-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Nonfunctioning parathyroid carcinoma associated with parathyromatosis. A case report
Nefunkční karcinom parathyroidey v terénu parathyreomatózy. Kazuistika
Prezentujeme případ 39-letého muže, který v roce 2013 prodělal thyreoidektomii s parathyreoidektomií následně s mylně diagnostikovaným medulárním karcinomem štítné žlázy. Štítnou žlázu i příštítná tělíska bylo obtížné exstirpovat pro obezitu pacienta a pro atypickou lokalizaci příštítného tělíska. Obě tyto struktury byly při operaci arteficiálně porušené. Tři roky po výkonu se u pacienta objevily v krčních měkkých tkáních uzly v lymfatických uzlinách suspektní z metastáz. Mikroskopický obraz však odpovídal uzlům parathyreomatózy a invazivnímu nádoru, který byl po doplnění klinických údajů a imunohistochemického vyšetření překlasifikován na nefunkční karcinom parathyreoidey.
V článku diskutujeme morfologické i imunohistochemické znaky parathyreomatózy a karcinomu parathyreoidey a možné diagnostické omyly.Klíčová slova:
parathyreomatóza – nefunkční karcinom parathyreoidey – štítná žláza – příštítné tělísko
Authors: Lucie Bartoňová 1; Vít Campr 1; Renata Chmelová 1; Miloš Taudy 2; Roman Kodet 1
Authors place of work: Department of Pathology and Molecular Medicine, nd Faculty of Medicine, Charles University and Faculty Hospital Motol, Prague, Czech Republic 1; Department of Otorhinolaryngology and Head and Neck Surgery, 1st Faculty of Medicine, Charles University and Faculty Hospital Motol, Prague, Czech Republic 2
Published in the journal: Čes.-slov. Patol., 54, 2018, No. 1, p. 37-42
Category: Původní práce
Summary
We report on the case of a 39-year old man who underwent a thyroidectomy and a parathyroidectomy with misdiagnosed medullary carcinoma of the thyroid in 2013. During the operation the thyroid gland and parathyroid glands were artificially damaged due to the complicated surgical access to the glands because of the obesity of the patient as well as the deep placement of the enlarged parathyroid glands. Three years later, the neck ultrasound showed bilateral nodules on the neck, suspected to be metastases of the medullary carcinoma. Microscopically, the nodules were found to be focuses of parathyromatosis, and there was also an infiltrating carcinoma. This lesion was reclassified after clinico-pathological correlation and immunohistochemical examination as nonfunctioning parathyroid carcinoma. This article discusses morphological and immunohistochemical features of parathyromatosis and parathyroid carcinoma and its separation from lesions with which it may be misdiagnosed.
Keywords:
parathyromatosis – nonfunctioning parathyroid carcinoma – thyroid gland – parathyroid glands
The pathology of parathyroid glands is considered to be a routine practice, usually lacking any surprising findings. The most common material examined is a pathologically enlarged parathyroid gland in the setting of hyperparathyroidism. The frequently required perioperative frozen section investigation is intended to prove that the pathological tissue was identified adequately during surgery. The definitive diagnosis is then usually either adenoma or hyperplasia. In contrast with the routine flow of the patients we present the case of a rare nonfunctioning parathyroid carcinoma with local metastases coexisting with parathyromatosis in a patient with a history of previous thyroidectomy with parathyroidectomy of the ectopic parathyroid glands.
CLINICAL HISTORY
The patient was a 39-year-old obese man, who was referred to the Ear, Nose and Throat (ENT) department for a nodule in the caudal part of the right lobe of the thyroid gland and for an enlarged ectopic parathyroid gland located in the deep cervical soft tissue on the right side, both found by neck ultrasound and scintigraphy. Fine needle aspiration cytology of the thyroid nodule was suspicious for malignancy (Bethesda V). The patient underwent a thyroidectomy and a parathyroidectomy in 2013. During the operation, the capsules of both the enlarged parathyroid gland and of the thyroid nodule were artificially damaged due to the complicated access to the lesions due to the obesity of the patient and the deep placement of the enlarged parathyroid gland. Surgical specimens consisted of a thyroid, a pathologically enlarged parathyroid gland and one normal parathyroid gland. The tumor of the thyroid gland was diagnosed as medullary carcinoma. Support for such a diagnosis was found in the strong positivity of chromogranin A and deposits of amyloid. A staining for calcitonin was interpreted as positive, though it was at the level of background. After the operation, the level of serum calcium was normal (2.25 mmol/L), the concentration of calcitonin was decreased (0.59 ng/L) (Tab. 1). The concentration of parathormon was not examined.
Tab. 1. Biochemical findings of iPTH, calcitonin and serum calcium level from 2013 to 2017. 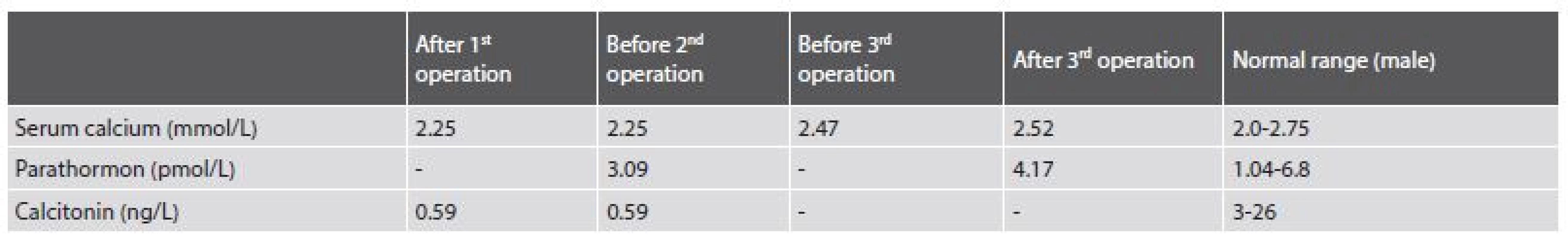
The patient was followed-up on at the ENT department and in 2015 the neck ultrasound showed enlarged cervical lymph nodes. The patient underwent bilateral cervical block dissection. Before the second surgery, his serum calcium (2.25 mmol/L) and parathormone (iPTH)(3.09 pmol/L) were within normal values, the concentration of calcitonin was decreased (0.59 ng/L). In the surgical specimen there were sixteen lymph nodes with features of hyperplasia and without metastases. One of the supposed nodes was found to be a fourth pathologically enlarged parathyroid gland.
