-
Medical journals
- Career
Progressive familial intrahepatic cholestasis in adulthood: 60 years’ follow-up
Authors: L. Husová 1; E. Sticová 2,3; V. Žampachová 4; Magdaléna Neřoldová 2,5; M. Jirsa 2,5
Authors‘ workplace: Center of Cardiovascular Surgery and Transplantation, Brno 1; Institute for Clinical and Experimental Medicine, Praha 2; Department of Pathology, 3rd Faculty of Medicine, Charles University and University Hospital Královské Vinohrady, Praha 3; Ist Institute of Pathology, Faculty of Medicine, Masaryk University, and St. Ann´s University Hospital, Brno 4; Institute of Medical Biochemistry and Laboratory Diagnostics, 1st Faculty of Medicine, Charles University and General University Hospital, Praha 5
Published in: Klin. Biochem. Metab., 28, 2020, No. 1, p. 11-14
Overview
Objective: Identify molecular basis of cholestatic cirrhosis with hepatocellular carcinoma (HCC) in a 55-year-old woman
Design: Case study
Settings: Center of Cardiovascular Surgery and Transplantation, Brno, Czech Republic
Material and Methods: A woman aged 60y had pruritus and icterus as an infant, with normal-range serum gamma-glutamyl transpeptidase activity, that were palliated effectively by biliary diversion and pharmacotherapy. Cirrhosis (diagnosed at age 20y) was complicated by portal hypertension with variceal bleeding (29y) and hypersplenism requiring splenectomy (34y). Jaundice recurred after a viral illness treated with acetaminophen, and worsened; serum alpha-fetoprotein was elevated, and magnetic resonance imaging found a 22mm left-lobe mass. At transplant hepatectomy (55y), HCC arisen in cirrhosis was confirmed. Immunostaining for bile salt export pump (BSEP), encoded by ABCB11, was conducted and ATP8B1, ABCB11 and TJP2 were analyzed in the patient and her first-degree relatives.
Results: BSEP was unremarkably expressed in the explanted liver. Compound heterozygosity for 2 pathogenic point mutations in ABCB11, encoding bile salt export pump (BSEP), was identified (c.2495G>A [p.Arg.832His] and c.2629G>A [p.Gly877Arg]), without ATP8B1 lesions. Both mutations are reported in association with progressive familial intrahepatic cholestasis.
Conclusion: HCC associated with PFIC due to ABCB11 mutations is recognized in children. To our knowledge, no report in an adult has yet appeared. Our patient had PFIC with functional but not absolute deficiency of BSEP. Unusually slow progression of her disease can be attributed to residual BSEP function, assisted by surgical and medical palliative interventions.
Keywords:
liver cirrhosis – liver transplantation – jaundice – Byler disease – Summerskill-Walshe syndrome
Introduction
Progressive familial intrahepatic cholestasis (PFIC) denotes a genetically heterogenous group of autoso-mal recessive liver diseases. PFIC was 30 years ago also called „Byler disease“, with Amish extended kindred in which it was first described supplying the eponym [1]. Clinical manifestations usually appear during the first year of life as permanent jaundice (albeit sometimes initially episodic), severe pruritus, steatorrhea, anorexia, growth retardation, and hepatosplenomegaly. Additional features resulting from fat-soluble vitamin malabsorption include coagulopathy, osteopenia, and neuromuscular disorders [2, 3].
ABCB11 or ATP8B1 mutations most frequently underlie PFIC with low serum gamma-glutamyltransferase (GGT) activity [2, 3]. ATP8B1, expressed body-wide, encodes an aminophospholipid-transporting flippase, ATP8B1 / familial intrahepatic cholestasis 1 (FIC1), that in the hepatocyte maintains compositional asymmetry between the two hemileaflets of the bile-canaliculus membrane. Functional or absolute deficiency of FIC1 impairs membrane stability [3]. How cholestasis follows is incompletely understood. ABCB11, expressed only in hepatocytes, a transporter, ABCB11 / bile salt export pump (BSEP), effectively the sole route for conjugated bile salts from cytoplasm into canalicular bile. Functional or absolute deficiency of BSEP impedes bile salt secretion. Hepatocellular injury and cholestasis ensue [3]. In both ATP8B1 disease and ABCB11 disease, fibrosis and cirrhosis develop, but biomarker patterns vary – in ATP8B1 disease reflecting bland cholestasis, in ABCB11 disease cholestatic hepatitis instead [3]. Hepatocellular carcinoma (HCC) may develop in early childhood, generally on a background of cirrhosis in ABCB11 disease or in the rare TJP2 disease [4-6].
