-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
Epoxyeicosatrienoic acids (EETs) confer vasoactive and cardioprotective functions. Genetic analysis of the contributions of these short-lived mediators to pathophysiology has been confounded to date by the allelic expansion in rodents of the portion of the genome syntenic to human CYP2J2, a gene encoding one of the principle cytochrome P450 epoxygenases responsible for the formation of EETs in humans. Mice have eight potentially functional genes that could direct the synthesis of epoxygenases with properties similar to those of CYP2J2. As an initial step towards understanding the role of the murine Cyp2j locus, we have created mice bearing a 626-kb deletion spanning the entire region syntenic to CYP2J2, using a combination of homologous and site-directed recombination strategies. A mouse strain in which the locus deletion was complemented by transgenic delivery of BAC sequences encoding human CYP2J2 was also created. Systemic and pulmonary hemodynamic measurements did not differ in wild-type, null, and complemented mice at baseline. However, hypoxic pulmonary vasoconstriction (HPV) during left mainstem bronchus occlusion was impaired and associated with reduced systemic oxygenation in null mice, but not in null mice bearing the human transgene. Administration of an epoxygenase inhibitor to wild-type mice also impaired HPV. These findings demonstrate that Cyp2j gene products regulate the pulmonary vascular response to hypoxia.
Published in the journal: . PLoS Genet 9(11): e32767. doi:10.1371/journal.pgen.1003950
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003950Summary
Epoxyeicosatrienoic acids (EETs) confer vasoactive and cardioprotective functions. Genetic analysis of the contributions of these short-lived mediators to pathophysiology has been confounded to date by the allelic expansion in rodents of the portion of the genome syntenic to human CYP2J2, a gene encoding one of the principle cytochrome P450 epoxygenases responsible for the formation of EETs in humans. Mice have eight potentially functional genes that could direct the synthesis of epoxygenases with properties similar to those of CYP2J2. As an initial step towards understanding the role of the murine Cyp2j locus, we have created mice bearing a 626-kb deletion spanning the entire region syntenic to CYP2J2, using a combination of homologous and site-directed recombination strategies. A mouse strain in which the locus deletion was complemented by transgenic delivery of BAC sequences encoding human CYP2J2 was also created. Systemic and pulmonary hemodynamic measurements did not differ in wild-type, null, and complemented mice at baseline. However, hypoxic pulmonary vasoconstriction (HPV) during left mainstem bronchus occlusion was impaired and associated with reduced systemic oxygenation in null mice, but not in null mice bearing the human transgene. Administration of an epoxygenase inhibitor to wild-type mice also impaired HPV. These findings demonstrate that Cyp2j gene products regulate the pulmonary vascular response to hypoxia.
Introduction
Human cytochrome P450 2J2 (CYP2J2) is abundant in cardiovascular tissues and pulmonary endothelium [1] and metabolizes arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs), short-lived mediators that have potent vascular protective properties [2]–[4]. The mouse chromosomal locus syntenic to human CYP2J2 contains 10 genes (8 presumed genes and 2 pseudogenes) spanning 626 kb on chromosome 4. Gene clusters in the mouse that are syntenic to a single human gene are not uncommon, but their study is rarely straightforward. Mutant mice with short gene deletions can be generated through the conventional gene targeting strategies [5], but the deleted region rarely exceeds ten kilobases in most applications of the existing technology. Bacterial artificial chromosomes (BACs), which can have lengths up to ∼250 kb, have been used for gene targeting [6]–[8], but even in these cases the length of the BAC creates a formal upper limit for the size of the deletion. Deletion of a large DNA region has been accomplished by sequential introduction of loxP sites followed by the expression of Cre recombinase in embryonic stem cells [9]–[11]. However, it is difficult to distinguish loxP sites integrated into the same autosome from those integrated into separate autosomes, and Cre-mediated recombination has relatively low efficiency when the distance between loxP sites is great [9]–[11]. Here we describe a method to join BACs using prokaryotic integrases to create a deletion replica in E. coli that is subsequently used to target the murine locus.
CYP2J2 products elicit a variety of effects including fibrinolysis, vasodilation, and inhibition of inflammation [12]–[14]. However, a definitive identification of the contributions of Cyp2j genes in the cardiovascular system has remained challenging due to the expansion of the locus in mice. Murine Cyp2j isoforms may act as epoxygenases and hydroxylases to metabolize AA into EETs and HETEs [15]. It has been shown that the pulmonary vasoconstrictor response to alveolar hypoxia is ablated in mice deficient for cytosolic phospholipase A2α (cPLA2α), an enzyme that lies upstream of Cyp2j and is responsible for liberation of AA from esterified forms of phospholipids in the cell membrane [16]. There are four pathways downstream of cPLA2α mediating the metabolism of AA, including the cyclooxygenase (COX), lipoxygenase (LO), epoxygenase, and ω-hydroxylase pathways. It has been shown that inhibition of COX or 5-LO pathways does not impair hypoxic pulmonary vasoconstriction (HPV) [17], [18]. Previous studies have demonstrated that products of CYP epoxygenases and hydroxylases can produce pulmonary vasoconstriction and vasodilation, respectively. However, it is unknown which cytochrome P450 is the major contributor to the regulation of HPV - a mechanism unique to the pulmonary vasculature, that diverts blood flow away from poorly ventilated lung regions, thereby preserving oxygenation of systemic blood [4], [16], [19]. The pulmonary vasoconstrictor response to alveolar hypoxia is crucial for maintaining arterial oxygenation during acute respiratory failure and lung injury. Due to the diversity of potential metabolites and the challenges associated with their measurement, stemming from their propensity for rapid metabolism and multiple functionally-relevant isomeric forms, it has been challenging to precisely identify which gene family and which eicosanoids are the most relevant modulators of HPV.
In this study, we describe a strategy to engineer large DNA fragments in bacteria and mammalian cells. We performed large scale ablation and human allelic complementation of the Cyp2j locus in mice using E. coli genetic techniques and bacterial artificial chromosome technology. Phenotypic characterization of the resulting Cyp2j-null and complemented mice showed that disruption of mouse Cyp2j genes did not alter pulmonary and systemic hemodynamic parameters at baseline. However, the increase in left lung pulmonary vascular resistance induced by selective left lung hypoxia was impaired in Cyp2j-null mice, but not in complemented mice, demonstrating that the Cyp2j genes contribute to hypoxic pulmonary vasoconstriction.
Results
Fusion of BACs using TP901 integrase in E. coli
Because no single BAC has been reported to span the entire mouse Cyp2j locus, two BACs that contain the termini of the Cyp2j gene cluster were separately modified to permit joining by site-specific recombination in E. coli (Figure 1A, 1B). Homologous arms were amplified from the BAC clones and subcloned into one targeting vector containing a kanamycin resistance element and the TP901-1 integrase attB site, and into another targeting vector containing an ampicillin resistance element and a TP901-1 attP site. Following homologous recombination, the selectable markers and integrase sites were integrated into the two BACs, forming MT5′BAC and MT3′BAC (Figure 1B), as identified using four PCR amplifications (using primers P1 to P8; Figure S1A). The PCR products were sequenced to confirm that the correct recombination products had formed.
Fig. 1. Construction of Cyp2j locus deletion replica. 
