-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Basal cell carcinoma of the skin with mixed histomorphology: a comparative study
Bazocelulárny karcinóm kože so zmiešaným histomorfologickým obrazom: porovnávacia štúdia
Bazocelulárny karcinóm (BCC) kože má veľmi rôznorodý histomorfologický obraz, na základe ktorého je klasifikovaný do viacerých typov a variantov. V mnohých prípadoch je však takéto zaradenie obtiažne, nakoľko môže pozostávať z kombinácie viacerých histologických typov. Informácie o charakteristických črtách týchto zmiešaných foriem BCC sú limitované, keďže doteraz neboli špecificky analyzované. Cieľom štúdie bolo sledovanie výskytu BCC kože so zmiešaným histomorfologickým obrazom v súbore diagnostikovaných primárnych BCC a porovnávanie ich klinicko-patologických nálezov s jednoduchými BCC tvorenými jedným histologickým typom. Vyšetrovaný súbor pozostával z 911 biopticky verifikovaných primárnych BCC získaných od 697 pacientov. Prevalencia jednoduchých a zmiešaných BCC bola 64,9 % a 35,1 %. V skupine zmiešaných BCC sme potvrdili veľmi rôznorodý histomorfologický obraz pozostávajúci z mixtúry dvoch až štyroch rozdielnych typov v rôznom zastúpení. Najčastejšie išlo o kombináciu nodulárno-infiltratívneho, superficiálno-nodulárneho, nodulárno-trichoepitelového a nodulárno-mikronodulárneho typu. Porovnávaním obidvoch hodnotených skupín sme zistili, že zmiešané BCC boli signifikantne častejšie lokalizované na mimotvárových častiach hlavy (30.0 % vs. 20.0 %, p = 0.02) a zriedkavejšie na tvári (37.2 % vs. 45.2 %, p = 0.03). Nepotvrdili sme evidentné rozdiely vo výskyte jednoduchých a zmiešaných BCC na iných častiach tela. Histologicky vykazovali zmiešané BCC omnoho častejšie agresívny rastový vzor (64.6 % vs. 13.0 %, p < 0.0001). V skupine zmiešaných BCC boli taktiež významne častejšie prítomné pozitívne resekčné okraje (17.8 % vs. 12.6 %, p = 0.02). BCC kože so zmiešaným histomorfologickým obrazom predstavujú približne jednu tretinu všetkých prípadov. V bioptickej praxi ide teda o častý nález, ktorý pravdepodobne svedčí o evolúcii a fenotypovej transformácii karcinómu. Keďže zmiešané BCC často pozostávajú z agresívnych histologických typov, bez ohľadu na osobné zvyklosti v deskripcii a terminológii medzi patológmi by mala byť prítomnosť agresívnej rastovej zložky vždy spomenutá v bioptickom náleze.