No further therapy was administered and the patient was frequently observed at the ENT department. In January 2016, the neck ultrasound showed bilateral hypoechoic nodules of 5 to 20 mm in diameter. The patient´s serum calcium was in a normal range (2.47 mmol/L). Serum iPTH and calcitonin were not examined. During the year 2016 the nodules continued enlarging and the patient underwent surgery again in December 2016.
During the operation, several nodules measuring from 10 to 20 mm were evident bilaterally, one of them invaded into the thyroid cartilage. The largest nodule measuring 40 mm was on the right side of the trachea. It was fixed to the arteria carotis communis, to the trachea and cricoid cartilage of the larynx. The nodules were removed, but the surgery was not considered radical. Postoperative serum calcium level (2.52 mmol/L) and iPTH (4.17 pmol/L) were in the normal range.
MATERIALS AND METHODS
The surgical specimen of the 2016 resection was delivered in two parts fixed in formalin. The first specimen measured 75x35x20 mm. It was composed of soft tissues with three white, round nodules 5 to 20 mm in diameter. The second specimen measured 45x25x20 mm and it was formed by a soft tissue with a nodule 30 mm in diameter. Both specimens were completely processed.
Histological slides were prepared and stained with hematoxylin-eosin. The Congo red stained slides were examined in polarized light for birefringence. Immunohistochemical stains for parathormon (clone105G7, dilution 1 : 150, Novocastra), cycline D1 (polyclonal and +3P4, dilution 1 : 80, Biogenex), galectin 3 (clone 9C4, dilution 1 : 200, Novocastra), chromogranin A (clone DAK-A3, dilution 1 : 150, Dako), synaptophysin (clone DAK-SYNAP, dilution 1 : 4000, Dako), TTF-1 (clone8G7G3/1, dilution 1 : 100, Cell Marque), thyreoglobulin (clone DAK-Tg6, dilution 1 : 50, Dako), CEA (clone II-7, dilution 1 : 50, Dako), CD34 (clone QBend/10, dilution 1 : 30, Biogenex), calcitonin (polyclonal, dilution 1 : 500, Dako) and Ki-67 (clone MIB-1, dilution 1 : 150, Dako) were used. For visualization of these reactions diaminobenzidine tetrahydrochloride was used. The proliferation index of Ki-67 was evaluated as an estimated percentage of positive cells (%).