(A) Schematic map of mouse Cyp2j cluster and the human syntenic locus CYP2J2. (B) Construction strategy. The WT 5′BAC and WT 3′BAC (location shown on A) were modified using 5′ and 3′ targeting vectors, respectively, through homologous recombination in E. coli. The resultant MT5′BAC and MT3′BAC lack sequences between the recombination arms (HR) and have acquired selectable markers (neomycin resistance, thymidine kinase sensitivity, and blasticidin resistance) and integrase sites (attB1 and attP1 of TP901, attB2 and attP2 of R4). The fused BAC (FS BAC) was generated through site-specific recombination between attB3 and attP3 sites carried by the MT5′BAC and MT3′BAC, respectively, and mediated by TP901 integrase. Ampr, Ampicillin resistance; Bsrr, blasticidin resistance; TK, herpes simplex thymidine kinase; neor, kanamycin/geneticin resistance. The MT5′BAC and the MT3′BAC containing the termini of the mouse Cyp2j locus were then fused in E. coli by site-specific recombination. A plasmid expressing TP901 integrase under control of the araBAD promoter was introduced into a bacterial strain harboring the BAC containing the kanamycin resistance element and the TP901 attB site. TP901 expression was induced prior to creation of electrocompetent cells, and the modified BAC bearing ampicillin resistance was introduced. Following selection for resistance to both kanamycin and ampicillin the fused BAC resulting from integrase was identified by PCR. Two pairs of primers (P9 to P12 shown in Figure 1B) were used to identify the integration events (PCR results shown in Figure S1B). PCR products were sequenced to confirm the desired TP901 attL and attR sites had formed (representative sequences shown in Figure S1B). The correctly fused BAC (FS BAC) was digested with SpeI and BamHI to confirm that no unwanted rearrangements had occurred (Figure S1C).
Excision of mouse Cyp2j gene cluster using a deletion replica in mouse ES cells
The fused BAC was electroporated into mouse ES cells and geneticin-resistant clones were screened by multiplex ligation-dependent probe amplification (MLPA) using five wild-type probes—5wt, 3wt, wtM1, wtM2 and wtM3, located within the region targeted for deletion of the Cyp2j gene locus, and three mutant probes—5 m, 3 m and neo—located within the engineered Cyp2j gene locus and recognizing vector sequences and the selectable marker (Figure 2A). Two internal control probes, HP1 and ITGB3, that detect genes located on chromosome 8 and 11, respectively, were used to normalize signal intensities from probes for Cyp2j wild-type and mutant genes. The desired clones showed the expected pattern, in which the wild-type signal intensity (5wt, 3wt, wtM1, wtM2 and wtM3) was decreased approximately by half, indicating disruption of one allele (Figure 2B, C). The areas of mutant signal intensities, including 5m, 3m, and neo, reflect the integrated copy numbers. Clones showing the lowest mutant signal intensity among the screened clones were considered likely to be single copy integrations (representative data shown in Figure 2B).
Fig. 2. Analysis of ES clones by multiplex ligation-dependent probe amplification (MLPA). 
(A) Schematic diagram of MLPA probes for WT and null alleles of the Cyp2j locus and of two control probes for chromosome 8 and 11. (B) Representative MLPA data of WT, non-target (12F12), and target (4G02) ES clones. The extra 5 m, 3 m and neo amplicons were detected in DNA from non-target and target ES clones but not from WT ES clones. The relative peak areas of 5wt and 3wt were decreased by approximately half in target ES clones (4G02) but not in non-target ES clones (12F12). (C) Representative MLPA data for three middle amplicons, wtM1, wtM2, and wtM3, which were reduced by approximately half in target ES clones (4G02) as well, is shown. MLPA reactions were repeated three times for all target ES clones. The selectable markers and vector sequences were removed from two ES clones, 1C04 and 4G02, using R4 integrase. A plasmid expressing the integrase under the control of the chicken actin-CMV hybrid promoter was transfected into the two ES cell lines. The action of the R4 integrase resulted in excision of the sequences located between the R4 attB and attP sites, as illustrated in Figure S2. The recombination between attB and attP sites gives rise to a chromosomal R4 attL site (sequences shown in Figure S2) and a circularized attR remnant that has no mechanism for persistence during cell division. The deletion events were initially identified by PCR using primers P13 and P14. Thirty of 43 clones for 1C04 and 29 of 37 clones for 4G02 showed the expected 561 bp PCR fragment (data not shown). Successful R4 integrase-mediated recombination was confirmed by sequencing the PCR products to detect the presence of the R4 attL site and by MLPA to confirm loss of the geneticin resistance allele (data not shown).
Generation of Cyp2j-null mice using mouse Cyp2j targeted ES cells
Four clones of mouse Cyp2j target ES cells were microinjected into C57BL/6 blastocysts. Four chimeric mice were born from 4 clones of which one, from B6-white ES cells, showed germ line transmission: among 20 litters, 20 pups from 148 offspring (13%) were derived from ES cells. Heterozygous mice were mated to generate homozygous mutant mice (Cyp2j−/−) and wild-type littermates (Cyp2j+/+). Genotyping by MLPA showed the absence of all internal regions located in the deletion region of the homozygotes (Figure S3A). Mouse genotypes were also tested by PCR, as shown in Figure S3B.
Generation of human CYP2J2 (hCYP2J2) transgenic mice using a modified BAC
A BAC clone was selected to provide the human CYP2J2 gene. A schematic diagram shows the procedure used to modify the BAC (Figure 3A). The E. coli recombination events were identified by four PCR amplifications using primers P15 to P22 shown in Figure 3A (Figure S4A). The PCR products were sequenced to confirm the anticipated recombination events. The correct recombinants were digested using EcoRI, NcoI, and HindIII, separately, to confirm the identity of the sequence (Figure S4B). The recombinant BAC DNA was used to produce hCYP2J2 transgenic mice (C57BL/6 background). Four founders were identified from 35 offspring by DNA blotting and PCR (Figure 3B). Three founders supported germ line transmission. These mice were backcrossed with Cyp2j−/− mice for more than 6 generations to derive Cyp2j−/− mice on the B6-white background carrying a transgene specifying human CYP2J2 (Cyp2j−/−-Tg).
