient group.
Statistical methods: The Student’s paired test was used to compare: demographic data. The predictive values, as well as sensitivity and specificity, as indicators of how well those patients with disease or non-disease were correctly classified, were analyzed according to the standard formulae [15].
- Positive predictive value (PPV) = individuals with ovarian malignancy and a positive test / all those with a positive test
- Negative predictive value (NPV) = individuals without ovarian malignancy and a negative test / all those with a negative test
- Sensitivity = individuals with ovarian malignancy and a positive test / all those with ovarian malignancy
- Specificity = individuals without ovarian malignancy and negative test / all those without ovarian malignancy
The Mantel-Haenzel’s X2 test was used to compare: demographic data, Serum CA-125 and ultrasound characteristics, according to the formula:
X2 = n ([AD–BC]–n/2)2
(A+B)(C+D)(A+C)(B+D)
Results
There were some significant differences in demographic data between the groups. Namely, puberty and adolescence age was most frequent among the patients in group B (p < 0.05), but senium in group A (p < 0.05). In total, the patients with ovarian malignancy were significantly older than those with benign ovarian tumors (p < 0.05) (Tab. 1).
1. Demographic data: age, gynecological age, parity, familiar history for ovarian tumor or cancer, habits of smoking and alcohol consuming, body mass index. 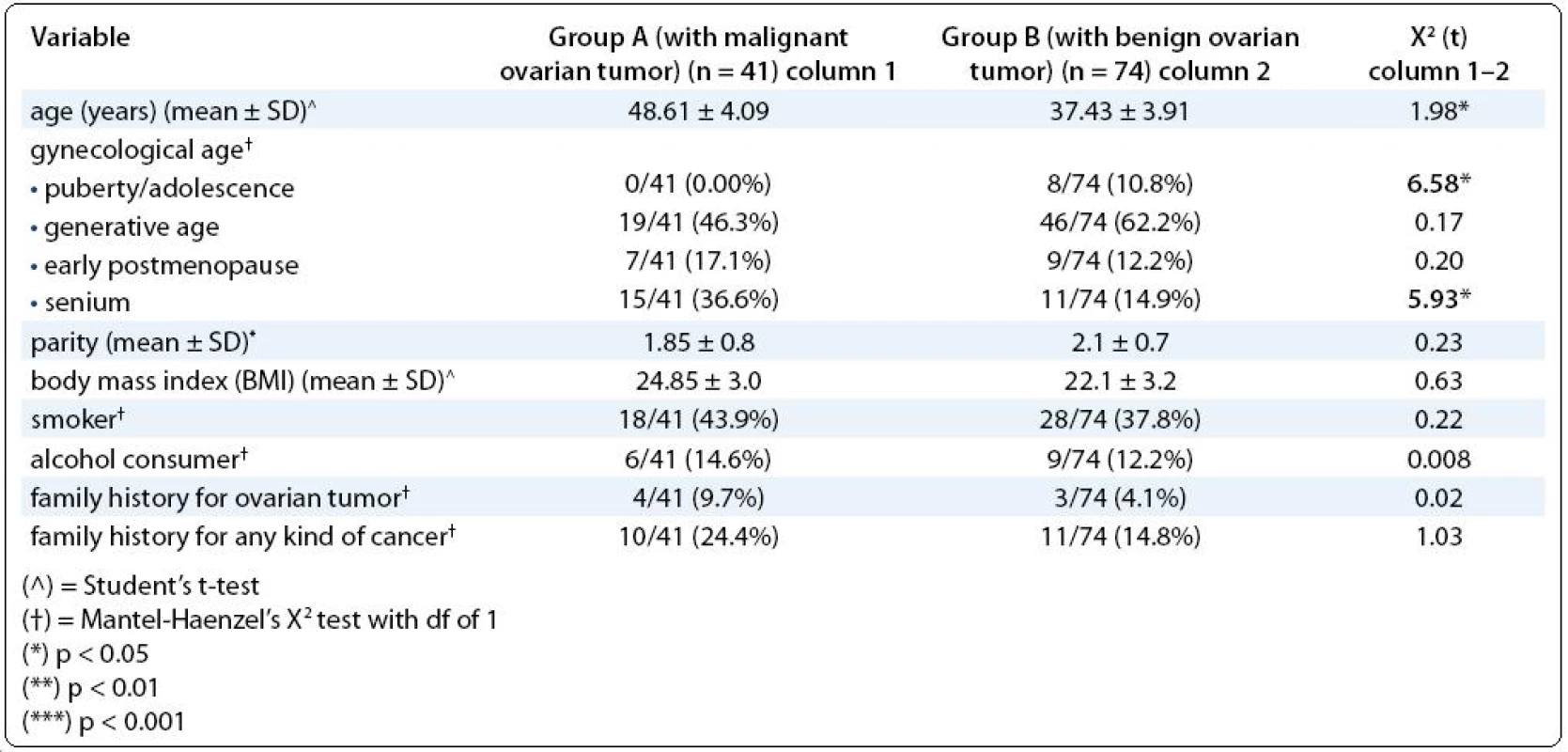