Conservative therapy of both ATP8B1 disease and ABCB11 disease includes administration of ursodeoxycholic acid, simvastatin, cholestyramine, and rifampicin. Its efficacy is generally low. Surgical procedures such as terminal-ileum bypass or partial cutaneous biliary diversion [7], which reduce the pool of circulating bile salts, can palliate PFIC. Liver transplantation (LTX), however, currently is the only definitive therapy.
Case study
A 60yo woman with an unremarkable family history suffered from jaundice and pruritus as an infant and toddler. After construction of a cholecystoduodenal anastomosis (age 2y), jaundice resolved. She experienced temporary cholestatic bouts thereafter, with long symptom-free periods. Aged 20y, after 5mo of non-remitting icterus and pruritus, surgical revision was undertaken. Whilst the biliary tract was patent, the liver appeared cirrhotic (confirmed histopathologically). PFIC was diagnosed and cholestyramine therapy was initiated, with near-complete resolution of symptoms and signs of cholestatic liver disease [8].
During the next cholestatic attack (29y) ascites developed and variceal hemorrhage occurred. LTX was considered, but cholestasis resolved, and supportive therapy was continued. Adhesions and splenomegaly were found on laparoscopy (31y) undertaken during general re-assessment, at which hypercholanemia without unusual bile-acid species was confirmed. Ursodeoxycholic acid was given. Splenectomy was undertaken for hypersplenism (34y).
Cirrhosis appeared adequately compensated on outpatient monitoring until, following an intercurrent viral illness for which paracetamol was given, jaundice and pruritus recurred and worsened rapidly (55y). Serum alpha-foetoprotein values were elevated (507µg/L). Magnetic resonance imaging showed a 22mm mass involving liver segments II and III. LTX was undertaken; the explanted liver contained a single HCC (G2, 22mm), with focal angioinvasion, corresponding to the mass, arisen against biliary cirrhosis with chronic cholestasis. On immunostaining, canalicular expression of BSEP was unremarkable (Fig. 1). Although recovery from LTX was both complicated and prolonged, at present (60y; 5y follow-up) the patient is well, without biomarker evidence of hepatobiliary disease.