Fig. 3. Creation of human CYP2J2 transgenic mice. 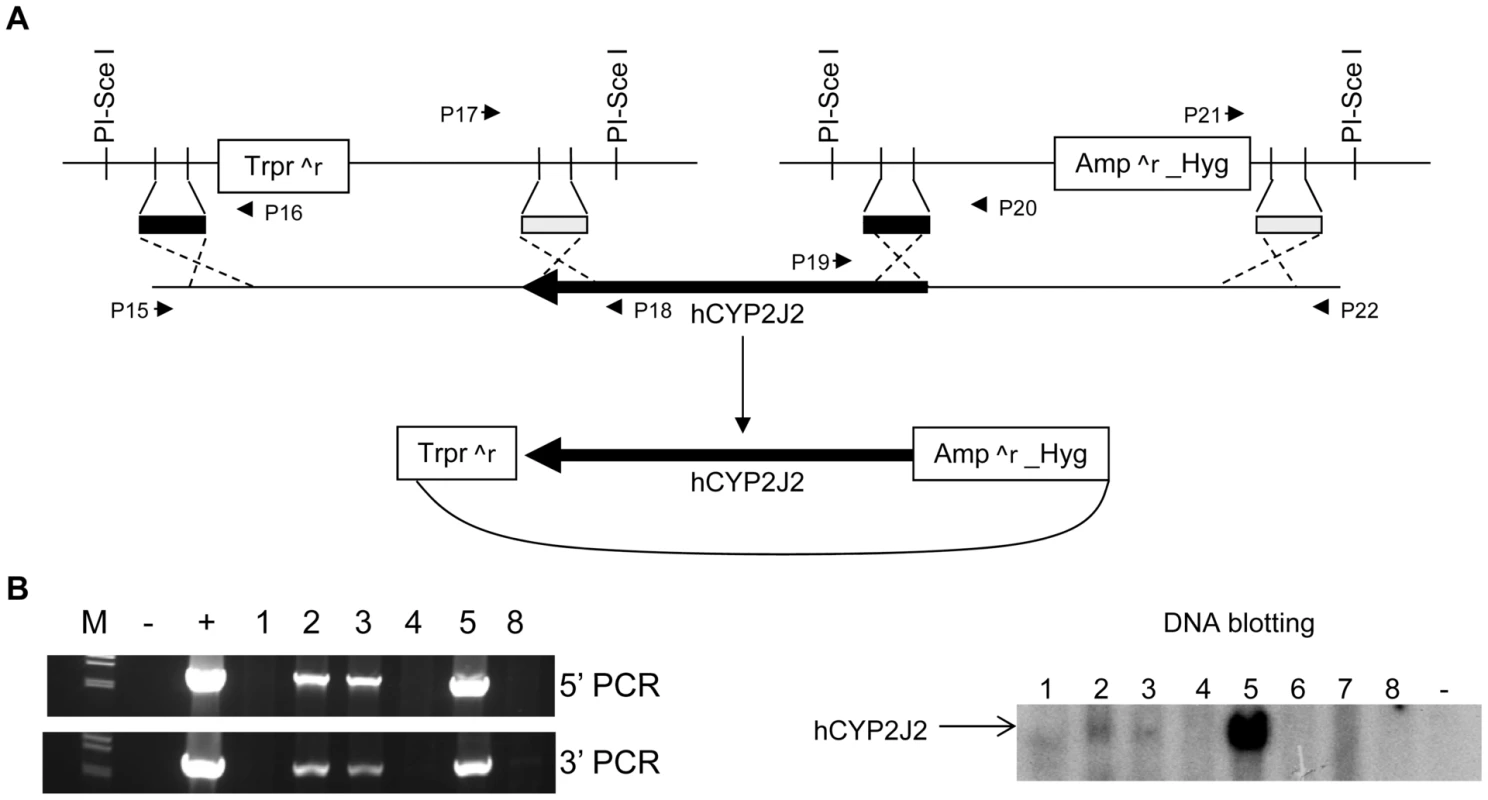
(A) Schematic diagram showing generation of the recombinant hCYP2J2 BAC. Two targeting vectors were constructed to remove the sequences flanking hCYP2J2 by homologous recombination in E. coli. Primers P15 to 22 were used to identify the recombinants in E. coli. PCR data are shown in Figure S5. Trpr∧r, trimethoprim resistance; Amp∧r, Ampicillin resistance; Hyg, Hygromycin. (B). Transgenic mice were identified by PCR and confirmed by DNA blotting. M, λ DNA marker/Hind III; −, negative PCR control; +, positive PCR control to amplify BAC; 1 to 5, 8, founder mice. Cyp2j gene expression in Cyp2j−/− and Cyp2j−/−-Tg mice
Human CYP2J2 and the eight mouse Cyp2js share 66–83% similarity in protein sequence and 55–88% sequence identity for mRNA sequence (Figure S5). RT-MLPA [20], a technology which allows detection and quantitation of nucleic acids having single nucleotide differences, as well as measurement of the expression of multiple genes in a single tube, was used to examine the expression of the eight Cyp2j genes in wild-type mice. Expression of 3 internal control genes, Tbp, Hprt, and Gapdh, was used to normalize the data. Each Cyp2j gene has a distinct expression pattern, as shown in Figure 4A. Kidney, liver, and gastrointestinal tissues are the major sites of Cyp2j isoform gene expression. Expression of six Cyp2j genes (2j5, 2j6, 2j8, 2j11, and 2j13) is detectable in liver and kidney. Cyp2j7 is expressed at low levels in the liver. Cyp2j13 is highly expressed in the kidney. Cyp2j9 shows expression in small intestine, liver, and brain. Cyp2j6 is broadly expressed: small intestine > stomach > thyroid > liver > large intestine > kidney > brain. Only low levels of expression of 2j5, 2j6, 2j9, 2j11, and 2j13 were detected in lung. RT-MLPA was also applied to RNA prepared from liver and kidney of Cyp2j−/− mice. No transcripts from Cyp2j genes were detected (Figure 4B).
Fig. 4. Representative data for quantitation of Cyp2j gene expression. 
(A) Mouse Cyp2j gene expression in different tissues was measured using RT-MLPA. (B) Mouse Cyp2j gene expression in liver and kidney of wild-type (WT) and null (KO) mice measured using RT-MLPA is shown. (C) CYP2J2 gene expression in human tissues. (D) Human CYP2J2 mRNA levels in lung and heart of Cyp2j+/+, Cyp2j−/− , Cyp2j+/+ -Tg and Cyp2j−/−-Tg mice quantified by RT-PCR are shown. The measurements were performed three times using pooled mouse RNA from three individual mice. To evaluate potential species differences in lineage-dependent expression, CYP2J2 gene expression was examined by quantitative reverse-transcriptase PCR (RT-PCR) using commercial pooled human cDNA preparations. The human gene is highly expressed in liver, and the abundance of transcripts in heart exceeds that in lung (Figure 4C). In contrast, in RNA prepared from Cyp2j−/−-Tg mice, CYP2J2 mRNA levels were substantially higher in lung than in heart in Cyp2j−/−-Tg mice but not in Cyp2j+/+-Tg mice (Figure 4D), an observation that was verified in mice derived from two independent founders (data not shown).
Effects of Cyp2j deletion on hemodynamic parameters
To investigate whether Cyp2j deficiency affects systemic hemodynamic measurements, the blood pressure (BP) and heart rate (HR) were measured in conscious male and female Cyp2j+/+ and Cyp2j−/− mice by tail cuff plethysmography. Systemic blood pressure and HR did not differ between genotypes (Table 1). Invasive hemodynamic measurements in anesthetized Cyp2j+/+ and Cyp2j−/− mice of both sexes also did not reveal differences in HR, BP, cardiac output, systemic vascular resistance, or left ventricular systolic or diastolic function (Table 2).