On the Tab. 2 we represent the differences between the groups regarding the preoperative Serum CA-125. The subjects with Serum CA-125 < 35 U/ml were more frequent in group B vs group A (p < 0.001), but those with Serum CA-125 ≥ 35 U/ml were more frequent in group A vs group B (p < 0.001). Nevertheless, there were no differences in prevalence of patients with mildly elevated CA-125 (35–130 U/ml) between the groups. The value of Serum CA-125 < 35 U/ml showed a relatively high NPV for malignancy, as well as sensitivity and specificity (82.8%; 0732; 0.716 respectively). The value of Serum CA-125 U/ml ≥ 35 U/ml showed the same high sensitivity and specificity as previous parameter. Nevertheless, our proposed graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL increased the specificity for both parameters: CA125 = 35–130 U/mL up to 0.811, and for CA-125 > 130 U/mL up to 0.905, as well as PPV for the latter parameter up to 72.0% (Tab. 2).
2. Differences among the both study groups regarding the preoperative CA-125 serum level (our proposed graduation of the serum CA-125 levels). 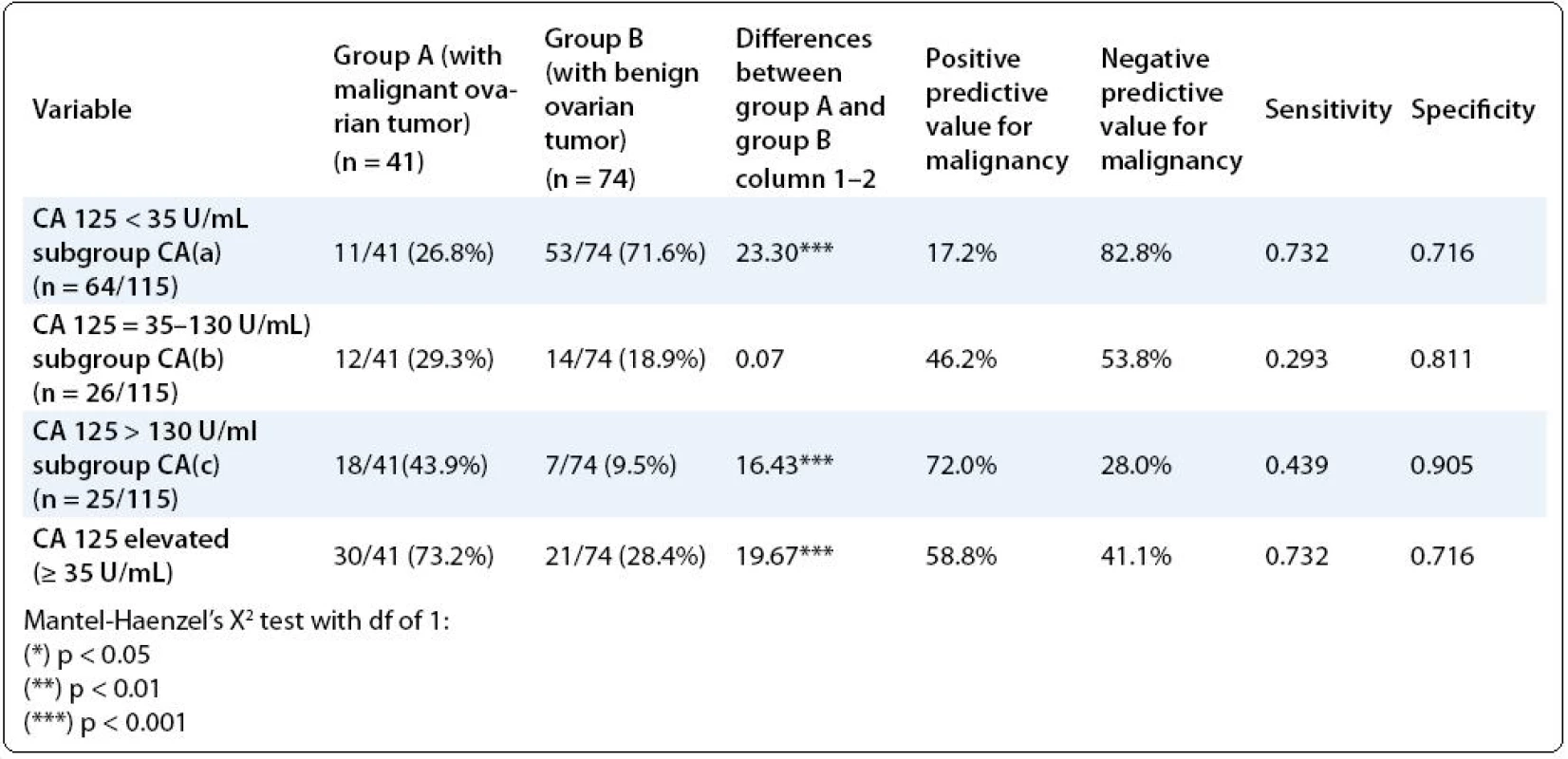
The Tab. 3 represents the differences between the groups regarding the ultrasound features of the ovarian tumors, as well as the predictive values of each particular one. Almost all ultrasound features showed very significant differences between the groups. So, the ultrasound characteristics such as: tumor size > 6 cm, structure of the tumor (solid/cystic), unclear distinguish ability of the tumor, presence of the ascites into the abdominal cavity, rugged margins, thickness of the capsule > 2 mm, presence