Kľúčové slová:
bazocelulárny karcinóm – jednoduchý a zmiešaný typ
Authors: Vladimír Bartoš 1; Milada Kullová 2
Authors place of work: Department of Pathology, Faculty Hospital in Žilina, Slovakia 1; Department of Dermatovenerology, Faculty Hospital in Žilina, Slovakia 2
Published in the journal: Čes.-slov. Patol., 52, 2016, No. 4, p. 222-226
Category: Původní práce
Summary
Basal cell carcinoma (BCC) of the skin exhibits a very heterogeneous histomorphology, on the basis of which it is classified into several subtypes and variants. In many cases, however, a definite categorization remains difficult, because BCC may consist of more than one histopathological subtype. There are limited data exploring the characteristics of these mixed BCCs, since they have not been specifically analysed. The aim of this study was to estimate the prevalence of BCCs with mixed histomorphology observed in a set of primary BCCs and to compare their clinicopathological features with a single type BCC subgroup. A total of 911 histologically proven primary BCCs from 697 patients were investigated. Prevalence of single and mixed type BCCs was 64.9 % and 35.1 %, respectively. In mixed type BCC subgroup, a very heterogeneous histomorphology was found comprising a mixture of two to four different subtypes in various proportions. The most frequent combinations included nodular-infiltrative, superficial-nodular, nodular-trichoepithelial and nodular-micronodular subtype. Comparative analysis of the two given subgroups showed that mixed type BCCs were significantly more frequently localized on the extrafacial regions of the head (30.0 % vs. 20.0 %, p = 0.02) and less often on the face (37.2 % vs. 45.2 %, p = 0.03). There were not convincing differences in the occurrence of single vs mixed type BCCs in other parts of the body. Histologically, mixed type BCCs exhibited an aggressive-growth pattern more frequently (64.6 % vs. 13.0 %, p < 0.0001). Positive surgical margins were significantly more common in mixed type BCC subgroup (17.8 % vs. 12.6 %, p = 0.02). Cutaneous BCCs with mixed histomorphology represented about one third of the cases. It is a common finding in routine pathological practice, probably suggestive of evolution and phenotypic transformation of the cancer. Since mixed type BCCs are frequently composed of aggressive histological subtypes, regardless the personal habits in description or terminology among pathologists, the presence of aggressive-growth component in tumor tissue should always be mentioned in final biopsy report.
Keywords:
basal cell carcinoma – single type basal cell carcinoma – mixed type basal cell carcinoma
Basal cell carcinoma (BCC) of the skin generally exhibits a very heterogeneous histomorphology. Therefore, an extremely broad spectrum of BCC subtypes and variants have been described so far, though this is partly due to the inconsistency in the nomenclature as well as due to the use of different classification schemes (1-3). More than sixty various subtypes of BCC have been presented in the scientific literature to date (2). However, their strict separation is somewhat artificial as there is an overlap due to the significant tumor plasticity (3). Thus, the terminology varies among authors reflecting their individual perceptions of the tumors (3). When classifying BCCs, most authors start from the growth pattern, which gives more information about biological behavior, and less often from the tumor differentiation (1).
There are variable treatment options for this neoplasia, in which histopathologic pattern is one of the most important factors in choosing the suitable therapeutic strategy (4). While some prognostically favorable subtypes, such as superficial or nodular ones, can be successfully treated non-invasively, the gold standard for more aggressive infiltrative (morpheic) BCC is a total surgical extirpation. Thus, recognition of the histological subtype of BCC is an important point for further clinical management. However, in many cases, it is difficult to classify BCC exactly, because it may consist of more than one histopathological subtype.
These mixed forms are interesting for many reasons. For example, since some BCC subtypes occur predominantly on distinct anatomic sites with certain age and gender differences, they may have different underlying causes and pathogenetic mechanisms (5,6). Currently, it is not definitively explained, whether they reflect a continuous process of carcinogenesis, in which morphological pattern changes over the time, or whether they represent separate developmental lines with stabile phenotype (7). Therefore, BCC with mixed histomorphology may represent a neoplasm with distinct etiopathogenesis and biology.
Based on some reports (8,9) there is an evidence that mixed type BCC may represent an individual subset of BCCs with potential aggressive behavior and tendency for local recurrences. Moreover, since different BCC subtypes need specific treatment interventions (4), improving the knowledge of the mixed type BCCs may lead to the development of a more effective treatment method for them. However, there are only limited data exploring the characteristics of mixed BCCs (10). The aim of this study was to estimate the prevalence of mixed BCCs in our files and to compare the clinicopathological features of mixed type vs. single type BCCs.