RESULTS
Histologically, in the first specimen there were fourteen nodules of the parathyroid tissue measuring 1 to 20 mm, found in fibrous soft tissue. The nodules were round, with smooth, noninfiltrative margins (Fig. 1). Most of the nodules were solidly arranged, composed of uniform round cells with eosinophilic or amphophilic cytoplasm and uniform nuclei with a fine structure of chromatin, corresponding to parathyroid chief cells (Fig. 2). Focally, cells with clear cytoplasm, with mild nuclear enlargement and with a visible nucleolus were detected. Mitotic activity was not evident. No vascular invasion was found.
Fig. 1. Parathyromatosis. In low magnifications, nodules of hyperplastic parathyroid tissue with smooth margins in cervical soft tissue (hematoxylin and eosin, magnifications x 40). 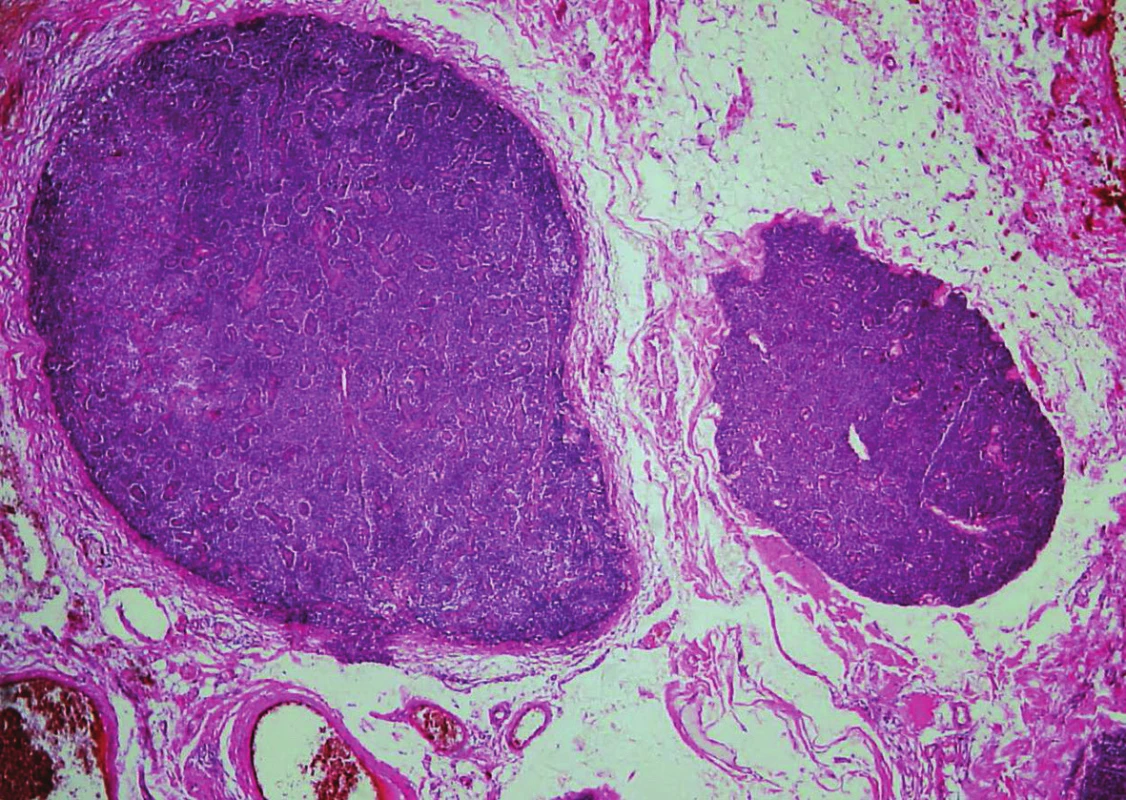
Fig. 2. Parathyromatosis shows a proliferation of parathyroid chief cells. Focally, the cells are oval, with clear or basophilic cytoplasm and with mild nuclear enlargement (hematoxylin and eosin, magnifications x 200). 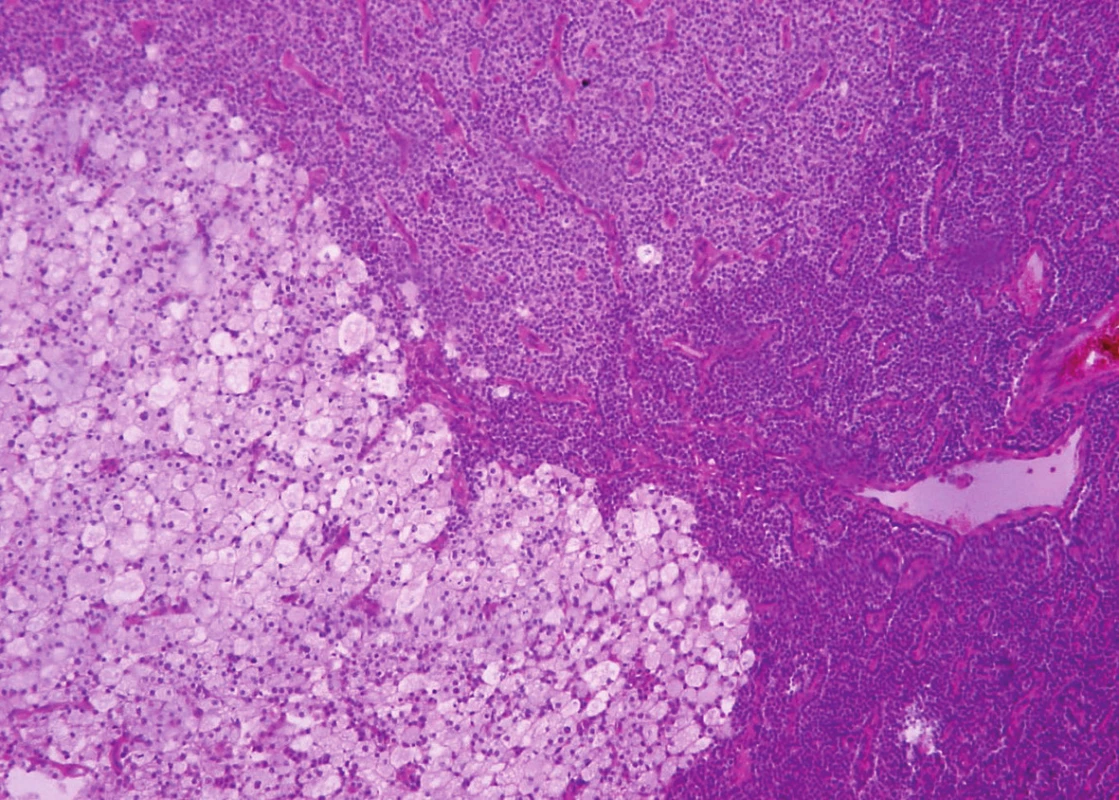
In immunohistochemistry, the cells expressed focally parathormon (Fig. 3A) and chromogranin A (Fig. 3D), and focal weak positivity of cyclin D1 was found (Fig. 3C). Galectin-3 (Fig. 3B); synaptophysin and CEA were all negative. A weak expression of calcitonin was considered to be background staining. The proliferation index determined by Ki-67 reached 15 %.
Fig. 3. Immunohistochemical findings of parathyromatosis. A: Focal strong cytoplasmic expression of parathormon (magnifications x 200). B: Negativity of galectin 3 (magnifications x 200). C: Nuclear weak or mild expression of cyclin D1 (magnifications x 200). D: Strong difuse cytoplasmatic expression of chromogranin (magnifications x 200). 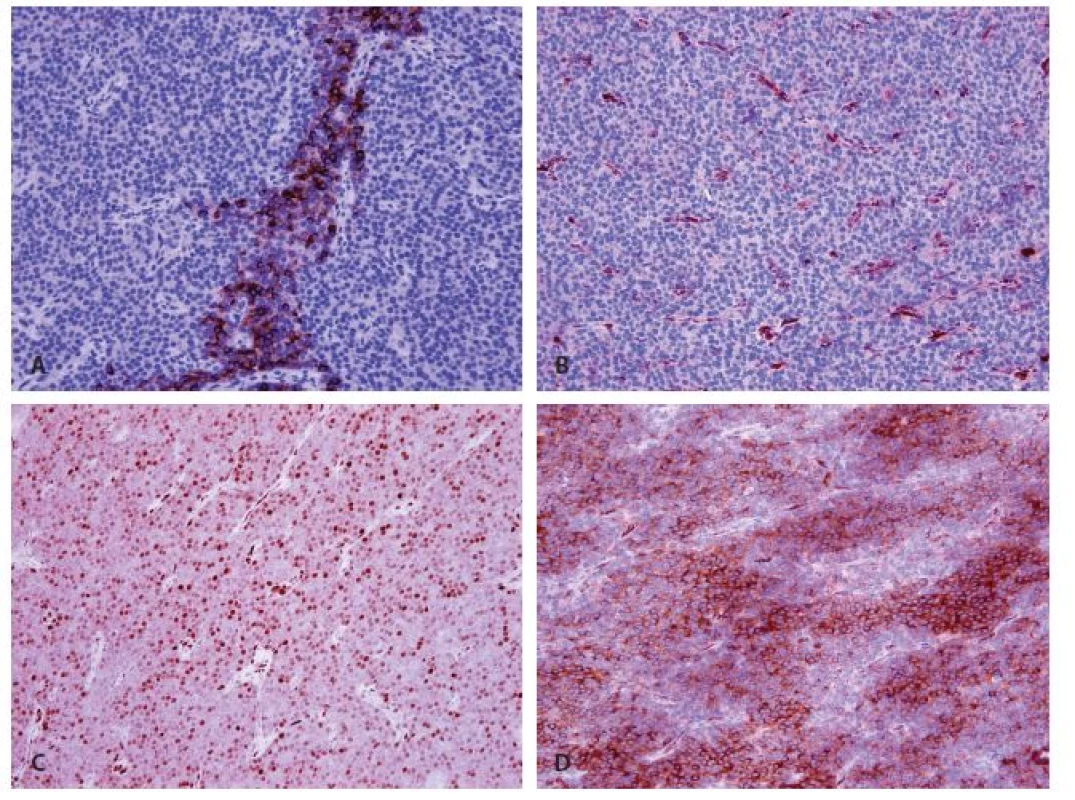
The findings were consistent with a diagnosis of parathyromatosis.