1. Explanted liver; septal fibrosis (A) and preserved immunohistochemical expression of BSEP along bile canaliculi (B). (A) Verhoeff’s van Gieson, original magnification x40. (B) Rabbit polyclonal anti-BSEP primary antibody (NBP1-89319, Novus Biologicals, Abingdon, UK), hematoxylin counterstain; original magnification x400. 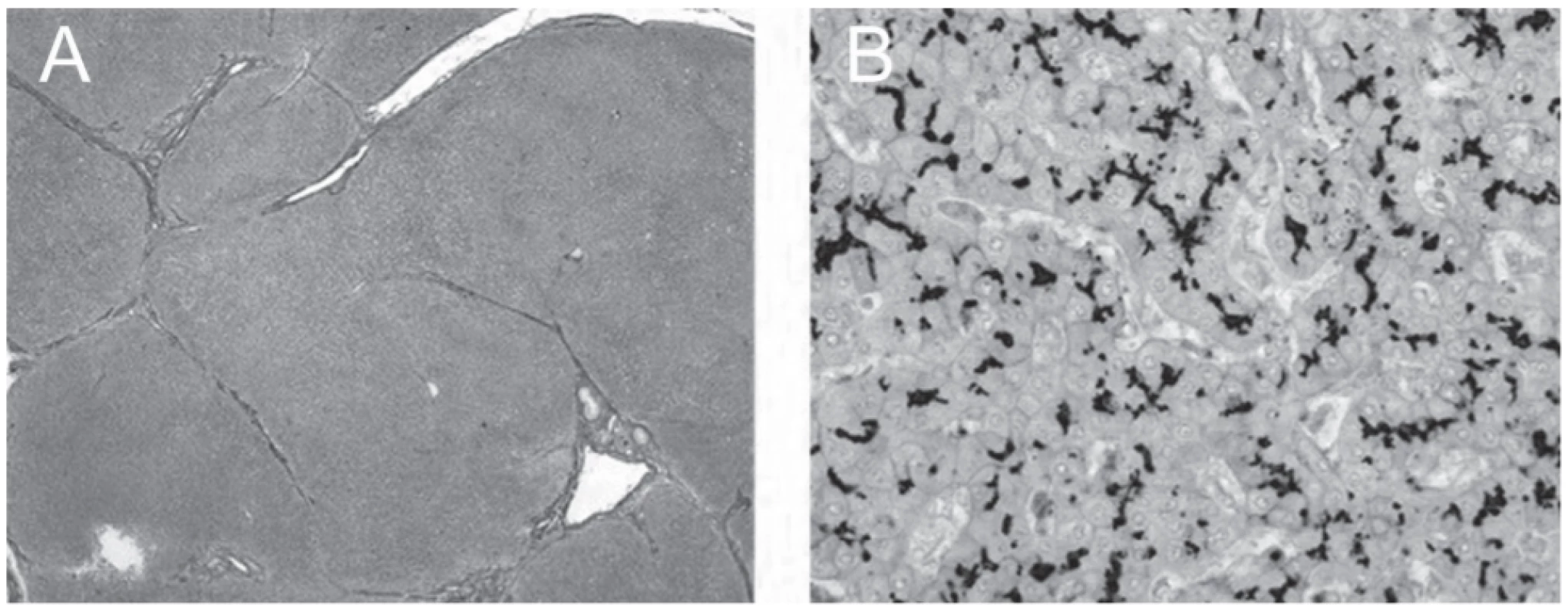
Whilst PFIC appeared an appropriate diagnosis, atypical clinicopathologic features were thought to warrant genetic study (HCC, unknown in ATP8B1 disease; age at development of HCC and unremarkable BSEP expression, both unusual in ABCB11 disease). Analysis of ATP8B1, ABCB11, and TJP2 was offered. The patient provided informed consent. The sequence of the coding regions of both genes, including untranslated regions and portions adjoining introns, was analysed in genomic DNA isolated from peripheral blood via cycle sequencing on an ABI3130 genetic analyzer (Applied Biosystems, Foster City, CA). Whereas no predictably pathogenic sequence variations were found in ATP8B1 (refseq NM_005603.4) and TJP2 (refseq NM_004817.3), two known pathogenic missense mutations were detected in ABCB11 (refseq NM_003742.4): c.2495G>A (p.Arg.832His) and c.2629G>A (p.Gly877Arg), listed in the Human Gene Mutation Database respectively as CM107193 and CM103695. The mutation c.2495G>A, predicted to yield p.Arg.832His, was found in combination with c.203G>A (p.Cys68Tyr) in an infant aged 6mo with cholestatic liver disease, histologically characterized as giant-cell cholestatic fibrosing hepatitis, with normal serum GGT activity [9]. The mutation c.2629G>A, predicted to yield p.Gly877Arg, was found in combination with a non-specified large deletion in trans in an infant aged 8mo with cholestatic liver disease [10]. Histopathologic correlation with BSEP expression was conducted in neither child.
The mutations were identified as in trans (compound heterozygosity) in our patient when, after obtaining informed consent, the patient’s healthy mother proved to carry the c.2495G>A mutation but not the c.2629G>A mutation, and the latter mutation was found in heterozygous state in a healthy brother, consistent with paternal origin (Fig. 2).