Tab. 1. Comparison of systemic hemodynamic measurements in conscious Cyp2j+/+ and Cyp2j−/− mice. 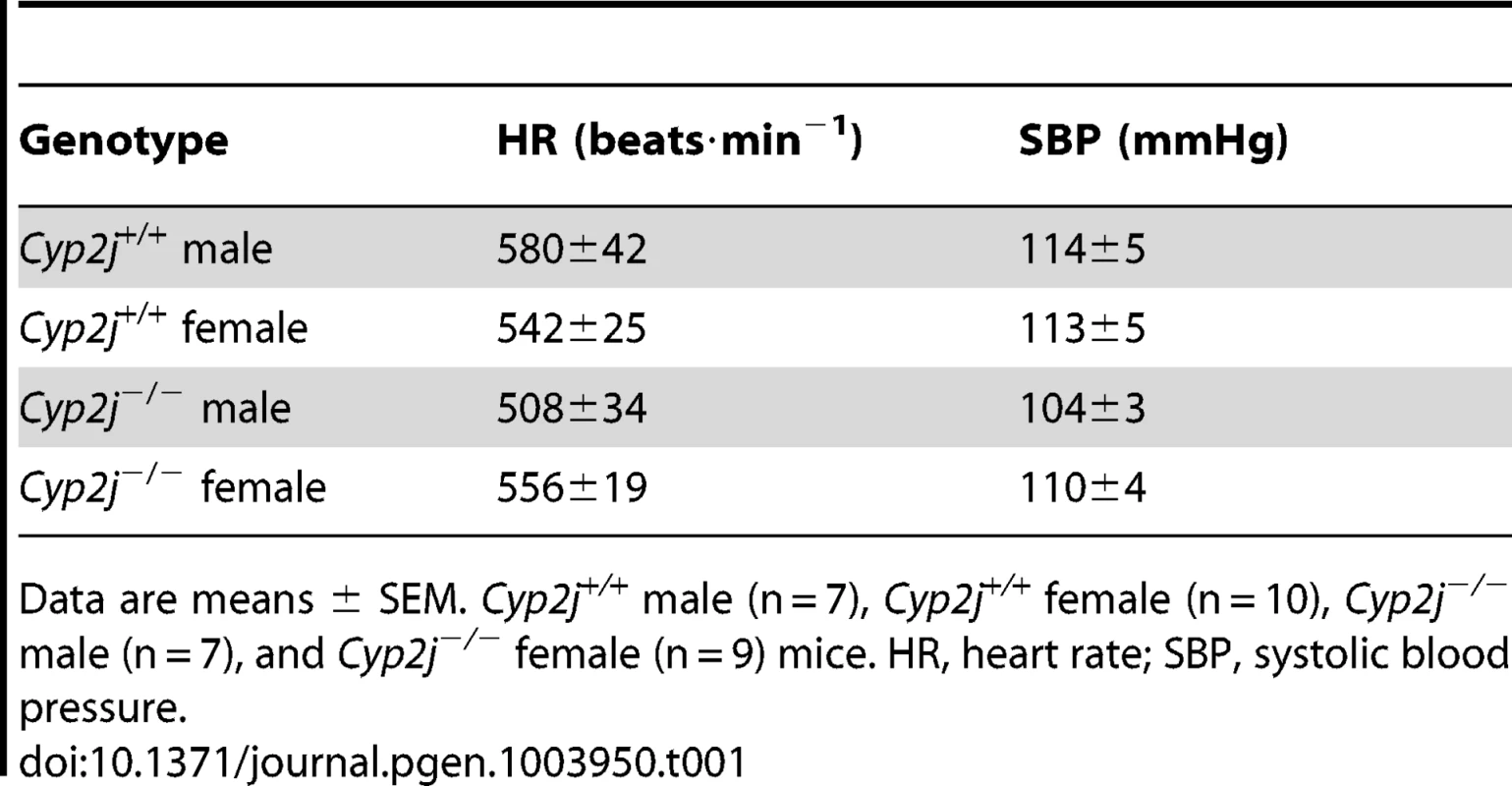
Data are means ± SEM. Cyp2j+/+ male (n = 7), Cyp2j+/+ female (n = 10), Cyp2j−/− male (n = 7), and Cyp2j−/− female (n = 9) mice. HR, heart rate; SBP, systolic blood pressure. Tab. 2. Comparison of systemic hemodynamic measurements in anesthetized Cyp2j+/+ and Cyp2j−/− mice. 
Data are means ± SEM. HR, heart rate; LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; CVP, central venous pressure; SVR, systemic vascular resistance; EF, ejection fraction; CO, cardiac output; SV, stroke volume; dP/dtmax, maximum rate of developed left ventricular pressure; dP/dtmin, minimum rate of developed left ventricular pressure; τ, time constant of isovolumic relaxation; SW, stroke work; Ea, arterial elastance; (n = 6 per group). Contribution of Cyp2j to hypoxic pulmonary vasoconstriction
To assess the contribution of Cyp2j to HPV, we measured changes in the left pulmonary vascular resistance (LPVR) in response to left mainstem bronchial occlusion (LMBO) in Cyp2j+/+ and Cyp2j−/− mice. We used dynamic measurements of pulmonary pressure and flow in the left pulmonary artery during transient inferior vena cava occlusion to estimate the LPVR [16]. Before LMBO, LPVR was similar in Cyp2j+/+ (80±5 mmHg⋅ml⋅min⋅g−1) and Cyp2j−/− mice (88±6 mmHg⋅ml⋅min⋅g−1). In Cyp2j+/+ mice, LMBO decreased the left pulmonary arterial blood flow (QLPA) without changing the pulmonary arterial pressure (PAP), doubling the LPVR (Figure 5A, Table S3). In contrast, LMBO did not change LPVR in Cyp2j−/− mice (Figure 5A, Table S3), consistent with the absence of HPV. To estimate the impact of impaired HPV on systemic arterial oxygenation, we measured arterial blood gas tensions 5 minutes after LMBO, while the right lung was ventilated at an inspired oxygen fraction (FIO2) of 1. Arterial oxygen partial pressure (PaO2) was higher in Cyp2j+/+ than in Cyp2j−/− mice (247±36 vs. 153±9 mmHg, respectively; P<0.05; Table S3). However, there was no difference in blood pHa, the arterial partial pressure of carbon dioxide, or the concentration of HCO3− (data not shown). Systemic oxygenation during LMBO was further assessed using an intra-arterial PaO2 probe in a subset of Cyp2j+/+ and Cyp2j−/− mice. No difference in PaO2 before LMBO was detected between Cyp2j+/+ and Cyp2j−/− mice (Figure 5B). After LMBO, PaO2 decreased in both genotypes to its new steady state within 2 min; however, Cyp2j−/− mice had a lower PaO2 than did Cyp2j+/+ mice during LMBO (Figure 5B). These observations confirm the presence of increased intrapulmonary shunting during LMBO in Cyp2j−/− mice and are consistent with absent HPV.
Fig. 5. (A) Percent increase in left lung pulmonary vascular resistance (LPVR) in response to left mainstem bronchial occlusion (LMBO) in Cyp2j+/+ and Cyp2j−/− mice (n = 10 per group). 
(B) Continuous tracings of arterial oxygen partial pressure (PaO2) measurements during LMBO in Cyp2j+/+ (n = 4) and Cyp2j−/− (n = 3) mice; (C) Percent increase in LPVR in response to LMBO in Cyp2j+/+ (n = 6) and Cyp2j−/−-Tg (n = 5) mice; (D) Percent increase in LPVR in response to LMBO in MS-PPOH or vehicle-treated Cyp2j+/+ mice (n = 5 per group); (E) Percent increase in LPVR in response to LMBO in L-NAME-treated Cyp2j+/+ (n = 5) and Cyp2j−/− (n = 6) and untreated Cyp2j+/+ and Cyp2j−/− mice (n = 10 per group); Data are means ± SEM. *P<0.05, **P<0.005. Human CYP2J2 restores HPV in Cyp2j−/− mice
Since CYP2J2 is the only human gene homologous or paralogous to multiple murine Cyp2j genes, we investigated whether or not complementation with CYP2J2 could restore HPV in Cyp2j−/− mice. At baseline, hemodynamic parameters did not differ between Cyp2j+/+ and Cyp2j−/−-Tg mice (Table S3). LMBO increased LPVR in Cyp2j+/+ and Cyp2j−/−-Tg mice to a similar extent (Figure 5C), indicating that HPV is preserved in Cyp2j−/−-Tg mice. Furthermore, during LMBO, arterial oxygen partial pressure (PaO2) did not differ between Cyp2j−/−-Tg and Cyp2j+/+ mice (Table S3), consistent with preserved HPV. These results suggest that presence of the single human CYP2J isoform in mice, in which the entire Cyp2j locus is deleted, is sufficient to permit pulmonary vasoconstriction.
Inhibition of cytochrome-P450 epoxygenase activity attenuates HPV
To exclude the possibility that the lifelong Cyp2j deficiency might lead to unanticipated compensatory mechanisms that could impair HPV in mice, we assessed HPV in Cyp2j+/+ mice treated with the epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH). The LMBO-induced increase in LPVR was markedly attenuated in a dose-dependent manner when mice were studied 90 minutes after treatment with MS-PPOH (Figure 5D). The PaO2 during LMBO was lower in MS-PPOH-treated than in vehicle-treated mice. These results further confirm that cytochrome P450 epoxygenase enzymatic activity contributes to HPV in mice.