Moreover, in the first specimen two small nodules with a trabecular pattern and with an invasion into the adjacent tissues were found. Vascular invasion was also present. The perineural spread was not detected. Admixed adipose tissue or deposits of amyloid were not obvious inside the lesions. The picture was similar to that of a larger nodule in the second specimen.
Trabeculae of neoplastic cells were separated by dense fibrous bands in-between (Fig. 4). The infiltrative growth into the surrounding soft tissues was obvious. The tumor cells were relatively uniform, with clear cytoplasm and regular vesicular nuclei with visible nucleoli (Fig. 5). Focally, the cells were polygonal or spindled, with eosinophilic cytoplasm and with irregular hyperchromatic nuclei. Mitotic figures were not present. Vascular invasion highlighted immunohistochemically by CD34 was found focally (Fig. 6). Necrosis, perineural spread and amyloid deposits were not evident.
Fig. 4. Parathyroid carcinoma. Tumor with infiltrative growth into the surronding soft tissue. Trabeculae of neoplastic cells with intralesional dense fibrous bands (hematoxylin and eosin, magnifications x 100). 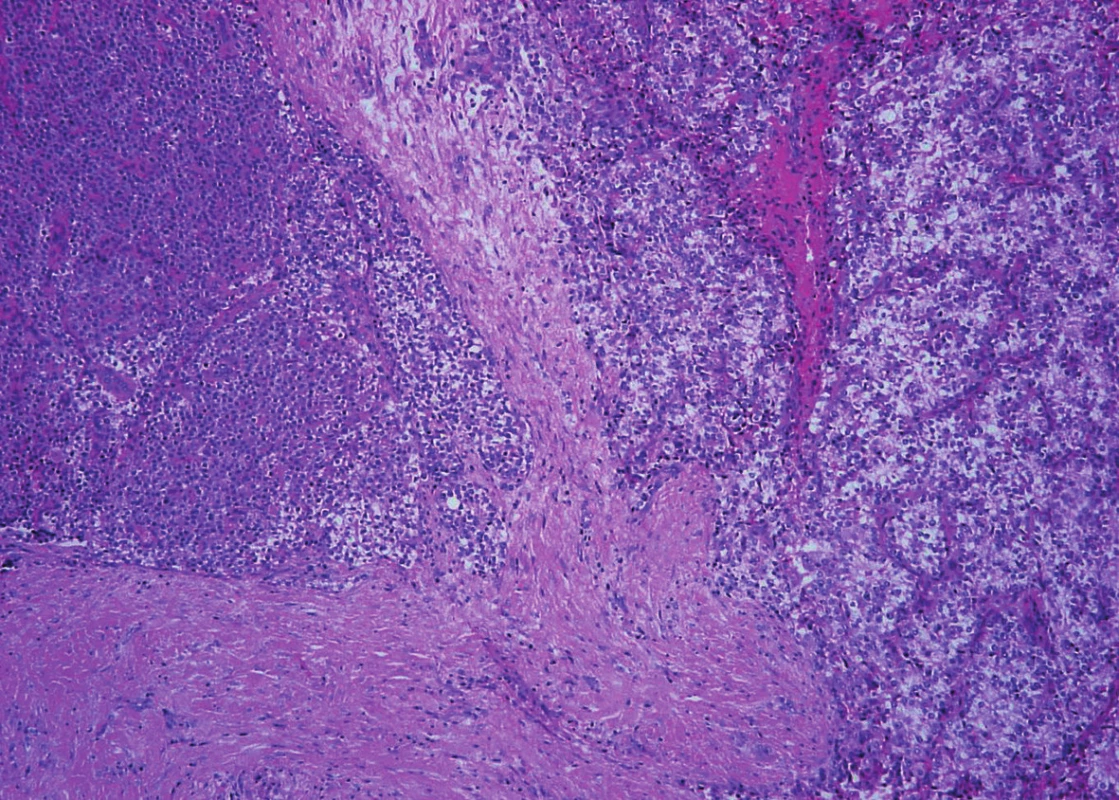
Fig. 5. Parathyroid carcinoma. Neoplastic cells are columnar, with clear cytoplasm and with regular vesicular nuclei (hematoxylin and eosin, magnifications x 200). 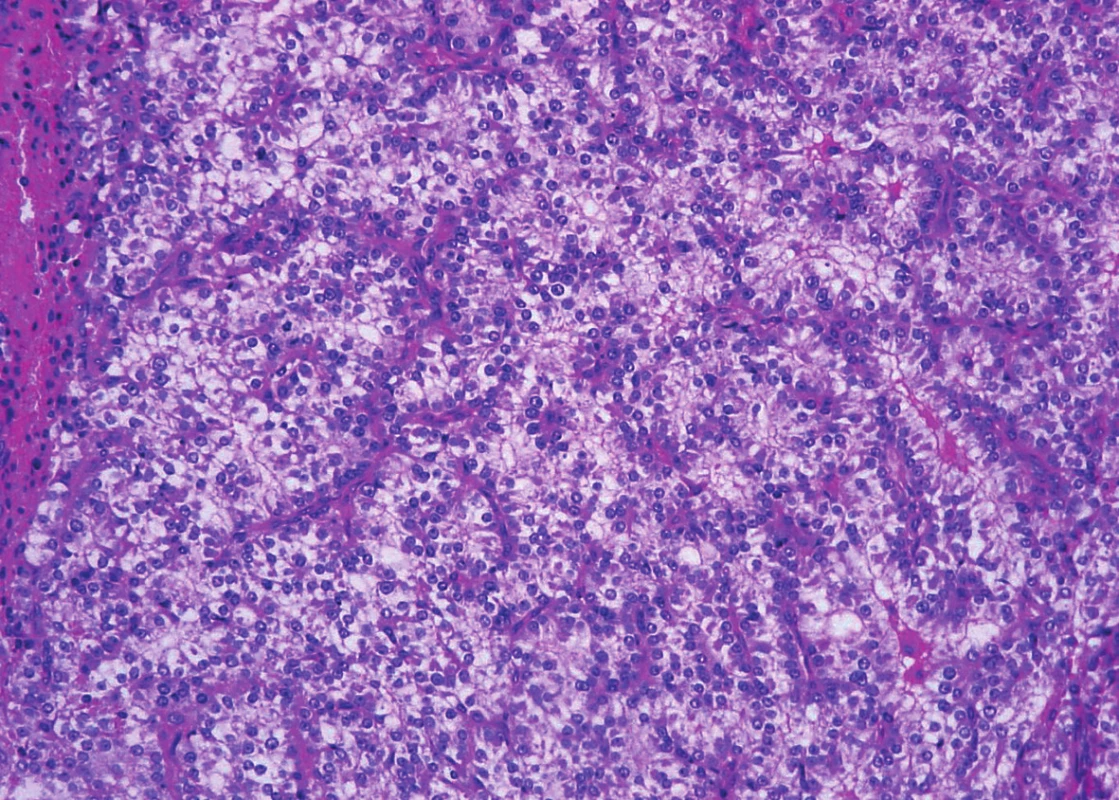
Fig. 6. Parathyroid carcinoma. The presence of vascular invasion in parathyroid carcinoma (hematoxylin and eosin, magnifications x 200). 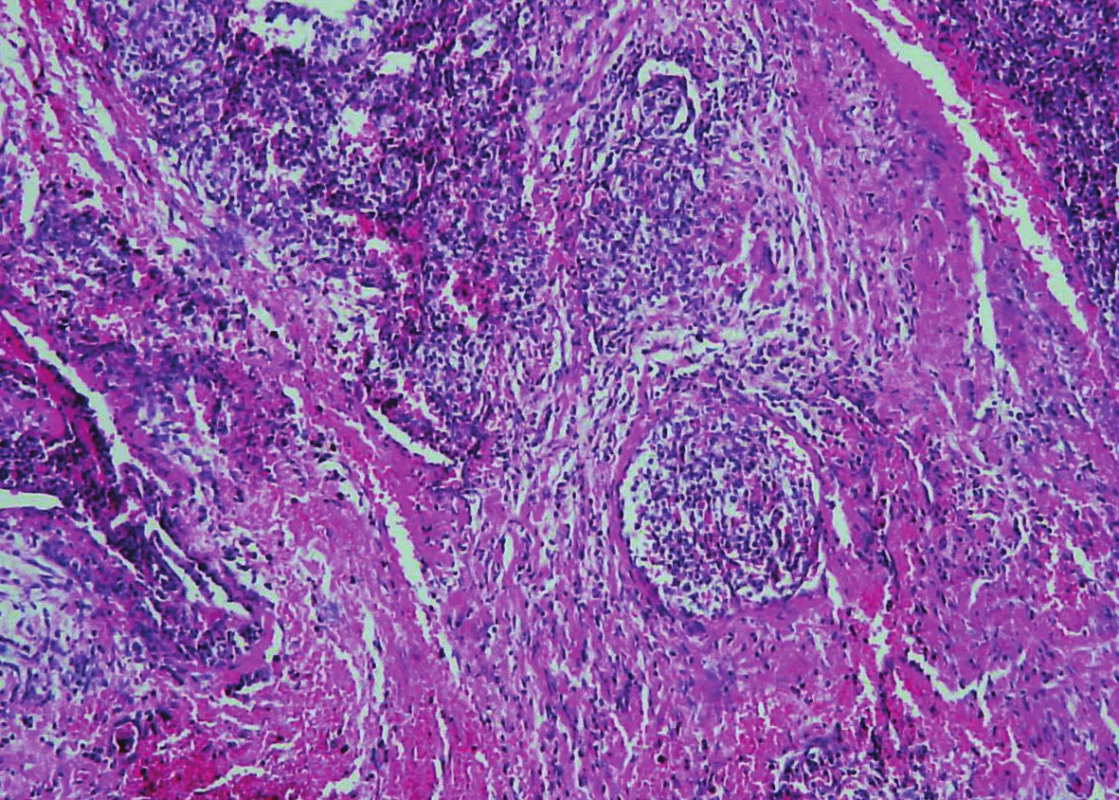
In immunohistochemistry, cells of the infiltrative tumor showed a diffuse cytoplasmic positivity of galectin-3 (Fig. 7B), weak cytoplasmic reactivity of chromogranin A (Fig. 7D) and a focal strong nuclear expression of cyclin D1 (Fig. 7C). Immunostaining of parathormon was negative as were further markers - synaptophysin, thyreoglobulin, TTF-1 and CEA (Fig. 7A). The proliferative index reached focally 10 %.