MATERIAL AND METHODS
For the purpose of the study, we retrospectively reviewed all consecutive BCCs of the skin, that were histologically diagnosed at the Department of Pathology in Faculty Hospital in Zilina (Slovakia) between January 2007 – September 2015. During this period, we examined total of 1105 cutaneous BCCs, of which 1033 were primary lesions and 72 recurrent ones. Then, we excluded recurrent tumors, as well as all cases, in which tissue sample was obtained by probatory excision (including shave and punch biopsies). Subsequent re-excisions after incomplete removal of tumor were also eliminated. Thus, the study group finally consisted of 911 primary BCCs of the skin. They were obtained from 697 subjects (350 men, 347 women) in the age range of 25 - 97 years (mean age 69.8 years). Biopsy material was fixed in buffered formalin, embedded in paraffin blocks and stained with hematoxylin and eosin.
In selected cases, immunohistochemical staining methods have also been applied, i.e. with antibodies against Epithelial Antigen (clone BerEP4, DAKO), E-cadherin (clone NCH-38, DAKO), High Molecular Weight Cytokeratins (clone 34βE12, DAKO) and Epithelial Membrane Antigen (clone E29, DAKO).
Clinical data of the patients were obtained from their medical records. Data on histological subtype, age, gender and topographical location were collected.
The histopathological classification of BCC subtypes was done using the latest World Health Organization classification of skin cancers (11). According to histomorphology, the cases were divided into the single type and mixed type BCC subgroups. The later one was composed of two or more histological subtypes in various proportions. Subsequently, indolent - (low-risk) and aggressive - (high-risk) growth phenotypes were determined according to the previous reports (2,12). While the infiltrative, morpheaform, micronodular and metatypical (basosquamous) subtypes of BCC were classified as aggressive-growth phenotypes, all others were considered as indolent. If the tumor contained (even focally) aggressive-growth features, it was finally categorized as a high-risk subtype. The anatomical sites were classified as follows: head, neck, trunk, upper extremities, and lower extremities. The head was additionally divided into facial and extrafacial part.
Data were collected in a databank, using a software SPSS Statistics version 19. For the statistical analysis, chi-square test was employed and P value < 0.05 was considered significant.
RESULTS
In our set of tumors, single type and mixed type BCCs represented 591 (64.9 %) and 320 (35.1 %) cases, respectively. The topographic distribution of all 911 lesions was as follows: face (n=386; 42.4 %), extrafacial regions of the head (n=201; 22.1 %), neck (n=32; 3.5 %), trunk (n=206; 22.6 %), upper extremities (n=65; 7.1 %), and lower extremities (n=21; 2.3 %). In single type BCC subgroup, we confirmed following histological subtypes in descending line: nodular (n=364; 61.6 %), superficial (n=107; 18.1 %), infiltrative (n=45; 7.6 %), BCC with adnexal differentiation (n=39; 6.6 %), morpheic (n=13; 2.2 %), micronodular (n=11; 1.9 %), basosquamous (n=8; 1.3 %), and Pinkus tumor (n=4; 0.7 %). In mixed type BCC subgroup, a very heterogeneous histomorphology was found comprising a mixture of two to four different histological subtypes. The four most frequent combinations were nodular-infiltrative (n=169; 52.8 %), superficial-nodular (n=64; 20.0 %), nodular-trichoepithelial (n=19; 5.9 %) and nodular-micronodular (n=16; 5.0 %). The remaining combinations occurred only in a very low percentage, including a few cases with specific cell line differentiation features (infundibulocystic BCC, BCC with focal glandular and sebaceous differentiation, BCC containing pleomorphic cell population). Comparative analysis of the two given subgroups showed that mixed type BCCs were significantly more frequently localized on the extrafacial regions of the head (30.0 % vs. 20.0 %, p = 0.02) and less often on the face (37.2 % vs. 45.2 %, p = 0.03). We did not observed differences in the incidence of single type vs. mixed type BCCs in other parts of the body. Histologically, mixed type BCCs exhibited an aggressive-growth pattern much more frequently (64.6 % vs. 13.0 %, p < 0.0001). Positive surgical margins detected microscopically were significantly more common in mixed type BCC subgroup (17.8 % vs. 12.6 %, p = 0.02). We were not able to explore reliably a frequency of local tumor recurrences due to incomplete follow-up data. A summary of the distribution of clinical and histopathological characteristics of our cohort of patients divided into single type and mixed type BCC subgroups is given in Table 1. The microscopic features of selected BCCs with mixed histomorphology are shown in Figures 1 - 5.