of septum/papillary vegetation into the cyst thicker than 2 mm, multi-locular feature and presence of intra-cystic liquor with high density were more frequent in patients with malignant ovarian tumor (p < 0.001, all of them). Regarding the PPV > 70.0%, as a likelihood of the ultrasound feature for that the individual has a malignant disease, only the presence of ascites into the abdominal cavity showed the high one. The NPV > 70.0% as a likelihood that the individual is free from malignant disease, was found for the following ultrasound features: tumor size ≤ 6 cm, solid tumor structure, cystic tumor structure, clear distinguish ability of the tumor from the surround tissue, unilateral tumor, absence of the ascites, smooth margins, capsule ≤ 2 mm, absence of septum/papillary vegetation into the cysts ≤ 2 mm, uni-locular cyst, intra-locular liquor with low density. The sensitivity > 0.700, as an indicator of how well those patients with disease are correctly classified according to the particular ultrasound feature, was noted only for: uni-laterality of the tumor, rugged margins, thicker than 2 mm capsule, septum/papillary vegetation thicker than 2 mm, and intra-locular liquor with high density. The specificity > 0.700, as an indicator of how well those without malignant disease are cor-rectly classified according to particular ultrasound feature, was noted for the following features: tumor size ≤ 6 cm, cystic structure, clear distinguish ability of the tumor from the surround tissue, absence of the ascites, smooth margins and uni-locular feature.