2. ABCB11 mutations detected in the index patient and her first-degree relatives. Heterozygosity for the mutation c.2495G>A (p.Gly877Arg) in one brother and its absence in the mother indicate paternal origin of this mutation. DNA not available, n.a. 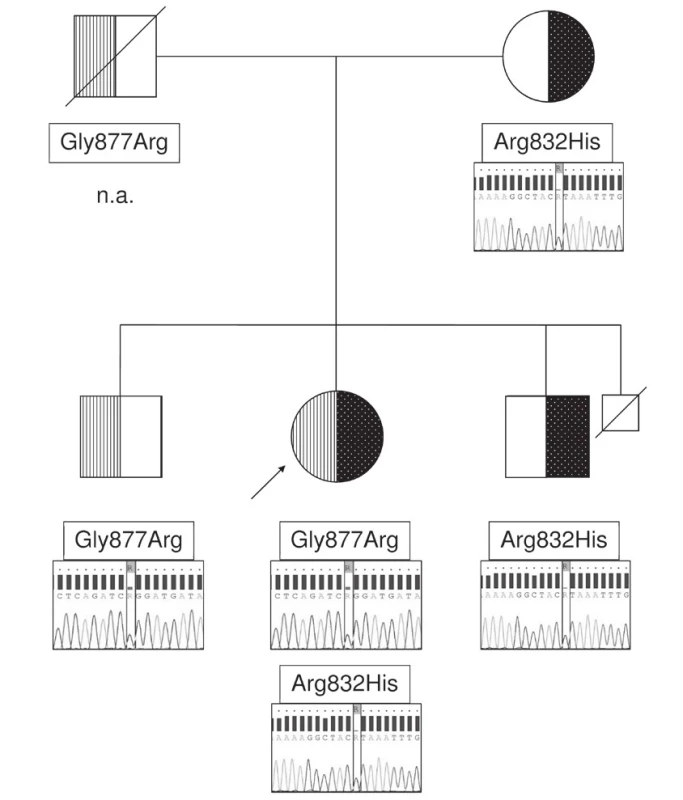
Discussion
Our patient’s disorder at onset met criteria for „benign“ recurrent intrahepatic cholestasis (BRIC; Summerskill-Walshe syndrome), with episodic cholestasis, low GGT values, and normal liver architecture; its prognosis is good, as regards longevity, although pruritus can make life a misery. The unexpected finding of cirrhosis at age 20y, however, warranted revision of her diagnosis to „adult Byler disease“ [8]. In 1982 this appellation was suitable; no longer. Both her disorder and its name evolved into PFIC, and she eventually developed HCC. Although HCC may complicate PFIC in both ABCB11 disease [4, 5] and TJP2 disease [6], it is not reported in ATP8B1 disease. Genetic stu-dies of ABCB11, ATP8B1, and TJP2 identified no di-sease-causing lesions in ATP8B1 and TJP2 and biallelic lesions in ABCB11. The two ABCB11 mutations that our patient harboured are reported in association with cholestatic liver disease of onset in infancy [9, 10]. Thus, although BSEP expression was preserved in our patient’s liver, we ascribe her hepatobiliary disorder to ABCB11 mutation, and report her progression to cirrhosis with HCC in late adulthood as unusual in ABCB11 disease.
Transformation from BRIC to PFIC is known in both ATP8B1 disease [11] and ABCB11 disease [12, 13], but in ABCB11 disease, at least, such slow progression from PFIC to HCC is not yet published and may well be extremely rare. Apart from the 5y between ages 29y and 34y, when cirrhosis was decompensated due to variceal bleeding and hypersplenism, our patient was stable and without complaints for almost 35y. Whilst freedom from icterus and pruritus likely was achieved in part by cholestyramine and ursodeoxycholic acid administration, that may have reflected the facts that neither of the patient’s ABCB11 lesions is a null mutation and that BSEP expression was not lost. We infer from the immunohistopathologic findings and the indolence of disease that some secretory capacity for conjugated bile salts remained at least partially preserved.
Partially preserved secretory function of mutated BSEP could also contribute to the late onset of HCC, in stark contrast with the early onset of HCC in children with complete biallelic inactivation of ABCB11 [4, 5]. Unfortunately, this is only speculation: Whilst interesting genetic abnormalities characterize HCC arisen in early childhood in association with ABCB11 mutations [14], the mechanisms of hepatocarcinogenesis in ABCB11 disease are still unknown. Further clouding the picture are the occurrence in ABCB11 of HCC with specific mutations associated with „routine“ HCC [15] and, at present considered non-specific, the occurrence of HCC in long-standing cirrhosis.
In conclusion, genetic testing in the proband and her family members confirmed and refined a clinical and histopathological diagnosis of intrahepatic cholestasis assigned over 35 years before HCC was identified. Residual secretory function of mutated BSEP likely permitted choleretics and bile-salt sequestrant therapy to palliate disease.
Acknowledgement
The authors thank Dr. A.S. Knisely from the Institute of Pathology, Medical University Graz, Austria, for careful revision and comments.
Supported by grant AZV MZČR no. NV18-06-00032.
Do redakce došlo 19. 9. 2019
Adresa pro korespondenci
Prof. MUDr. Mgr. Milan Jirsa, CSc.