Fig. 7. Immunohistochemical findings of recurrent parathyroid carcinoma. A: Negativity of parathormon (magnifications x 200). B: Strong difuse cytoplasmatic expression of galectin 3 (magnifications x 200). C: Focal strong nuclear expression of cyclin D1 (magnifications x 200). D: Weak cytoplasmatic expression of chromogranin (magnifications x 200). 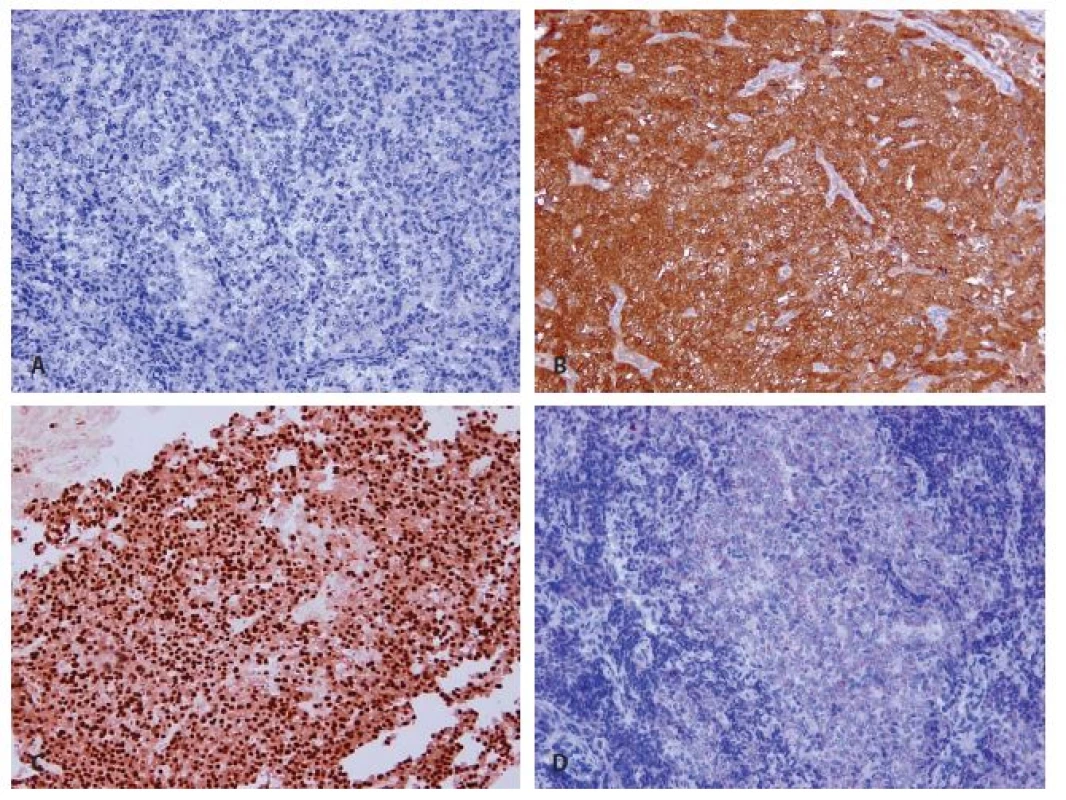
In the adjacent soft tissues of the second specimen further small nodules of hyperplastic parathyroid tissue measuring 2 mm in diameter and three lymph nodes without metastases were found.
On the basis of these findings the material from 2013 was reviewed and the same markers which we used for the lesion resected in 2016 were added. In the right lobe of the thyroid gland the tumor, with an artificially damaged capsule, with vascular invasion and with deposits of amyloid was found. The expression of chromogranin A was diffusely positive and immunostaining of parathormon and galectin-3 both showed focal positivity. Cyclin D1 was weakly positive. The expression of calcitonin was only very weak and rather questionably positive. The Ki-67 proliferative index did not exceed 5 %. The immunostaining for synaptophysin and CEA were negative. The tumor originally supposed to be a medullary carcinoma of the thyroid was reclassified as a parathyroid carcinoma.
DISCUSSION
Parathyromatosis is a rare condition in which nodules of hyperfunctioning parathyroid tissue are formed in the neck or mediastinum (1). Rarely, it has been described in the forearm after the autotransplantation of the gland (2,3). The lesions tend to affect females more frequently than males.
There are three theories trying to explain the development of parathyromatosis. The first speculates that it is a result of the dissemination of parathyroid cells during the surgery. The second theory attributes the origin of the parathyromatosis to embryonic rests during the ontogenesis, and the third theory considers parathyromatosis to be a low grade malignancy (1,3). The first theory is supported by the fact that the parathyromatosis occurs as a secondary finding after surgery, especially when the capsule of a parathyroid gland is damaged. It can be found from 5 months to 19 years after the surgical extirpation (4). It is speculated that it is not as uncommon as generally thought, but it is often underdiagnosed (4).
Parathyromatosis is a rare cause of recurrent hyperparathyroidism with the serum calcium level only mildly increased or being normal (2). The first clinical signs are psychological or there are metabolic disturbances associated with hypercalcemia (2). Clinically it presents as several nodules palpable during physical examination or found on the neck via ultrasound or by other imaging methods.
In light microscopy, nodules or nests of a variable size are sharply circumscribed and they are composed of hyperplastic parathyroid tissue. The nodules are usually surrounded by fibrous tissue corresponding to scar tissue. Trabecular growth, nuclear atypia or increased mitotic activity may be seen, but these features are not considered signs of malignancy.
Surgery is the treatment of choice and follow-up of the patients is necessary for early resection of newly found nodules (1).
Parathyroid carcinoma (PC) is a rare endocrine malignancy accounting for less than 1 % of cases of primary hyperparathyroidism (5,6). PC is usually a sporadic disease, but it has also been described within the familial endocrine neoplasia syndrome type 1 or within jaw tumor syndrome (2). It is more prevalent in males within the age range of 27 - 71 years (1).