3. Ultrasound characteristics of the ovarian tumors. 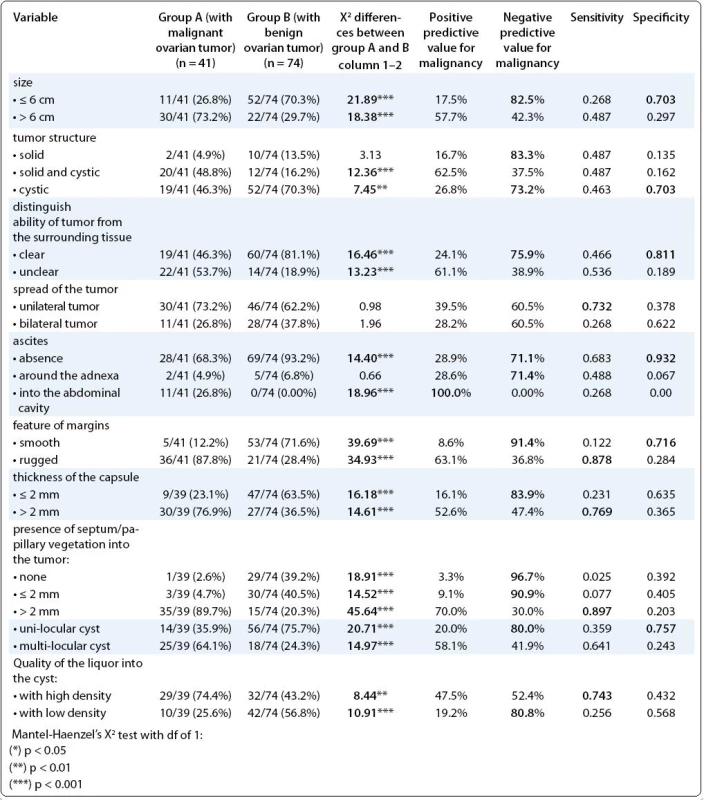
Nevertheless, the highest sensitivity, as an indicator for best distinguishing the diseased patients, showed the following parameters: rugged margins and presence of septum/vegetations, (0.878; 0.897, respectively). The following ultrasound parameters showed the highest specificity, as an indicator for best distinguishing the healthy patients: clear distinguish ability of tumor from the surrounding tissue and absence of ascites (0.811; 0.932, respectively). The parameter: presence of the ascites into the abdominal cavity showed the highest PPP (100%) i.e. it was the best ultrasound predictor for ovarian malignancy (Tab. 3).
The histological features in the first study group-A (n = 41) consisted of patients with malignant ovarian tumor was as follows: serous adenocarcinoma (12/41), mucinous cystadenocarcinoma (7/41), endometrioid adenocarcinoma (12/41) clear cell ovarian carcinoma (5/41), others ovarian carcinomas (5/41). The stage of spreading was: borderline (IA) (10/41); stage IC (13/41); stage II (2/41); IIIA (4/41); IIIB (3/41); III C (9/41). The nuclear grade in the same group was as follows: G1 (10/41), G2 (14/41, G3 (17/41).
The histological features in the first study group-B (n = 74), consisted of patients with benign ovarian tumor was as follows: serous ovarian cystadenoma (12/74), mucinous ovarian cystadenoma (13/74), benign terathoma (12/74) and simplex cysts (11/74) and endometrioma (26/74).
In order to estimate whether our proposed graduation of serum CA-125 levels into abovementioned three grades could improve the accuracy of RMI in prediction of ovarian malignancy, we made:
- the predictive values for RMI for entire study group;
- the predictive values for the three particular parts of RMI: menopausal status, ultrasound features and Ca-125 levels, as a single criterion;
- the predictive values for RMI for the three abovementioned subgroups according to our proposed graduation of CA-125.
So:
- the pre-menopausal status as a single criterion, noted with point 1 in RMI, was more frequent in group B (p < 0.01) and showed relatively high NPV for malignancy (74.0%). The post-menopausal status, noted with point 3 in RMI, was more frequent in group A (p < 0.01), and showed relatively significant specificity for malignancy (0.729);
- the ultrasound criteria, noted with point 1 in RMI, as an indicator for benign tumor nature, predominated in group A (p < 0.001) and showed high NPV of 81.3%. On the contrary, the ultrasound criteria, noted with point 3 in RMI, as an indicator for malignant tumor nature, predominated in group B (p < 0.001), but their predictive values were low. Nevertheless, they showed relatively high sensitivity and specificity (0.707 and 0.703, respectively);
- RMI for entire study group, as an indicator for risk of tumor malignancy showed only relatively high NPV for RMI ≤ 200 (76.8%);
- when we additionally analyzed RMI in correlation with CA-125 levels, we found that its predictive values and sensitivity/specificity increased. So, in subgroup CA(a) with CA-125 < 35 U/ml we noted this situation: high NPV and sensitivity (81.6% and 0.818, respectively) for RMI ≤ 200; but also high NPV (86.7%) for RMI > 200. In subgroup CA(b) with CA-125 between 35 and 130 U/ml, we noted very high specificity, as an indicator for good distinguishing of healthy patients (0.929) for RMI ≤ 200. In patients with CA-125 > 130 U/mL, RMI > 200 showed very high sensitivity, as an indicator for good distinguishing of diseased patients (0.944) (Tab. 4).
