-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Side Effects: Substantial Non-Neutral Evolution Flanking Regulatory Sites
article has not abstract
Published in the journal: . PLoS Genet 9(5): e32767. doi:10.1371/journal.pgen.1003528
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003528Summary
article has not abstract
In the pre-genome era, most of what we knew about molecular evolution could be traced to our knowledge of the genetic code, and the impact of DNA sequence variation on protein structure and, by inference, protein function [1]. But in the post-genome era, it has become clear that the fraction of functional sequence—estimated using comparative approaches to identify residues that “escape” genetic drift—far exceeds the fraction explained by protein-coding genes. In mammals, somewhere between 5% and 15% of the genome is evolutionarily constrained, and is presumably functional [2]. Drosophila and other invertebrate genomes may have much larger proportions (47%–70%) of constrained nucleotides [3]; in all cases, the proportions of nucleotides found to be conserved dwarves those encoding proteins (around 1% in humans and 20% in flies)—which prompts the question: what aspects of genomic function might explain these apparent excesses of conserved sequence?
Over the past few years, it has become clear that the physical organization and structure of the genome within cells, over a range of scales, also casts discernable shadows on the sequence. This is the realm of chromatin structure (the many combinations of proteins associated with the DNA), which adopts an undulating landscape along chromosomes associated with cellular functions such as transcription. The binding of a range of proteins to eukaryotic genomes has been shown to be linked to variation in the underlying DNA sequence. The specific regions of the human genome known to be bound by transcription factors often display remarkable patterns of conservation that parallel the structure of the DNA-binding interface of the protein involved [4]. More broadly, characteristic fluctuations in sequence divergence have been observed corresponding to nucleosome cores and intervening linker sequences across a variety of species [5], and there is evidence that this reflects the action of selection [6]. However, recent data from the ENCODE Consortium has suggested that perhaps 80% of the human genome is functional, in the sense that it is subject to a biochemical modification in at least one cell type [7]. This substantially exceeds all estimates of the proportion of human nucleotides under constraint, including those used by the ENCODE Consortium [8], and the discrepancy has led to some notably animated discussion [9]. There is therefore a large gap between the proportion of the genome thought to be functional via evolutionary studies and the proportion that appears functional, according to the presence of particular chromatin features. This gap also appears to exist, though to a lesser extent, in Drosophila, where over 90% of the genome has been assigned a biochemical role of some description [10]. In this issue of PLOS Genetics, Kenigsberg and Tanay [11] have investigated the links between chromatin and sequence evolution from the point of view of conserved noncoding elements (CNEs), and may have found a way to begin to bridge the gap. Rather than examining DNA sequence conservation at the sites of a particular chromatin state, they have investigated the characteristics of CNEs in the Drosophila genome, within their genomic and chromatin context.
Kenigsberg and Tanay first identified approximately 68,000 short (mean length of 50 bp) regions of the genome whose rate of divergence was at least two times lower than expected. These CNEs were observed to coincide with the location of a range of chromatin features, suggesting underlying DNA sequence conservation is a feature of a range of functional chromatin states in Drosophila. Although these CNEs covered only around 3% of the Drosophila genome, they were found to have characteristic sequence compositional biases. The vast majority of these short elements were centered upon a small (20–30 bp), unusually AT rich, focal region. However, it was found these short AT rich regions were embedded in larger (several hundred base pairs), relatively GC rich regions. Surprisingly, these patterns were observed at CNEs irrespective of the functional chromatin state seen at the CNE, including states associated with promoters, enhancers, repressed sites, and insulator sites. Compositional biases have previously been noted as a common feature of some regulatory sites, and this study shows these compositional biases are linked to the positioning of nucleosomes on either side of such sites. Nucleosomes have been shown to preferentially associate with GC rich regions of DNA, and, in species from yeast to humans, nucleosome positioning appears to be maintained by a balance in the number of A/T relative to G/C base pair gaining substitutions maintained by selection [6], [12]. Kenigsberg and Tanay report a similar balance in the gain and loss of GC dinucleotides, maintaining elevated GC content on either side of the relatively AT rich Drosophila CNEs, and suggest this balance is also likely to be maintained by selection. They conclude that although only a small proportion of the genome (within CNEs) displays evidence for strong evolutionary constraint, a substantially larger proportion, approximately 25%, is evolving non-neutrally due to the milder selective constraints imposed to maintain the surrounding local chromatin structure (Figure 1). This raises the possibility that large swathes of any genome may be subject to rather modest, and often elusive, levels of constraint on sequence composition as an extended side effect of the presence of neighbouring regulatory sites.