Tab. 1. A summary of the clinical and histopathological characteristics of our cohort of patients categorized into single type and mixed type BCC subgroups. Symbol * indicates a statistical significance (P < 0.05). 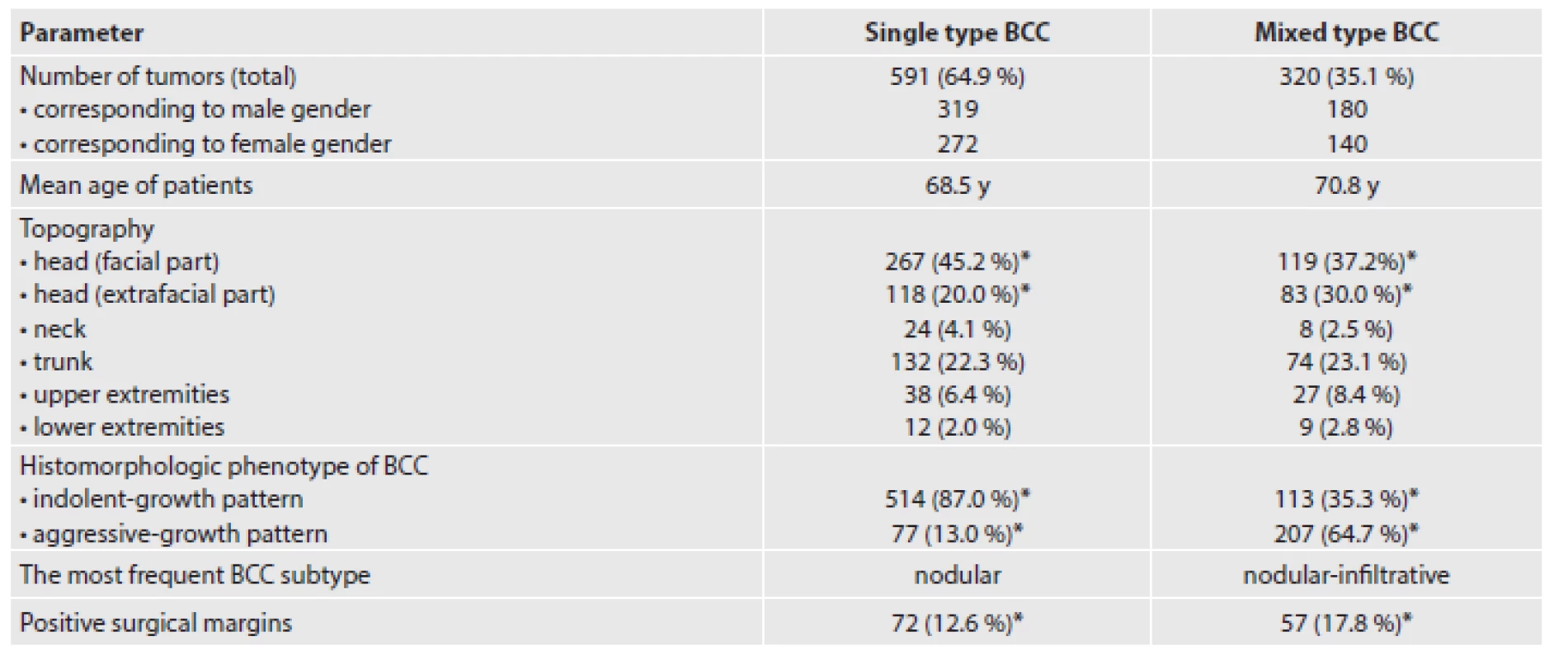
Fig. 1. Mixed type BCC composed of nodular (upper right) and superficial (left) pattern (hematoxylin & eosin staining, original magnification 120x). 