4. Risk of malignancy index (RMI), its particular parts as a single criterion, such as menopausal status and ultrasound features, and the correlation between RMI and serum CA-125 levels. 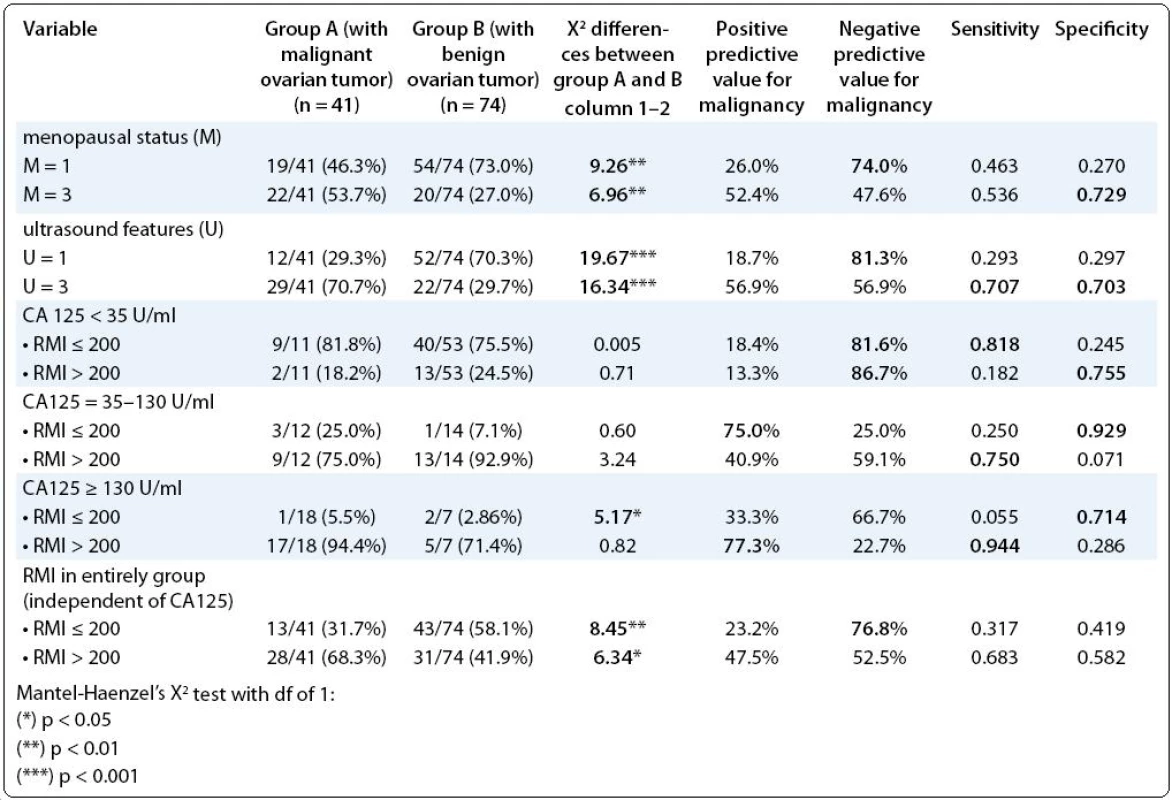
Discussion
Estimating the predictive values of different parameters for tumor malignancy: such as: serum CA-125, ultrasound findings and RMI, as a single criterion, we noted them relatively insignificant, that pertain to the uncertain diagnosis for ovarian malignancy. These findings confirmed our first specific hypothesis.
Nevertheless, when we divided all patients into three subgroups, according to our new proposed graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL, we noted:
- increased specificity of RMI ≤ 200 up to 0.929 for patients with mildly elevated CA-125 (35–130 U/mL), as an indicator for good distinguishing of healthy patients;
- increased sensitivity of RMI > 200 up to 0.944 for patients with highly elevated CA-125(> 130 U/mL), as an indicator for good distinguishing of diseased patients. These findings confirmed our second specific hypothesis that the RMI > 200 in cases with mildly elevated Serum CA-125 (35–130 U/ml), is not enough reliable parameter for ovarian malignancy; These results also confirmed our forth hypothesis that our proposed graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL could improve the accuracy of RMI in prediction of ovarian malignancy. Therefore, our opinion is that patients with mildly elevated CA-125 (35–130 U/mL) and RMI > 200 necessarily should be undergone additional diagnostic methods, including operation and histological verification. Tsukishiro et al [16], using an serum secretory leukocyte protease inhibitor levels (SLPI) cut-off of 50 ng/ml and a CA-125 cut-off of 30 U/mL, found that with both markers elevated, the sensitivity was 95%, the specificity was 100%, the PPV was 100%, and the NPV 89% between the malignant and benign cysts. Rzymski et al [17], evaluating the serum concentrations of soluble intracellular adhesion molecule-1 (sICAM-1), as well as CA-125 in 45 women with benign ovarian tumors, observed higher sICAM-1 concentrations in fibrothecomas and lower in endometrial and dermoid cysts. Serum ICAM-1concentrations correlate with some histological types of benign tumors, but not with tumor volume. Levels of CA-125 were more effective than ICAM-1 in ovarian tumors differentiation.