PC arises de novo more often than from pre-existing adenoma or hyperplasia (7). Clinically, PC presents as a solitary lesion, sometimes with small lesions in adjacent tissues or as a metastatic disease in cervical lymph nodes. In some cases PC is detected due to severe hypercalcemia because it usually increases the serum calcium level and iPTH to extreme levels.
Nonfunctioning PCs are very rare and they behave more aggressively than the carcinomas with hormonal production (8). The diagnosis depends on correlation of clinical data and histopathological findings. Clinically, the tumor presents as a palpable neck mass or as a metastatic disease in the absence of hypercalcemia.
Histologically, PC is a tumor with a solid or trabecular growth pattern permeated with dense fibrous bands. The capsule of the tumor is thick, often with extensive local invasion. Cytoarchitectural features may be similar to a parathyroid adenoma. The tumor cells are polygonal or spindled, with eosinophilic cytoplasm, sometimes with extracellular mucin. Some cells may have clear or occasionally oxyphilic cytoplasm. Nuclei are uniform or pleomorphic, nucleoli may not be visible. The microscopic features of nonfunctioning PC are identical.
Recurrences occur in about half of patients (2,5). According to the WHO classification the recurrence rate is 30 to 67 %, and recurrences are more common in younger men (2). Metastases develop most often in cervical and mediastinal lymph nodes or at distant sites such as the lungs, bones or liver (2).
Patients are treated with extensive surgery, in recurrent or metastatic cases of the disease by chemotherapy and/or radiotherapy, but with an unsatisfactory response (9). The treatment of complications of hypercalcemia is also important.
The prognosis depends on the stage at primary diagnosis. Patients with severe hypercalcemia (higher than 3.75 mmol/L), metastatic disease or invasion into the adjacent organs have an unfavourable prognosis (2,5,9). WHO classification reports a 10-year survival rate in approximately 50 % of patients.
The distinction of parathyromatosis from PC is based on the correlation of clinical and radiological features, biochemical findings and histopathological examination supported by immunohistochemistry. Histopathological criteria for PC are the presence of intralesional fibrous bands, vascular or lymphatic invasion, invasion into the cervical soft tissues, or metastases (2). In case of uncertainty the nature of the lesion must be demonstrated by immunohistochemistry.
In proving the parathyroid origin of the tumor the immunostaining of parathormon is helpful. In nonfunctioning PC the demonstration of parathormon is a potential diagnostic aid, but in a substantial proportion of tumors, especially the recurring ones, the result may be negative. It is also possible to identity mRNA for PTH by in situ hybridization (7,8). Nonfunctioning parathyroid carcinoma is reported infrequently in the medical literature and other markers are helpful for diagnosis. In the reported case, primary nonfunctioning PC was focally positive for PTH and the recurrent tumor was PTH negative.
The overexpression of galectin-3 distinguishes PC from benign parathyroid lesions (1, 3, 6, 10). In parathyromatosis, galectin-3 has been shown only weakly positive or negative (1). Fernandez et al. found in his study positivity of galectin-3 in 93.3 % of PC and only in 14.3 % of cases of parathyromatosis. Bergero et al. reviewed 26 cases of PC and 30 parathyroid adenomas. Positivity of galectin-3 was found in 92.3 % of PC, and only in 3.3 % of adenomas. Diffuse and strong positivity of galectin-3 was present in 100 % of metastatic PC. The galectin-3 positivity indicates a resistance to apoptosis or the ability of the tumor to avoid it (6). These results confirm that galectin-3 expression as demonstrated by immunohistochemistry is a most sensitive (92.3 %) and specific (96.7 %) marker for the diagnosis of PC (11). In the reported case, the revised primary tumor considered originally as medullary carcinoma of the thyroid gland was focally galectin-3 positive, but the recurrent and metastatic tumor was strongly and diffusely positive. The nodules of parathyromatosis were negative. These findings correspond with the literature.
The proliferation index determined by Ki-67 is usually higher than 4 – 6 % in PC, the parathyromatosis and adenomas usually have a lower level of Ki-67 positivity. The proliferation index of parathyromatosis is usually below 5 % (10). To separate the entities it is necessary to combine Ki-67 with other markers, especially with Gal-3 (6,7,10,11).
Cyclin D1 positivity is present in 91 % of PC (8) and there is only a weak expression in parathyromatosis (7).
In the differential diagnosis one must not forget chromogranin A and synaptophysin expression in PC, but reliable data on the results of these markers in parathyromatosis are not available (5,7). In our case, the primary tumor (PC) was chromogranin strongly positive, the recurrent carcinoma was negative.
In our case, it turned out to be important to make the differential diagnosis between medullary carcinoma and parathyroid carcinoma. The investigation should be complex and should include a comparison of clinical and biochemical data with morphological and immunohistochemical findings. Biochemically, for medullary carcinoma, concentrations of calcitonin are higher and serum calcium level is lower than the normal limits.
At light microscopy, neoplastic cells in medullary carcinoma may be round, polygonal or spindled with granular cytoplasm and round nuclei, without nucleolus. In the stroma, there are often deposits of amyloid. Immunohistochemistry for calcitonin and CEA are the most helpful markers for this diagnosis (7,8). Further positive markers are chromogranin A and synaptophysin (7,8).
In the reported case, the encapsulated tumor was located inside the thyroid gland. Morphologically, the primary tumor was a solid nodule with deposits of amyloid in the stroma, with growth beyond the capsule. Neoplastic cells were predominantly uniform, with eosinophilic or amphophilic cytoplasm and with round nuclei with fine chromatin. Focally, we found groups of clear cells with irregular, larger nuclei without nucleolus. Vascular invasion was found. Immunohistochemical reactivity of calcitonin was interpreted as weakly positive at the time of diagnosis in 2015, but after the review we considered the reaction as a misleading background staining. Chromogranin A was positive and thyroglobulin was negative. The former diagnosis of medullary carcinoma was reclassified for parathyroid carcinoma in the light of the new findings in 2016 as well as on the reinterpretation of the previous histopathological and immunohistochemical results.