L-NAME restores HPV in Cyp2j−/− mice
To examine the possibility that HPV is impaired in Cyp2j−/− mice due to an alteration in the balance of vasoconstrictors and vasodilators, we studied the effects of enhancing pulmonary vascular tone by inhibiting nitric oxide (NO) production on HPV in Cyp2j−/− mice. At 30 minutes after administration of NG-nitro-L-arginine methylester (L-NAME, an inhibitor of NO synthases), before LMBO, hemodynamic parameters did not differ between Cyp2j+/+and Cyp2j−/− mice (Table S3). During LMBO, inhibition of NO synthesis with L-NAME augmented the increase in LPVR in Cyp2j+/+ mice and restored the ability of LMBO to increase LPVR in Cyp2j−/− mice (Figure 5E, Table S3). These findings demonstrate that the Cyp2j−/− mice retain the mechanisms necessary for the pulmonary vascular response to hypoxia and that HPV can be restored in these mice by enhancing vasoconstriction.
EET levels in bronchial alveolar lavage fluid and oxygenase activity in pulmonary microsomes
The plasma and urine EET levels did not differ between Cyp2j+/+and Cyp2j−/− mice (data not shown). Levels of 11, 12 - and 14, 15-EET, as reflected by the difference of 11, 12 - and 14, 15-DHET levels before and after hydrolysis of EETs (measured by ELISA) were evaluated in bronchoalveolar lavage fluid (BALF) from Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice. BALF EET levels did not differ among the genotypes (Figure 6A, B). Moreover, the generation of EETs and DHETs by pulmonary microsomes from Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice did not differ (Figure 6C).
Fig. 6. 11, 12- and 14, 15-EETs measurements in BALF (A, B) and the generation of EETs and DHETs by pulmonary microsomes (C) of Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice. 
The black bar represents the DHET quantity before EET hydrolysis, and the grey bar represents the DHET quantity after EET hydrolysis in the samples. Triplicate measurements were performed for each mouse. Data are means ± SEM. n = 3 for each group in A, B; n = 4 for each group in C. Cyp2c44, 2c38, and 2c29 gene expression in lung and heart of Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice
In addition to members of the Cyp2j subfamily, members of the Cyp2c family of cytochrome P450 enzymes, including Cyp 2c44, 2c38, and 2c29, are able to metabolize AA to EETs in endothelial cells [21], [22]. Expression of these three Cyp2c family members in lung and heart of Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice was measured using quantitative RT-PCR. Pulmonary expression of the Cyp2c genes did not differ among genotypes, but deletion of the Cyp2j locus led to increased expression of the three Cyp2c genes in the heart (Figure 7).
Fig. 7. Gene expression by quantitative RT-PCR. 
(A, B, C) Cyp2c44, Cyp2c38, and Cyp2c29 mRNA levels in lung and heart of Cyp2j+/+, Cyp2j−/− and Cyp2j−/− -Tg mice. Experiments were run in triplicate. Mouse tissue RNAs were pooled from three individual mice. Discussion
Allelic expansion in the rodent genome is a commonly encountered phenomenon that has the potential to reduce the utility of rodent models for understanding human gene function. At a minimum, the presence of multiple functional murine paralogs confounds the extrapolation to the human context of the results of single gene ablations in mice. An alternative to single gene analysis is the inactivation of an entire locus syntenic to the human gene of interest. For the most part, the genetic tools to undertake such inactivations have been relatively underdeveloped. In this report, we demonstrate the feasibility of joining multiple bacterial artificial chromosomes using site-specific recombination to form a deletion replica that can be used to induce genomic rearrangement in mice. Previous approaches for the deletion of large DNA fragments required two targeting vectors harboring loxP sites [9]–[11]. These approaches require sequential gene targeting. The fused BAC targeting approach represents a powerful and efficient method for developing genetically-modified mice for the purpose of characterizing the function of gene clusters or studying genetic diseases associated with large chromosomal DNA deletions. Using this technology, we generated Cyp2j-null mice in which the 626-kb Cyp2j locus is deleted, as well as mice carrying a transgene specifying the human CYP2J2 allele in context of a Cyp2j-null allele.
It has previously been shown that overexpression of human CYP2J2 has cardiovascular protective effects in mice [13], [14], [23]. CYP2J synthesizes EETs in vascular endothelial cells [12], [13]. Epoxygenase-derived EETs hyperpolarize vascular smooth muscle cells in kidney, brain, and heart, resulting in vasorelaxation [24]–[27]. Previously Athiracul et al. reported that female mice deficient in the Cyp2j5 gene on a 129/SvEv background exhibit increased systemic blood pressure [28]. We therefore expected that mice lacking the entire Cyp2j gene family would show systemic vascular effects. However, deletion of the Cyp2j locus did not affect baseline systemic hemodynamic parameters or left ventricular contractile function in either sex. The variance with previous observations might be attributable to differences in strain background or to the actions of other Cyp2j enzymes in Cyp2j5−/− mice which are not present in the Cyp2j−/− mice. Effects of Cyp2j5 deletion on pulmonary vascular function have not been reported for Cyp2j5−/− mice.
In the pulmonary circulation, EETs enhance vasoconstrictor tone [3], [19], [29], [30], likely via activation of TRPC6 channels in vascular smooth muscle cells [30]. Epoxygenase-derived EETs are reported to contribute to the pulmonary vascular response to hypoxia [30]. Moreover, 11, 12 - and 14, 15-EET levels were recently reported to be increased in isolated-perfused murine lungs following exposure to hypoxia (FIO2 0.01) for 10 minutes [31]. Previous studies of the roles of epoxygenases in the regulation of HPV have relied on chemically-synthesized EETs, pharmacological activators or inhibitors of cytochrome P450 enzymes, or lineage-restricted overexpression of CYPs [3], [30], which cannot distinguish between the contributions of CYP2J and CYP2C. We did not detect an effect of deleting the Cyp2j locus on pulmonary vascular tone at baseline, but the pulmonary vasoconstrictor response to hypoxia was absent in Cyp2j−/− mice. There are several possible mechanisms by which the Cyp2j subfamily might regulate HPV. It is probable that Cyp2j−/− mice have reduced ability to generate pulmonary vasoconstricting EETs. Alternatively, it is possible that the deletion of Cyp2j subfamily shunts arachidonic acid into other pathways leading to the increased synthesis of cyclooxygenase, lipoxygenase, and ω-hydroxylase products. Some of these products, such as prostacyclin or 20-HETE are known to be pulmonary vasodilators [32], [33] and could impair HPV.
We were unable to detect differences in EET levels in the plasma, urine, and BALF of wild-type and Cyp2j−/− mice. Moreover, we did not observe differences in the generation of EETs and DHETs by microsomes extracted from the lungs of wild-type, Cyp2j−/−, or Cyp2j−/−-Tg mice. Previous studies have shown that multiple cytochrome P450 enzymes, including CYP1A, CYP2B, CYP2C, CYP2D, CYP2G, CYP2J, CYP2N, and CYP4A subfamilies, are capable of EET biosynthesis in vitro [34]. It is conceivable that EET production by CYP isoforms other than Cyp2j could obscure the impact of Cyp2j deficiency on pulmonary and systemic EET generation. In lung tissues, immunohistochemistry studies detected CYP2C proteins exclusively in the serous cells of bronchial glands, whereas CYP2J proteins were detected in a variety of cell types including pulmonary vascular smooth muscle and endothelial cells [1], [35]. We observed that Cyp2c genes were expressed in the lungs of wild-type and Cyp2j−/− mice with or without the human CYP2J2 transgene.
In addition to actions mediated by their enzymatic activity, Cyp2j isoforms could function in signaling circuits via other mechanisms, for example, serving as scaffold proteins or mediators in signal transduction complexes that regulate HPV via mechanisms not dependent on epoxygenases or hydroxylases. The proposal that the catalytic activity of Cyp2j family members regulates HPV is supported by the finding of the present study that administration of an epoxygenase inhibitor, MS-PPOH, to wild-type mice impaired HPV in a dose-dependent manner.