Regarding the ultrasound features, in our study:
- presence of ascites into the abdominal cavity showed highest PPV (100%) i.e. it was the best ultrasound predictor for ovarian malignancy;
- the parameters: clear distinguish ability of tumor from the surrounding tissue and absence of ascites showed highest specificity, i.e. they were the best parameters for good distinguishing of healthy patients (0.811; 0.932, respectively). These findings only partially confirmed our third specific hypothesis.
Sagiv et al [18], in their study of 21 patients with extremely large cystic/complex ovarian cysts, reached the umbilicus or higher, and were not associated with ascites or enlarged pelvic or para-aortic lymph nodes on computed tomography scan, found Serum CA-125 levels within the normal range or mildly elevated (< 130 U/mL). In one of them (1/21, 4.8%) they found ovarian malignancy. Geomini et al [19], estimating 181 women with ovarian masses with three-dimensional ultrasonography and three-dimensional power Doppler, found that: central vessels were present in 15% of the benign masses, 69% of the malignant masses and 27% of the masses of borderline malignancy; and the likelihood ratios for presence of central vessels for a mass being malignant and//or borderline was 4.9 (95%, CI 2.1–12).
In order to realize our second aim of the work, we analyzed the predictive values for RMI in cases with mildly elevated Serum CA-125 (35–130 U/ml), levels, which are doubtful for malignancy and found very high specificity (0.929) for RMI ≤ 200; and high sensitivity (0.750) for RMI > 200; but also high PPV for RMI ≤ 200 (75.0%), the fact which confirmed our suspicion that this group of patients could be the most confounding regarding the nature of the ovarian tumor, and the decision for operative treatment. On the other hand, we found high NPV (86.7%) for RMI > 200 in subgroup CA(a) with CA-125 < 35 U/ml. These abovementioned findings: high PPV for RMI ≤ 200 in subgroup CA(b) with slightly elevated CA-125 and also high NPV for RMI > 200 in subgroup CA(A) with low CA-125 were in favor of the parameter CA-125 vs the parameter RMI in patients with Ca-125 lower than 130 U/mL regarding the prediction of ovarian malignancy.
Ulusoy et al [20], assessing the ability of the RMI in 296 women with adnexal masses found that the RMI with cut-off level of 153 identified malignant cases more accurately than any individual criterion (PPV of 65.9%; NPV of 85.5%; sensitivity of 0.764 and specificity of 0.779). Bailey et al [21] in their series of 182 patients with a pelvic mass found that 24% patients had benign tumors, 6% had tumors of borderline malignancy, and 70% had invasive tumors. An RMI > 200 had a sensitivity of 88.5% for diagnosing invasive lesions. The overall sensitivity of this algorithm for diagnosing all borderline, invasive ovarian tumors, or primary peritoneal lesions was 87.4% and the PPV was 86.8%. Therefore, they recommend the RMI for continued use. Szpurek et al [22] assessing the usefulness of their artificial neural network computer model, which included: age, menopausal status, BMI, grayscale and Doppler ultrasonographic features, as well as levels of CA-125 and tissue polypeptide specific antigen, in series of 686 women, found very high sensitivity and specificity of this method in prediction of ovarian tumor malignancy (96.0% and 97.7%, respectively). Roupa et al [23] proposed the combination of their Transvaginal Ultra Sonography score ≥ 35 points and CA-125 ≥ 30 U/ml as an accurate screening procedure for ovarian malignancy. In their retrospective case-control pilot study of 120 women with ovarian neoplasia, they found a sensitivity of 81.7% and specificity of 100.0% of this combination. Moolthiya et al [24] compared the ability of two risk of malignancy indices to discriminate between