Fig. 2. BCC with a mixture of nodular and infiltrative pattern (monoclonal mouse anti-human antibody against E-cadherin, clone NCH-38, DAKO, dilution 1:50, original magnification 120x). 
Fig. 3. Mixed type BCC consisted of nodular (left) and micronodular (right) histological component. A micronodular component exhibits infiltrative-growth pattern (hematoxylin & eosin staining, original magnification 240x). 
Fig. 4. Mixed BCC with features of basosquamous (mostly upper part) and infiltrative (mostly lower part) subtype (hematoxylin & eosin staining, original magnification 240x). 
Fig. 5. Immunohistochemical detection of EMA (the case presented in Figure 4). While basosquamous parts of tumor focally exhibit EMA expression (upper), „pure“ infiltrative component (lower) is completely negative (monoclonal mouse anti-human antibody against EMA, clone E29, DAKO, dilution 1:100, original magnification 240x). 
DISCUSSION
The total prevalence of BCCs with mixed histomorphology is hardly to estimate exactly, because the published studies have provided different and inconsistent results representing 32.4 – 43 % of all cases (6,8,10,13,14). In our analysis, mixed BCCs accounted for 35.1 %, which is in accordance with that percentage range. However, a recent study published by Roozeboom et al. (15) has shown that approximately 74 % of all primary BCCs consisted of more than one single histological subtype. Conversely, Hakverdi et al. (16) found only 44 mixed BCCs (22.3 %) in a set of 144 lesions and Betti et al. (9) showed prevalence reaching merely 17.8 %. These discrepancies are probably caused by disunity in classification approaches, since there is no consensus about from which percentage of an additional morphological subtype should BCC be determined as a different histological subtype. It mostly depends on personal habits of pathologists, whether they definitively designate BCC with one name based on its predominant subtype, or whether they classify it as mixed type BCC with accompanying description of all histological components found.
A mixture of histopathological subtypes within one lesion may be variable and this phenomenon is likely suggestive of an evolution and of a phenotypic transformation of the cancer. In a detailed analysis of 267 mixed type BCCs of the skin, Ghanadan et al. (10) confirmed 31 various combinations, of which about one third consisted of three distinct histological subtypes. The most common mixed BCCs included nodular-infiltrative, superficial-nodular, and nodular-micronodular subtype. Our results are consistent with these data. To date, there are only few reports specifically focused on the analysis of BCC with mixed histomorphology trying to explore their specific features. Among them, some confirmed significant differences in certain clinico-pathological parameters between single type and mixed type BCC group (9,10). For example, in the study of Ghanadan et al. (10), mixed type BCCs were more commonly located on the scalp and less frequently on the face in comparison to single type BCCs. The mean diameter and prevalence of necrosis were significantly higher in mixed type BCCs. Betti et al. (9) found that prevalence of mixed type BCCs was higher on the upper limbs and on the lower back. They showed that mixed BCCs had been associated with tumor aggressiveness, lateral margin involvement and male gender. While an aggressive-growth pattern was found only in 16.0 % of single type BCCs, it was present in 59.1 % of BCCs with combined histomorphology. Furthermore, surgical margin involvement was more prevalent in mixed type than in single type BCCs.
In our series, mixed BCCs occurred significantly more frequently on the extrafacial regions of the head and less often on the face. Histologically, mixed type BCCs had an aggressive-growth pattern, as well as positive surgical margins more commonly. Several studies have shown, so as we did, that the majority of mixed type BCCs (up to 70 %) contain aggressive-growth features (9,10,17). From the prognostic point of view, they usually represent an unfavorable form of the cancer with higher propensity for local recurrences (8,9). Unfortunately, we could not objectively compare a relapse rate between both given BCC groups, because we did not have an adequate clinical follow-up of such a large cohort of the patients.