Transgenic introduction of human CYP2J2 into Cyp2j-deficient mice did not affect baseline hemodynamic parameters but restored the pulmonary vasoconstrictor response to LMBO. These results suggest that the human CYP2J2 functions in a manner similar to one or more of the murine Cyp2j isoforms in the regulation of pulmonary vascular tone by hypoxia. It is of note that the expression of human CYP2J2 transgene was greater in the lungs of Cyp2j−/− mice than in wild-type mice, suggesting the existence of a feedback loop designed to maintain expression of Cyp2j.
Administration of the NO synthase inhibitor, L-NAME, restored HPV in Cyp2j-null mice, indicating that Cyp2j-deficient mice retain the ability to constrict their pulmonary vasculature in response to alveolar hypoxia. This result is in agreement with observations of Ichinose et al., who reported that L-NAME restores HPV in cPla2-deficient mice [16]. These observations suggest that HPV is highly sensitive to the balance of vasoconstrictors and vasodilators in the lung. Enhancing vasoconstrictor tone or reducing vasodilation restores HPV in a variety of settings [36]. Taken together, these findings suggest that, although EET biosynthesis potentially increases in response to hypoxia [31] can enhance HPV, cPLA2/CYP2J signaling is not required for the pulmonary vasculature to sense and respond to regional hypoxia.
After genes duplicate, they often diverge in ways that can lead to new functions that were not exhibited by the parental gene. Mice have evolved eight Cyp2j genes and two pseudogenes. The results of this study have shown that the expression profile for each Cyp2j gene is distinct. CYP genes may also become specialized with respect to substrate specificity or product distribution. Cyp2j5 shares the highest nucleic acid sequence similarity with the human CYP2J2 gene, whereas Cyp2j6 and Cyp2j9 have the highest similarity with the sequence of the human protein (Fig. S5). It is conceivable that one or more of the mouse Cyp2j isoforms may have functions that differ from the single human CYP2J2 enzyme. However, our observations that both human and mouse CYP2J enzymes contribute to HPV suggest that the function of human CYP2J2 and one or more mouse Cyp2j isoforms has been conserved as the two genomes diverged during evolution.
In conclusion, ablation of a large gene family through fused BAC-mediated homologous recombination in ES cells has generated mice in which the 626-kb murine Cyp2j gene cluster was deleted. The single human ortholog/paralog CYP2J2 was introduced transgenically to complement the deleted mouse locus. Surprisingly, genetically modulating Cyp2j activity did not affect baseline vascular function. However deletion of the Cyp2j gene locus resulted in a compromise of the pulmonary vasoconstrictor response to hypoxia.
Materials and Methods
BAC clones
Mouse BAC clones RP23-24J24 and RP23-70M4 and the human BAC clone RP11-163O24 were obtained from the Children's Hospital Oakland Research Institute.
Sequences of oligonucleotides for PCR and probes for multiplex ligation-dependent probe amplification
Primers P1 through P22, mouse genotyping primers, and RT-PCR primer sequences are shown in Table S1. The MLPA probe sequences are shown in Table S2.
Integrase
A codon-optimized TP901 integrase was designed by Dr. Changhong Pang and placed in an E. coli expression vector (pacycTP901_ermb) under the control of the araBAD promoter. A codon-optimized R4 integrase was inserted in the mammalian expression vector pEAK15 under the control of the chicken actin-CMV hybrid promoter.
Animals
All the animal studies conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital.
Reagents
The non-selective nitric oxide synthase (NOS) inhibitor, NG-nitro-L-arginine methylester (L-NAME); kanamycin; and ampicillin were purchased from Sigma-Aldrich, St. Louis, MO. The selective epoxygenase inhibitor, N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH), was purchased from Cayman Chemical, Ann Arbor, MI. Ganciclovir and blasticidin were purchased from Novagen. Hygromycin and geneticin were obtained from Invitrogen.
Modification of BACs flanking the Cyp2j locus or containing human CYP2J2
The mouse Cyp2j and human CYP2J2 gene structures are shown in Figure 1A. Target vectors were endowed with four restriction enzyme sites to allow insertion of the recombination homology arms (Figure 1B). Arms, 200–2000 bp in length, were amplified from BAC DNA and subcloned into the desired target vector. The target vector was cut with PI-SceI, and a fragment containing the homology arms and the selection cassette was recovered by gel purification and electroporated into competent cells containing the target BAC clone and a recombinase expression vector (pacycredabsce_ermb) bearing the bacteriophage λ redα and β genes under the control of the araBAD promoter [6]. Electrocompetent cells were prepared by growing the BAC strain bearing the recombinase expression vector in LB medium containing 0.1% arabinose. Candidate recombinant clones were identified by growth on selective medium (kanamycin or ampicillin and chloramphenicol) and screened by PCR using primers flanking the arms (P1–8) (Figure 1B). The authenticity of candidate clones was confirmed by sequencing of the resulting PCR products. DNA from a verified clone was electroporated into DH10B competent cells and individual colonies were re-streaked on different selection plates to confirm the removal of the recombinase plasmid. The resultant recombinant BACs MT5′BAC and MT3′BAC were digested with restriction enzymes chosen to distinguish the recombinant from the original BAC sequences (data not shown).
A similar process was followed to trim the sequences flanking human CYP2J2 gene from a human BAC (Figure 3A). Homologous arms were amplified from the wild-type BAC and subcloned into two target vectors containing two different selectable markers, conferring trimethoprim and ampicillin resistance.
Integrase-mediated fusion of two modified BACs
A plasmid expressing TP901 integrase under araBAD promoter control was introduced into bacteria harboring the recombinant BAC bearing the TP901 attB site. Electrocompetent cells were prepared from cells propagated in 0.1% arabinose. The recombinant BAC bearing the TP901 attP site and expressing ampicillin resistance was electroporated into cells that were then spread on plates containing kanamycin, ampicillin, and chloramphenicol. The fused BAC clones were screened by PCR, and PCR products were sequenced to confirm the formation of TP901 attL and attR sites. DNA was prepared from a correctly fused clone and electroporated into DH10B competent cells to remove the TP901 expression plasmid. DNA from the fused BAC was digested with diagnostic restriction enzymes to confirm the structural integrity of the fused BAC.
Mouse ES cell culturing and targeting
ES cell lines were cultured with irradiated fibroblast feeder cells in Knock-Out Dulbecco's Modified Eagle's Medium (KO-DMEM) supplemented with 15% Fetal Bovine Serum and 1000 units Leukemia Inhibitory Factor (LIF) per mL. BAC DNA (5–20 µg) was digested with NotI or PI-SceI in 30–50 µL volume overnight and then electroporated into 107 ES cells at 0.25 kV, 960 µF with a Bio-Rad Gene Pulser. Transformants were selected for 8–10 days with 250 µg/mL geneticin (Invitrogen).
Removal of the vector and selectable sequence by R4 integrase action in ES cells
A transfection mixture containing a plasmid capable of expressing R4 integrase and Lipofectamine 2000 (Invitrogen) was prepared according to the manufacturer's instructions and incubated for 20–30 min at room temperature. Target ES cells at 80–90% confluence were trypsinized using 0.1% trypsin in 10 mM EDTA and resuspended in fresh ES cell culture medium with low (2%) serum at 3×105 cells per mL. The ES cell suspension (10 mL) was mixed with the transfection mixture and replated on a 10 cm feeder plate. After 24 h, the negative selective agent, ganciclovir (2.5 µM), was added. Transformant colonies were visible after 8–10 days of culture.