benign and borderline/malignant ovarian tumor in 209 women with pelvic masses and found that cut-off 200 gave sensitivity of 70.6%, specificity of 83.9%, PPV of 75% and NPV of 80.6% for RMI 1, and sensitivity of 80%, specificity of 78.2%, PPV of 71.6% and NPV of 85.1% for RMI 2. In our study of 115 women with ovarian masses, the Tingulstad’ RMI gave sensitivity of only 47.5%, specificity of 52.5%, PPV of 68.3% and NPV of 58.2% for RMI > 200 and CA-125 > 35 UI/ml, but sensitivity of 94.4% and PPV of 77.3% for RMI > 200 and CA-125 > 130 UI/ml. Van den Akker et al [25] in series of 548 women with ovarian masses found PPV of 48% and NPP of 96% for RMI > 200 in detection of ovarian cancer. Montagnana et al [26] evaluated the predictive values of their ROMA (Risk of malignancy algorithm) as separate logistic regression algorithm in 104 women with a pelvic mass (55 with epithelial ovarian cancer and 49 benign cases) and found that ROMA had high predictive values (84.6% specificity and 82.5% sensitivity) only for postmenopausal women, but not in pre-menopausal women.
Conclusion
We found Serum CA-125, ultrasound findings and RMI, as single criteria for malignancy not enough accurate. In the subgroup of patients with CA-125 between 35 and 130 U/ml we noted high PPV and specificity (75.0% and 0.929, respectively) for RMI < 200; and high sensitivity (0.750) for RMI > 200. These results for RMI confirmed our suspicion that this subgroup of patients could be the most confounding regarding the nature of the ovarian tumor, and the decision for operative treatment. We recommend that the RMI should be interpreted carefully and only in correlation with serum levels of CA-125, for every case individually. For that reason we propose a graduation of serum CA-125 levels into three grades: less than 35 U/mL; between 35 and 130 U/mL; and more than 130 U/mL and analyzing the RMI in light of this graduation. This procedure could improve the accuracy of RMi in prediction of ovarian malignancy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Vesna S. Antovska, PhD, Prof.
Department for Urogynaecology and Pelvic Floor Disorders
University Clinic for Gynaecology and Obstetrics
Medical Faculty University Saint Cyril and Methodius
Skopje
Republic of Macedonia
e-mail: vantovska@yahoo.com
Submitted: 30. 5. 2011
Accepted: 29. 7. 2011
Sources
1. Fields MM, Chevlen E. Ovarian cancer screening: a look at the evidence. Clin J Oncol Nurs 2006; 10(1): 77–81.
2. Berkenblit A, Cannistra SA. Advances in the management of epithelial ovarian cancer. J Reprod Med 2005; 50(6): 426–438.
3. Timor-Tritsch IE, Peisner DB, Montegudo A. Vaginal sonographic puncture procedures. In: Timor-Tritsch IE, Rottem S (eds). Transvaginal Sonography. New York: Elsevier 1991.
4. Hurwitz A, Yagel S, Zion I et al. The management of persistent clear pelvic cysts diagnosed by ultrasonography. Obstet Gynecol 1988; 72 (3 Pt 1): 320–322.
5. Hill LM, Nyberg DA. Transvaginally guided procedures. In: Nyberg Da, Hill LM, Böhm-Velez M et al (eds). Transvaginal Ultrasound. St Lous: Mosby Year Book 1992.
6. Kurjak A, Shalan H, Matijevic R et al. Stage I ovarian cancer by transvaginal color Doppler sonography: a report of 18 cases. Ultrasound Obstet Gynecol 1993; 3(3): 195–198.
7. Woolas R, Jacobs I, Davies A et al. What is the true incidence of primary fallopian tube carcinoma? Int J Gynecol Cancer 1994; 4(6): 384–388.
8. Bast RC Jr, Klugg TL, St John E et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309(15): 883–887.
9. Hawkins RE, Roberts K, Wittshaw E et al. The clinical correlates of serum CA125 in 169 patients with epithelial ovarian carcinoma. Br J Cancer 1989; 60(4): 634–637.
10. Maggino T, Gadducci A, D’Addario V et al. Prospective multicentar study on CA125 in postmenopausal pelvic masses. Gynecol Oncol 1994; 54(2): 117–123.