As pointed above, the recognition of histopathologic patterns is an important element in BCC treatment algorithms. Some BCC subtypes possess slow indolent growth, while others may grow aggressively with extensive tissue damage, which complete healing is difficult (1,7,18). However, in a routine biopsy practice, many mixed type BCCs contain both indolent - and aggressive-growth pattern, for example nodular-infiltrative or nodular-micronodular subtypes. The last consensus of The Royal College of Pathologists (19) advise, that the overall clinical risk status of BCC is best judged from „the highest“ risk subtype that is present in tumor tissue, irrespective of percentage. On that basis, BCC containing any aggressive-growth component should be histomorphologically considered and clinically managed as high-risk variant.
CONCLUSION
Cutaneous BCCs with mixed histomorphology represent about one third of cases indicating, that it is a frequent finding in routine pathological practice. There is no agreement about how should be these lesions described in the biopsy report at best, but description of all present histological components seems to be the most appropriate choice. Since mixed type BCCs frequently involve aggressive histological subtypes, the presence of aggressive-growth component in tumor tissue should always be mentioned in final biopsy report. Furthermore, given relatively high rate of BCCs with mixed histomorphology, a limited value of probatory biopsy samples for the determination BCC subtype should be kept in mind.
ACKNOWLEDGMENTS
The authors wish to thank MUDr. Zacharová Oľga and MUDr. Pokorný Dušan for their educational support and MUDr. Doboszová Jana and MUDr. Rychlý Boris, PhD. for technical assistance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
Correspondence address:
Bartoš Vladimír, M.D., Ph.D., MSc.
Björnsonova 3/5
Martin, 036 01,
Slovakia
e-mail: vladim.bartos@gmail.com,
phone: +421908386352
Zdroje
1. Vantuchová Y, Čuřík R. Histological types of basal cell carcinoma. Scripta Medica (Brno) 2006; 79(5-6): 261-270.
2. Nedved D, Tonkovic-Capin V, Hunt E et al. Diagnostic concordance rates in the subtyping of basal cell carcinoma by different dermatopathologists. J Cutan Pathol 2014; 41(1): 9-13.
3. Kazakov DV, Michal M, Kacerovska D, McKee, PH. Cutaneous Adnexal Tumors, Wolters Kluwer Health / Lippincott Williams & Wilkins, Philadelphia, 2012. 814 pages. ISBN 978-1605478548
4. Telfer NR, Colver GB, Morton CA. Guidelines for management of basal cell carcinoma. Br J Dermatol 2008; 159(1): 35-48.
5. Bastiaens MT, Hoefnagel JJ, Brujin JA, Westendorp RG, Vermeer BJ, Bouwes Bavinck JN. Differences in age, site distribution, and sex between nodular and superficial basal cell carcinoma indicate different types of tumors. J Invest Dermatol 1998; 110(6): 880-884.
6. Ghanadan A, Abdollahi P, Rabet M et al. Different anatomical distribution of basal cell carcinoma subtypes in Iranian population: association between site and subtypes. Ann Dermatol 2014; 26(5): 559-563.
7. Bartoš V, Adamicová K, Kullová M, Péč M. Bazocelulárny karcinóm kože – biologické správanie nádoru a prehľad najvýznamnejších molekulových ukazovateľov progresie ochorenia v praxi patológa. Klin Onkol 2011; 24(1): 8-17.
8. Cohen PR, Schulze KE, Nelson BR. Basal cell carcinoma with mixed histology: a possible pathogenesis for recurrent skin cancer. Dermatol Surg 2006; 32(4): 542-551.
9. Betti R, Radaelli G, Crosti C, Ghiozzi S, Moneghini L, Menni S. Margin involvement and clinical pattern of basal cell carcinoma with mixed histology. J Eur Acad Dermatol Venereol 2012; 26(4): 483-487.