ES clone screening by MLPA
MLPA was performed as described previously [37]. Fragment analysis was carried out on an ABI 3730XL DNA analyzer.
Blastocyst injection and testing for germ line transmission
ES cells were injected into C57/BL6 mouse blastocysts to generate chimeric mice. Chimeras from ES cell clones derived from the B6-white ES cell line were mated with wild-type B6-white mice (B6(Cg)-Tyrc-2J/J, Jackson Laboratory, Bar Harbor, Maine, USA) to test germ line transmission identifiable by coat color difference. Heterozygous mice (Cyp2j+/−) were identified by MLPA. Cyp2j−/− and littermate-matched wild-type (Cyp2j+/+) mice were obtained by mating of pairs of Cyp2j+/− mice.
Generation of human CYP2J2 transgenic mice
The recombinant human CYP2J2 BAC DNA was linearized by PI-SceI digestion. The purified DNA was microinjected into pronuclear zygotes from (C57BL/6×DBA) F1 mice and embryos were transplanted into recipients for the generation of transgenic mice. The resultant transgenic mice (Cyp2j−/−-Tg) were backcrossed with B6-white Cyp2j−/− mice for more than 6 generations prior to molecular and physiological characterization.
RT-MLPA
The RT-MLPA probes used in this study are shown in Table S2. The RT-MLPA procedure was performed as described previously [20].
RNA preparation and real-time quantitative PCR
Total RNA was extracted and purified using an RNeasy kit (Qiagen) from mouse tissues (6–8 week-old). The primers used are detailed in Table S1. Reverse transcription and real-time quantitative PCR (qPCR) reactions were prepared with SuperScript II Reverse Transcriptase (Invitrogen) and iQ SYBR Green Supermix (Bio-Rad) and run in triplicate on a Bio-Rad iQ5. The cDNA panels of human adult tissue were obtained from Clontech.
Hemodynamic studies
We studied mice of both sexes with an age range of 2–5 months, weighing 20–30 g. Animals in each experimental group were matched for body weight.
Hemodynamic measurements in conscious Cyp2j+/+ and Cyp2j−/− mice
Systolic blood pressure (SBP) and heart rate (HR) were measured using a non-invasive blood pressure system (XBP 1000, Kent Scientific, Torrington, Conn) in awake Cyp2j+/+ and Cyp2j−/− mice, as described previously [38].
Hemodynamic measurements in anesthetized Cyp2j+/+ and Cyp2j−/− mice
Invasive hemodynamic measurements were performed in anesthetized Cyp2j+/+ and Cyp2j−/− mice, as described previously [39]. Briefly, mice were anesthetized by intraperitoneal injection of ketamine (120 mg·kg−1), fentanyl (0.05 mg·kg−1), and pancuronium (2 mg·kg−1). After intubation, animals were mechanically ventilated inspired oxygen fraction (FIO2) 1.0, tidal volume 10 µL·g−1, respiratory rate 120 breaths·min−1, and a fluid-filled catheter was inserted into the left carotid artery for infusion of saline (0.5 µL·g−1·min−1). A second fluid-filled catheter was inserted into the right jugular vein for measurement of central venous pressure (CVP). A thoracotomy was performed, and a pressure-volume conductance catheter (Size 1F, Model PVR-1030, Millar Instruments, Inc., Houston, TX) was inserted via the apex into the left ventricle. Systemic vascular resistance (SVR) was calculated based on mean arterial pressure (MAP), CVP, and cardiac output (CO) using following formula: SVR = ([MAP-CVP]·CO−1). The following parameters were derived from left ventricular pressure-volume curves: LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; EF, ejection fraction; SV, stroke volume; dP/dtmax, maximum rate of developed left ventricular pressure; dP/dtmin, minimum rate of developed left ventricular pressure; τ time constant of isovolumic relaxation; SW, stroke work; Ea, arterial elastance.
Measurement of HPV and arterial blood gases in mice
To assess HPV, left lung pulmonary vascular resistance (LPVR) was estimated before and during left mainstem bronchial occlusion (LMBO) in Cyp2j+/+, Cyp2j−/−, and Cyp2j−/−-Tg mice (n = 10, 10, and 5, respectively), using methods described previously [16]. Briefly, mice were anesthetized, mechanically ventilated at FIO2 of 1.0, and then subjected to a thoracotomy. An arterial line was inserted into the right carotid artery, a custom-made catheter was placed into the main pulmonary artery, and a flow probe was positioned around the left pulmonary artery. MAP, pulmonary arterial pressure (PAP), and left pulmonary arterial blood flow (QLPA) were continuously measured and recorded before and during LMBO. To estimate the LPVR, the inferior vena cava was partially occluded to transiently reduce CO until QLPA was reduced by approximately 50%. LPVR was calculated from the slope of the PAP/QLPA relationship. The increase in LPVR induced by LMBO (ΔLPVR) was obtained by calculating the change in the mean value of the PAP/QLPA slopes in each mouse. Five minutes after LMBO, arterial blood was sampled from the right carotid artery. Blood gas tension analyses were measured by using an ABL800 FLEX analyzer (Radiometer America, Inc., Westlake, USA). To further assess the impact of Cyp2j deficiency on systemic oxygenation during LMBO in 4 Cyp2j+/+ and 3 Cyp2j−/− mice, a flexible polarographic Clark-type oxygen micro probe (0.5 mm OD; LICOX CC1.R, GMS, Kiel-Mielkendorf, Germany) was advanced into the aortic arch via the right carotid artery. Arterial oxygen partial pressure (PaO2) was measured in real time and recorded continuously. The PaO2 electrodes were calibrated before and after each experiment in air at ambient pressure according to the manufacturer's instructions.
Effects of cytochrome-P450 epoxygenase inhibition on HPV
Wild-type (C57BL/6-W (B6(Cg)-Tyrc-2J/J)) mice received MS-PPOH (30 or 60 mg·kg−1 dissolved in 50 µL dimethyl sulfoxide (DMSO)) or vehicle alone (equal volume of DMSO) (n = 5 per group) via tail vein 90 minutes before measurement of ΔLPVR. The dose and timing of administration were chosen based on data published previously [3].
Measurements of effects of NOS inhibition on HPV
ΔLPVR was measured 30 minutes after intravenous administration of L-NAME, dissolved in 50 µL vehicle (normal saline) at a dose of 100 mg·kg−1 in Cyp2j+/+ mice (n = 5) and Cyp2j−/− mice (n = 6). The dose was chosen based on results from a previous study [40].
Evaluation of 11, 12 - and 14,15-EET in BALF by ELISA
Collection of BALF was performed as previously described [41]. To assess in vivo 11,12 - and 14,15-EETs production, an enzyme-linked immunosorbent assay (ELISA) kit (Detroit R&D, Inc., Detroit, MI) was used to determine concentrations of the stable 11,12 - and 14,15-EETs metabolites 11,12 - and 14, 15-dihydroxyeicosatrienoic acid (11,12-DHET and 14, 15-DHET) in BALF of Cyp2j+/+, Cyp2j−/− and Cyp2j−/−-Tg mice (n = 3 per group). 11,12 and 14,15-DHET were quantified by ELISA according to the manufacturer's instructions and normalized by protein content in BALF samples. The difference of the quantity of DHET before and after EET hydrolysis represents EET quantity in the samples.
Preparation of pulmonary microsomal fractions and quantification of microsomal EET and DHET synthesis
Microsomal fractions were prepared from both lungs of Cyp2j+/+, Cyp2j−/−, and Cyp2j−/−-Tg mice (n = 4 per group) as described previously [42], [43]. To determine the eicosanoids generated, samples containing microsomal fractions were incubated with 1 µg of arachidonic acid and 1mM NADPH for 1 hour at 37C in a shaking water bath. Reactions were terminated by adding 2 volume of HPLC grade methanol and stored at −80C prior to analysis to each sample. The generated eicosanoid profiles were determined by LC-MS-MS, as previously described [44].