11. Jacobs I, Oram D, Fairbanks J et al. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990; 97(10): 922–929.
12. Obeidat BR, Amarin ZO, Latimer JA et al. Risk of malignancy index in the preoperative evaluation of pelvic masses. Int J Gynaecol Obstet 2004; 85(3): 255–258.
13. Tingulstad NS, Hagen B, Skjeldstad FE et al. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol 1996; 103(8): 826–831.
14. Bailey J, Tailor A, Naik R et al. Risk of malignancy index for referral of ovarian cancer cases to a tertiary center: does it identify the correct cases? Int J Gynecol Cancer 2006; 16 (Suppl 1): 30–34.
15. Daya S. Diagnostic test–predictive values. Evidence-based Obstet Gynec 2005; 7 : 71–73.
16. Tsukishiro S, Suzumori N, Nishikawa H et al. Use of serum secretory leukocyte protease inhibitor levels in patients to improve specificity of ovarian cancer diagnosis. Gynecol Oncol 2005; 96(2): 516–519.
17. Rzymski P, Opala T, Woźniak J et al. Assessment of soluble intracellular adhesion molecule-1 (sICAM-1) in women with benign ovarian tumors. Ginekol Pol 2004; 75(10): 785–792.
18. Sagiv R, Golan A, Glezerman M. Laparoscopic management of extremely large ovarian cysts. Obstet Gynecol 2005; 105(6): 1319–1322.
19. Geomini PM, Kluivers KB, Moret E et al. Evaluation of adnexal masses with three-dimensional ultrasonography. Obstet Gynecol 2006; 108(5): 1167–1175.
20. Ulusoy S, Akbayir O, Numanoglu C et al. The risk of malignancy index in discrimination of adnexal masses. Int J Gynaecol Obstet 2007; 96(3): 186–191.
21. Bailey J, Tailor A, Naik R et al. Risk of malignancy index for referral of ovarian cancer cases to a tertiary center: does it identify the correct cases? Int J Gynecol Cancer 2006; 16 (Suppl 1): 30–34.
22. Szpurek D, Moszynaski R, Smolen A et al. Artificial neural network computer prediction of ovarian malignancy in women with adnexal masses. Int J Gynaecol Obstet 2005; 89(2): 108–113.
23. Roupa Z, Faros E, Raftopoulos V et al. Serum CA 125 combined with transvaginal ultrasonography for ovarian cancer screening. In Vivo 2004; 18(6): 831–836.
24. Moolthiya W, Yuenyao P. The risk of malignancy index (RMI) in diagnosis of ovarian malignancy. Asian Pac J Cancer Prev 2009; 10(5): 865–868.
25. van den Akker PA, Aalders AL, Snijders MP et al. Evaluation of the Risk of Malignancy Index in daily clinical management of adnexal masses. Gynecol Oncol 2010; 116(3): 384–388.
26. Montagnana M, Danese E, Ruzzenente O et al. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med 2011; 49(3): 521–525.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2011 Issue 6-
All articles in this issue
- Plasminogen Activator System and its Clinical Significance in Patients with a Malignant Disease
- Castleman Disease
- Naše päťročné výsledky in vitro testovania chemorezistencie u onkologických pacientov
- The Late Effects in Patients Treated with Allogeneic Hematopoietic Stem Cell Transplantation
- The Role of Chemotherapy and Targeted antiVEGF- and antiEGFR-Therapy in Metastatic Colorectal Cancer: a Case Report of Long-Term and Intensive Response
- Trabectedin Registry
- Positron Emission Tomography in the Diagnosis and Monitoring of Patients with Nonseminomatous Germ Cell Tumours
- Predictive Values of the Ultrasound Parameters, CA-125 and Risk of Malignancy Index in Patients with Ovarian Cancer
- Recent Patterns in Stomach Cancer Descriptive Epidemiology in the Slovak Republic with Reference to International Comparisons
- Long Term Follow up of Eosinophilic Granuloma of the Rib
- HER2 positive T1N0M0 tumours: Time for a change?
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Castleman Disease
- Long Term Follow up of Eosinophilic Granuloma of the Rib
- Trabectedin Registry
- Predictive Values of the Ultrasound Parameters, CA-125 and Risk of Malignancy Index in Patients with Ovarian Cancer
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