Fig. 1. Non-neutral evolution within large regions flanking CNEs in <i>Drosophila</i> acts to maintain sequence composition and favourable nucleosome positioning. 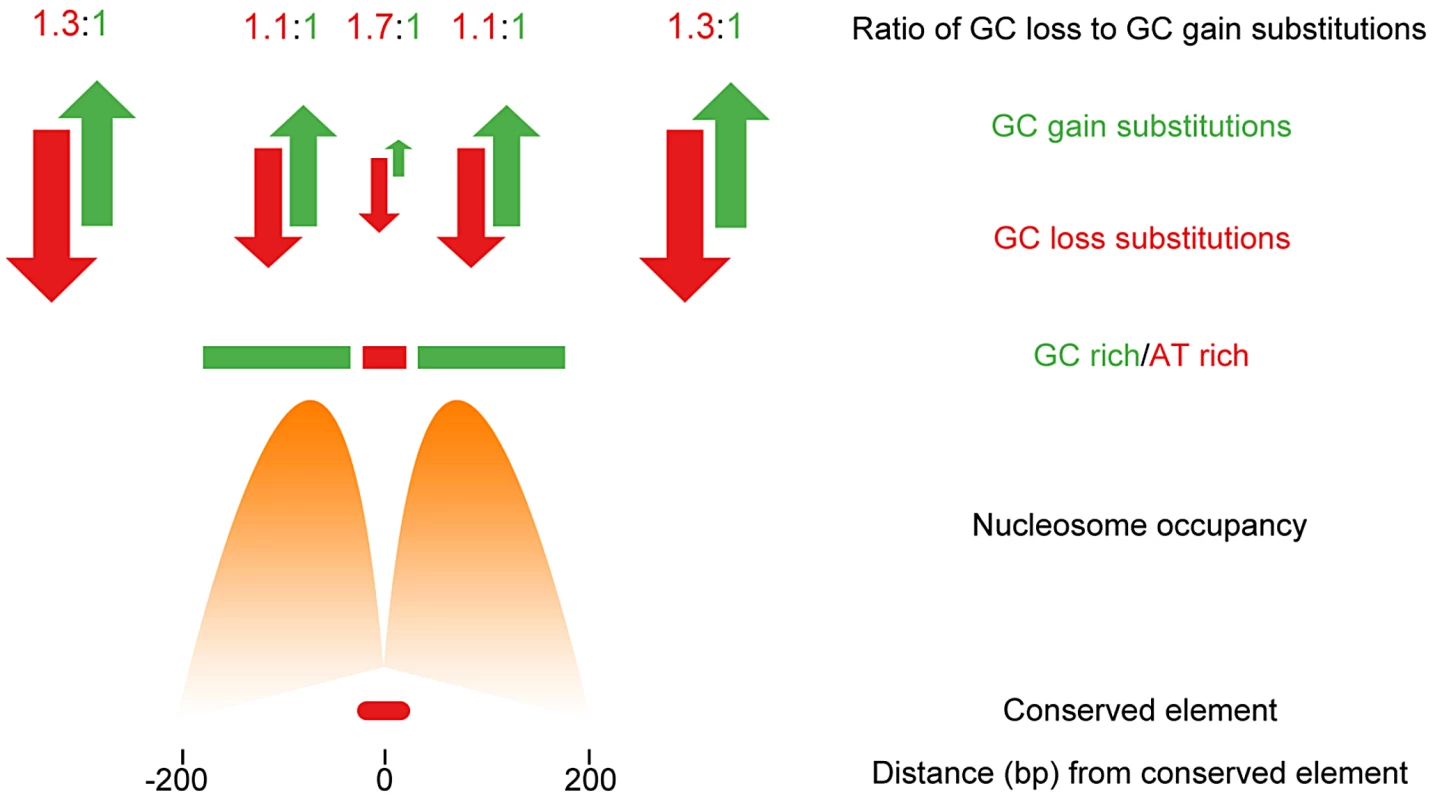
Kenigsberg and Tanay go on to show that the rate of base substitutions, as measured by population polymorphisms, is also dependent on the base composition of the region considered. For instance, GC depleting substitutions were observed to be underrepresented at GC rich regions. This was found to be the case not only in flies, but also when mouse and human data were examined. Together, these data suggest that structural constraints are impacting the evolutionary dynamics of current populations across a range of eukaryotic organisms. They also support a new worldview in evolutionary genomics, where a complete understanding of sequence variation and its effects on function is only possible by considering the genome as a physical molecule. Genome evolution may be seen more clearly seen through the lens of the epigenome.
Zdroje
1. FitchWM, MargoliashE (1967) Construction of phylogenetic trees. Science 155 : 279–284.
2. PontingCP, HardisonRC (2011) What fraction of the human genome is functional? Genome Res 21 : 1769–1776.
3. PontingCP, NellakerC, MeaderS (2011) Rapid turnover of functional sequence in human and other genomes. Annu Rev Genomics Hum Genet 12 : 275–299.