In the differential diagnosis of PC, parathyroid adenoma (PA) and parathyromatosis have to be considered from the beginning. Parathyromatosis was described above. PA lacks features of malignancy.
Furthermore, there is a lesion called atypical parathyroid adenoma (APA) and it has to be ruled out as well. APA is a term for a neoplasia with histological features suggestive of malignancy (atypias, mitoses), but without diagnostic signs of malignancy (vascular or lymphatic invasion, infiltrative margins or metastases). APA is often found in females and the serum calcium level is usually lower than that in cases of functioning PC. Immunohistochemical results of APA are intermediate between parathyroid carcinoma and adenoma (8).
In a broader differential diagnosis, other carcinomas of the thyroid gland should also be considered. The diagnosis of thyroid follicular carcinoma can be confirmed by positive immunostaining of thyreoglobulin and TTF-1 (8). The correlation with biochemical findings is also important for a diagnosis. Another, diagnostic possibility represents an undifferentiated carcinoma of the thyroid, which can be recognized on the basis of clinical data and immunohistochemical findings (8).
CONCLUSION
We present a case of recurrent, nonfunctioning parathyroid carcinoma with neck metastases in association with parathyromatosis which developed after the extirpation of a pathologically enlarged parathyroid gland. Distinguishing between these two conditions may pose a diagnostic problem. The reported case supports the postsurgical theory of the origin of parathyromatosis and stresses the necessity of a careful extirpation of pathologically enlarged parathyroid glands during the surgery. The case also demonstrates the histological similarity and easy misdiagnosis with other malignancies especially of the thyroid origin. In case of doubts a thorough sampling must be performed and an extended panel of immunohistochemical markers for confirmation of the origin of the lesions must be utilized.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Lucie Bartoňová, MD
Department of Pathology and Molecular Medicine,
2nd Faculty of Medicine, Charles University and Faculty Hospital Motol
V Úvalu 84
150 06 Prague, Czech Republic
tell.: +420 224 435 645
email: Lucie.Bartonova@fnmotol.cz
Zdroje
1. Mohammadi A, Ghasemi-Rad M. Parathyromatosis or recurrent multiple parathyroid adenomas? A case report. Maedica (Buchar) 2012; 7(1): 66-69.
2. Gustavo G. Fernandez-Ranvier MD, et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? http://onlinelibrary.wiley.com.
3. Aksoy-Altinboga A, Akder Sari A, Rezanko T, Haciyanli M, Orgen Calli A. Parathyromatosis: critical diagnosis regarding surgery and pathologic evaluation. Korean J Pathol 2012; 46(2): 197-200.
4. Tsung-Jui Wu, Ying-Tang Wang, Hung Chang, Shih-Hua Lin. Parathyromatosis. http://www.sciencedirect.com.
5. Cetani F, Frustaci G, Torregrossa L, et al. A nonfunctioning parathyroid carcinoma misdiagnosed as a follicular thyroid nodule. World J Surg Oncol 2015; 13 : 270.
6. Karaarslan S, Yurum FN, Kumbaraci BS, Pala EE, Sivrikoz ON, Akyildiz M, Bugdayci MH. The Role of Parafibromin, Galectin-3, HBME-1, and Ki-67 in the Differential Diagnosis of Parathyroid Tumors. Oman Med J 2015; 30(6): 421-427.
7. DeLellis R, Lloyd RV, Heitz PU, Eng Ch. World Health Organisation; International Agency for Research on Cancer. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press 2004 : 124-132.
8. Fletcher CDM. Diagnostic histopathology of tumors (4th edition). Volume 2. Elsevier, Philadelphia 2013; 1281-1289.
9. Keith AA, Robert CH, Naguib AS. Parathyroid carcinoma: A clinical study of seven cases of functioning and two cases of nonfunctioning parathyroid cancer. http://onlinelibrary.wiley.com.
10. Fernandez-Ranvier GG, Khanafshar E, Tacha D, Wong M, Kebebew E, Duh QY, Clark OH. Defining a molecular phenotype for benign and malignant parathyroid tumors. Cancer 2009; 115(2): 334-344.
11. Bergero N, De Pompa R, Sacerdote C, Gasparri G, Volante M, Bussolati G, Papotti M. Galectin-3 expression in parathyroid carcinoma: immunohistochemical study of 26 cases. Hum Pathol 2005; 36(8): 908-914.
Štítky
Genetika Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseČesko-slovenská patologie
Nejčtenější tento týden
2018 Číslo 1- Transthyretinová amyloidóza z pohledu neurologa a kardiologa aneb jak se vyhnout „misdiagnostice“?
- Akutní intermitentní porfyrie
- Primární hyperoxalurie – aktuální možnosti diagnostiky a léčby
- Etiologie opakovaných reprodukčních ztrát – zaostřeno na genetiku
- Klinický exom v prenatální diagnostice – kazuistika
-
Všechny články tohoto čísla
- Predictive diagnosis in breast cancer - What‘s new in 2018?
- Prediction of EGFR blockade responses in metastatic colorectal carcinoma
- Predictive diagnostics of gastric cancer in 2018
- Evaluation of inflammatory cells (tumor infiltrating lymphocytes – TIL) in malignant melanoma
- Update terapeuticko-indikační patologie (2. díl)
- Dedifferentiated carcinoma of the ovary. A case report
- Prof. MUDr. Alena Linhartová, DrSc.
- Nonfunctioning parathyroid carcinoma associated with parathyromatosis. A case report
- S novou předsedkyní akreditační komise našeho oboru o specializačním vzdělávání
- Marginálie z 13. kongresu EADO v Aténach
- MONITOR ANEB NEMĚLO BY VÁM UNIKNOUT, ŽE...
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evaluation of inflammatory cells (tumor infiltrating lymphocytes – TIL) in malignant melanoma
- Dedifferentiated carcinoma of the ovary. A case report
- Predictive diagnosis in breast cancer - What‘s new in 2018?
- Prediction of EGFR blockade responses in metastatic colorectal carcinoma
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