IKEM
Vídeňská 1958/9
140 21 Praha 4
e-mail: milan.jirsa@ikem.cz
Sources
1. Clayton, R.J., Iber, F.L., Ruebner, B.H., McKusick, V.A. Byler disease. Fatal familial intrahepatic cholestasis in an Amish kindred. Am J Dis Child 1969, 117, 1, p. 112-124.
2. Baker, A., Kerkar, N., Todorova, L., Kamath, B.M., Houwen, R.H.J. Systematic review of progressive fami-lial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2019, 43(1), p. 20-36.
3. Bull, L.N., van Eijk, M.J., Pawlikowska, L., et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet 1998, 18, 3, 219 - 224.
4. Knisely, A.S., Strautnieks, S., Meier, Y., et al. Hepatocellular carcinoma in ten children under five years old with bile salt export pump deficiency. Hepatology 2006, 44(2), p. 478-486.
5. Strautnieks, S.S., Byrne, J.A., Pawlikowska, L., et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 2008, 134(4), p. 1203-1214.
6. Zhou, S., Hertel, P.M., Finegold, M.J., et al. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology 2015, 62, 6, p. 1914-1916.
7. Kalicinski, P.J., Ismail, H., Jankowska, I., et al. Surgical treatment of progressive familial intrahepatic cholestasis: comparison of partial external biliary diversion and ileal bypass. Eur J Pediatr Surg 2003, 13, 5, p. 307-311.
8. Seidlová, V., Duda, M., Jezdinská, V., Dušek, J. Progresivní intrahepatická cholestáza (M. Byler) v dospělosti. Česká a slovenská gastroenterologie 1982, 36, 2, p. 109.
9. Matte, U., Mourya, R., Miethke, A., et al. Analysis of gene mutations in children with cholestasis of undefined etiology. J Pediatr Gastroenterol Nutr 2010, 51, 4, p. 488-493.
10. Shapiro, R., Anikster, Y., Yardeni, T., et al. DHPLC screening for mutations in progressive familial intrahepatic cholestasis patients. J Hum Genet 2010, 55, 5, p. 308-313.
11. van Ooteghem, N.A., Klomp, L.W., van Berge-Henegouwen, G.P., Houwen, R.H. Benign recurrent intrahepatic cholestasis progressing to progressive familial intrahepatic cholestasis: low GGT cholestasis is a clinical continuum. J Hepatol 2002, 36, 3, p. 439-443.
12. van Mil, S.W., van der Woerd, W.L., van der Brugge, G., et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology 2004, 127, 2, p. 379-384.
13. Fotoulaki, M., Giza, S., Jirsa, M., et al. Beyond an obvious cause of cholestasis in a toddler: compound heterozygosity for ABCB11 mutations. Pediatrics 2019, 143, 5.
14. Iannelli, F., Collino, A., Sinha, S., et al. Massive gene amplification drives paediatric hepatocellular carcinoma caused by bile salt export pump deficiency. Nat Commun 2014, 5, p. 3850.
15. Vilarinho, S., Erson-Omay, E.Z., Harmanci, A.S., et al. Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. J Hepatol 2014, 61, 5, p. 1178-1183.
Labels
Clinical biochemistry Nuclear medicine Nutritive therapist
Article was published inClinical Biochemistry and Metabolism

2020 Issue 1-
All articles in this issue
- Doporučení ČSKB
- Nový koronavirus 2019. Pár základních informací a jejich dostupnost.
- Pathobiochemistry of inhibin A and its use in the screening of congenital developmental defects.
- Nephrocalcinosis after kidney transplantation as a rare manifestation of parathyroid carcinoma
- RNDr. Ivan Bilyk
- Report on biotin and its interferences in immunoanalytical methods
- Doporučení České společnosti klinické biochemie k jednotkám výsledků měření
- Doporučení České společnosti klinické biochemie a České myelomové skupiny k laboratorní diagnostice monoklonálních gamapatií
- Doporučení: Systém externího hodnocení kvality (EHK)
- Progressive familial intrahepatic cholestasis in adulthood: 60 years’ follow-up
- Clinical Biochemistry and Metabolism
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Doporučení: Systém externího hodnocení kvality (EHK)
- Doporučení ČSKB
- Doporučení České společnosti klinické biochemie k jednotkám výsledků měření
- Pathobiochemistry of inhibin A and its use in the screening of congenital developmental defects.
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