4. NephS, VierstraJ, StergachisAB, ReynoldsAP, HaugenE, et al. (2012) An expansive human regulatory lexicon encoded in transcription factor footprints. Nature 489 : 83–90.
5. SempleCA, TaylorMS (2009) Molecular biology. The structure of change. Science 323 : 347–348.
6. PrendergastJG, SempleCA (2011) Widespread signatures of recent selection linked to nucleosome positioning in the human lineage. Genome Res 21 : 1777–1787.
7. ENCODE Project Consortium (2012) DunhamI, KundajeA, AldredSF, CollinsPJ, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
8. HoffmanMM, ErnstJ, WilderSP, KundajeA, HarrisRS, et al. (2013) Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res 41 : 827–841.
9. GraurD, ZhengY, PriceN, AzevedoRB, ZufallRA, et al. (2013) On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biol Evol 5 : 578–590.
10. modENCODE Consortium (2010) RoyS, ErnstJ, KharchenkoPV, KheradpourP, et al. (2010) Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330 : 1787–1797.
11. KenigsbergE, TanayA (2013) Drosophila functional elements are embedded in structurally constrained sequences. PLoS Genet 9: e1003512 doi:10.1371/journal.pgen.1003512.
12. KenigsbergE, BarA, SegalE, TanayA (2010) Widespread compensatory evolution conserves DNA-encoded nucleosome organization in yeast. PLoS Comput Biol 6: e1001039 doi:10.1371/journal.pcbi.1001039.