10. Ghanadan A, Abbasi A, Rabet M, Abdollahi P, Abbasi MA. Characteristics of mixed type basal cell carcinoma in comparision to other BCC subtypes. Indian J Dermatol 2014; 59(1): 56-59.
11. LeBoit P, Burg G, Weedon D et al. (Eds). World Health Organization Classification of Tumours, Pathology and Genetics of Skin tumours, IARCPress, Lyon, 2006; 355 pages. ISBN 92-832-2414-0
12. Bartoš V, Adamicová K, Kullová M, Péč M. Immunohistochemical evaluation of proliferative activity (Ki-67 index) in different histological types of cutaneous basal cell carcinoma. Biologia 2012; 67(3): 610-615.
13. Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol 1990; 23(6 Pt 1): 1118-1126.
14. Mantese SAO, Berbert ALC, Gomides MDA, Rocha A. Basal cell Carcinoma - Analysis of 300 cases observed in Uberlândia - MG, Brazil. An Bras Dermatol 2006; 81(2): 136-142.
15. Roozeboom MH, Mosterd K, Winnepenninckx VJ, Nelemans PJ, Kelleners-Smeets NW. Agreement between histological subtype on punch biopsy and surgical excision in primary basal cell carcinoma. J Eur Acad Dermatol Venereol 2013; 27(7): 894-898.
16. Hakverdi S, Balci DD, Dogramaci CA, Toprak S, Yaldiz M. Retrospective analysis of basal cell carcinoma. Indian J Dermatol Venereol Leprol 2011; 77(2): 251.
17. Kamyab-Hesari K, Seirafi H, Naraghi ZS et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol 2014; 28(2): 250-253.
18. Bartoš V, Adamicová K, Mačuga I, Pokorný D, Zacharová O, Péč M. „Gigantický” bazocelulárny karcinóm kože hlavy s intrakraniálnou propagáciou - kazuistika. Cesk Patol 2011; 47(4): 178-182.
19. Slater D, Walsh M. Standards and dataset for reporting cancers. Dataset for the histological reporting of primary cutaneous basal cell carcinoma. The Royal College of Pathologists. 3
rd ed., May 2014. 29 pages.
Štítky
Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseČesko-slovenská patologie

2016 Číslo 4-
Všechny články tohoto čísla
- Mutace genů BRCA ... i co dalšího život patologům dal a vzal
- Nikdo by neměl podléhat iluzi, že to bez něj dál nepůjde
- MONITOR - aneb nemělo by vám uniknout že ...
- BRCA1 and BRCA2 – pathologist’s starting kit
- Problematika mutací BRCA z klinického pohledu
- Oncopathological aspects of BRCA1 and BRCA2 genes inactivation in tumors of ovary, fallopian tube and pelvic peritoneum
- Breast cancer in BRCA1/2 mutation carriers
- Testing of mutations in BRCA1 and BRCA2 genes in tumor tissues - possibilities and limitations
- Prof. MUDr. Blahoslav Bednář, DrSc. – 100 let od narození
- Clear cell sarcoma of vulva. A case report
- MONITOR - aneb nemělo by vám uniknout že ...
- Diffuse tenosynovial giant cell tumor of the cervical spine destroying vertebra C6 - a case report
- Basal cell carcinoma of the skin with mixed histomorphology: a comparative study
- Zemřel neurohistolog a neuropatolog prof. MUDr. Stanislav Němeček, DrSc. (4. 11. 1931 – 17. 8. 2016)
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Testing of mutations in BRCA1 and BRCA2 genes in tumor tissues - possibilities and limitations
- Diffuse tenosynovial giant cell tumor of the cervical spine destroying vertebra C6 - a case report
- BRCA1 and BRCA2 – pathologist’s starting kit
- Breast cancer in BRCA1/2 mutation carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