Štítky
Genetika Reprodukční medicína
Článek Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific RecombinationČlánek Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding YeastČlánek High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple IsolatesČlánek Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance inČlánek Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 5- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Functional Elements Are Embedded in Structurally Constrained Sequences
- RNA–Mediated Epigenetic Heredity Requires the Cytosine Methyltransferase Dnmt2
- Loss of Expression and Promoter Methylation of SLIT2 Are Associated with Sessile Serrated Adenoma Formation
- Attachment Site Selection and Identity in Bxb1 Serine Integrase-Mediated Site-Specific Recombination
- Human Genetics in Rheumatoid Arthritis Guides a High-Throughput Drug Screen of the CD40 Signaling Pathway
- Genome-Wide Analysis in German Shepherd Dogs Reveals Association of a Locus on CFA 27 with Atopic Dermatitis
- Liver X Receptors Protect from Development of Prostatic Intra-Epithelial Neoplasia in Mice
- Chromosomal Organization and Segregation in
- A Statistical Framework for Joint eQTL Analysis in Multiple Tissues
- Cell Polarity and Patterning by PIN Trafficking through Early Endosomal Compartments in
- Bck2 Acts through the MADS Box Protein Mcm1 to Activate Cell-Cycle-Regulated Genes in Budding Yeast
- High-Resolution Transcriptome Maps Reveal Strain-Specific Regulatory Features of Multiple Isolates
- Neuropeptides Function in a Homeostatic Manner to Modulate Excitation-Inhibition Imbalance in
- A Compendium of Nucleosome and Transcript Profiles Reveals Determinants of Chromatin Architecture and Transcription
- Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation
- Filamin and Phospholipase C-ε Are Required for Calcium Signaling in the Spermatheca
- The Specificity and Flexibility of L1 Reverse Transcription Priming at Imperfect T-Tracts
- Imputation-Based Meta-Analysis of Severe Malaria in Three African Populations
- Implicates Tyrosine-Sulfated Peptide Signaling in Susceptibility and Resistance to Root Infection
- Clathrin and AP2 Are Required for Phagocytic Receptor-Mediated Apoptotic Cell Clearance in
- Encodes CDF Transporters That Excrete Zinc from Intestinal Cells of and Act in a Parallel Negative Feedback Circuit That Promotes Homeostasis
- Global Properties and Functional Complexity of Human Gene Regulatory Variation
- DNA Binding of the Cell Cycle Transcriptional Regulator GcrA Depends on N6-Adenosine Methylation in and Other
- Side Effects: Substantial Non-Neutral Evolution Flanking Regulatory Sites
- From Paramutation to Paradigm
- From Mouse to Human: Evolutionary Genomics Analysis of Human Orthologs of Essential Genes
- Distinct Translational Control in CD4 T Cell Subsets
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- The Majority of Primate-Specific Regulatory Sequences Are Derived from Transposable Elements
- Identification of Meiotic Cyclins Reveals Functional Diversification among Plant Cyclin Genes
- EGL-13/SoxD Specifies Distinct O and CO Sensory Neuron Fates in
- Congruence of Additive and Non-Additive Effects on Gene Expression Estimated from Pedigree and SNP Data
- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- Ikbkap/Elp1 Deficiency Causes Male Infertility by Disrupting Meiotic Progression
- Analysis of the Genetic Basis of Disease in the Context of Worldwide Human Relationships and Migration
- Duplication and Retention Biases of Essential and Non-Essential Genes Revealed by Systematic Knockdown Analyses
- Strong Purifying Selection at Synonymous Sites in
- , a Susceptibility Gene for Type 1 and Type 2 Diabetes, Modulates Pancreatic Beta Cell Apoptosis via Regulation of a Splice Variant of the BH3-Only Protein
- Chromosome Movements Promoted by the Mitochondrial Protein SPD-3 Are Required for Homology Search during Meiosis
- The Secretory Pathway Calcium ATPase PMR-1/SPCA1 Has Essential Roles in Cell Migration during Embryonic Development
- The Genomic Signature of Crop-Wild Introgression in Maize
- CDK4 T172 Phosphorylation Is Central in a CDK7-Dependent Bidirectional CDK4/CDK2 Interplay Mediated by p21 Phosphorylation at the Restriction Point
- Genome-Wide Identification of Regulatory RNAs in the Human Pathogen
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Extended Genealogy to Estimate Components of Heritability for 23 Quantitative and Dichotomous Traits
- HDAC7 Is a Repressor of Myeloid Genes Whose Downregulation Is Required for Transdifferentiation of Pre-B Cells into Macrophages
- Female Bias in and Regulation by the Histone Demethylase KDM6A
- ATM–Dependent MiR-335 Targets CtIP and Modulates the DNA Damage Response
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