Statistical analysis
All data are expressed as means ± SEM. P values<0.05 were considered statistically significant. Statistical analyses were performed using Prism 5 software (GraphPad Software Inc., La Jolla, CA). For hemodynamic experiments, a two-way ANOVA with repeated measures was used to compare differences between groups. However, when the interaction P value between time and condition was significant, a one-way ANOVA with post hoc Bonferroni tests (two-tailed) for normally distributed data or a Kruskal-Wallis test (two-tailed) with a post hoc Dunn's test for data that was not normally distributed was used. Measurements within the same experimental group were compared by a paired t-test. If the normality test failed, Mann-Whitney rank sum test was applied.
Supporting Information
Zdroje
1. ZeldinDC, FoleyJ, MaJ, BoyleJE, PascualJM, et al. (1996) CYP2J subfamily P450s in the lung: expression, localization, and potential functional significance. Mol Pharmacol 50 : 1111–1117.
2. GrossGJ, FalckJR, GrossER, IsbellM, MooreJ, et al. (2005) Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 68 : 18–25.
3. PokreiszP, FlemingI, KissL, Barbosa-SicardE, FisslthalerB, et al. (2006) Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47 : 762–770.
4. VoelkelNF, MorganrothM, FeddersenOC (1985) Potential role of arachidonic acid metabolites in hypoxic pulmonary vasoconstriction. Chest 88 : 245S–248S.
5. CapecchiMR (2005) Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet 6 : 507–512.
6. YangY, SeedB (2003) Site-specific gene targeting in mouse embryonic stem cells with intact bacterial artificial chromosomes. Nat Biotechnol 21 : 447–451.
7. BarakatTS, RentmeesterE, SleutelsF, GrootegoedJA, GribnauJ (2011) Precise BAC targeting of genetically polymorphic mouse ES cells. Nucleic Acids Res 39: e121.
8. KlymiukN, MundhenkL, KraeheK, WuenschA, PlogS, et al. (2012) Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J Mol Med (Berl) 90 : 597–608.
9. LiZW, StarkG, GotzJ, RulickeT, GschwindM, et al. (1996) Generation of mice with a 200-kb amyloid precursor protein gene deletion by Cre recombinase-mediated site-specific recombination in embryonic stem cells. Proc Natl Acad Sci U S A 93 : 6158–6162.
10. Van DeursenJ, FornerodM, Van ReesB, GrosveldG (1995) Cre-mediated site-specific translocation between nonhomologous mouse chromosomes. Proc Natl Acad Sci U S A 92 : 7376–7380.
11. Ramirez-SolisR, LiuP, BradleyA (1995) Chromosome engineering in mice. Nature 378 : 720–724.
12. NodeK, HuoY, RuanX, YangB, SpieckerM, et al. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285 : 1276–1279.
13. SpieckerM, LiaoJK (2005) Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys 433 : 413–420.
14. YangB, GrahamL, DikalovS, MasonRP, FalckJR, et al. (2001) Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol 60 : 310–320.
15. GravesJP, EdinML, BradburyJA, GruzdevA, ChengJ, et al. (2013) Characterization of four new mouse cytochrome P450 enzymes of the CYP2J subfamily. Drug Metab Dispos 41 : 763–773.
16. IchinoseF, UllrichR, SapirsteinA, JonesRC, BonventreJV, et al. (2002) Cytosolic phospholipase A(2) in hypoxic pulmonary vasoconstriction. J Clin Invest 109 : 1493–1500.
17. FischerLG, HollmannMW, HorstmanDJ, RichGF (2000) Cyclooxygenase inhibitors attenuate bradykinin-induced vasoconstriction in septic isolated rat lungs. Anesth Analg 90 : 625–631.
18. IchinoseF, ZapolWM, SapirsteinA, UllrichR, TagerAM, et al. (2001) Attenuation of hypoxic pulmonary vasoconstriction by endotoxemia requires 5-lipoxygenase in mice. Circ Res 88 : 832–838.
19. ZhuD, BirksEK, DawsonCA, PatelM, FalckJR, et al. (2000) Hypoxic pulmonary vasoconstriction is modified by P-450 metabolites. Am J Physiol Heart Circ Physiol 279: H1526–1533.
20. ElderingE, SpekCA, AbersonHL, GrummelsA, DerksIA, et al. (2003) Expression profiling via novel multiplex assay allows rapid assessment of gene regulation in defined signalling pathways. Nucleic Acids Res 31: e153.
21. MichaelisUR, FisslthalerB, MedhoraM, HarderD, FlemingI, et al. (2003) Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J 17 : 770–772.
22. PozziA, Macias-PerezI, AbairT, WeiS, SuY, et al. (2005) Characterization of 5,6 - and 8,9-epoxyeicosatrienoic acids (5,6 - and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem 280 : 27138–27146.
23. SeubertJ, YangB, BradburyJA, GravesJ, DegraffLM, et al. (2004) Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res 95 : 506–514.
24. GebremedhinD, MaYH, FalckJR, RomanRJ, VanRollinsM, et al. (1992) Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol 263: H519–525.
25. ImigJD, SimpkinsAN, RenicM, HarderDR Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med 13: e7.
26. RomanRJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82 : 131–185.
27. SchwartzmanML, MartasekP, RiosAR, LevereRD, SolangiK, et al. (1990) Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney Int 37 : 94–99.
28. AthirakulK, BradburyJA, GravesJP, DeGraffLM, MaJ, et al. (2008) Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J 22 : 4096–4108.
29. LosapioJL, SpragueRS, LonigroAJ, StephensonAH (2005) 5,6-EET-induced contraction of intralobar pulmonary arteries depends on the activation of Rho-kinase. J Appl Physiol 99 : 1391–1396.
30. KeseruB, Barbosa-SicardE, PoppR, FisslthalerB, DietrichA, et al. (2008) Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J 22 : 4306–4315.
31. WangL, YinJ, NicklesHT, RankeH, TabuchiA, et al. (2012) Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 122 : 4218–4230.
32. BirksEK, BousamraM, PresbergK, MarshJA, EffrosRM, et al. (1997) Human pulmonary arteries dilate to 20-HETE, an endogenous eicosanoid of lung tissue. Am J Physiol 272: L823–829.
33. RubinLJ, GrovesBM, ReevesJT, FrosolonoM, HandelF, et al. (1982) Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation 66 : 334–338.
34. ZeldinDC (2001) Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276 : 36059–36062.
35. YokoseT, DoyM, TaniguchiT, ShimadaT, KakikiM, et al. (1999) Immunohistochemical study of cytochrome P450 2C and 3A in human non-neoplastic and neoplastic tissues. Virchows Arch 434 : 401–411.
36. CaironiP, IchinoseF, LiuR, JonesRC, BlochKD, et al. (2005) 5-Lipoxygenase deficiency prevents respiratory failure during ventilator-induced lung injury. Am J Respir Crit Care Med 172 : 334–343.
37. LangerakP, NygrenAO, SchoutenJP, JacobsH (2005) Rapid and quantitative detection of homologous and non-homologous recombination events using three oligonucleotide MLPA. Nucleic Acids Res 33: e188.
38. YuB, RaherMJ, VolpatoGP, BlochKD, IchinoseF, et al. (2008) Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117 : 1982–1990.
39. BuysES, CauwelsA, RaherMJ, PasseriJJ, HobaiI, et al. (2009) sGC(alpha)1(beta)1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol 297: H654–663.
40. SteudelW, IchinoseF, HuangPL, HurfordWE, JonesRC, et al. (1997) Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 81 : 34–41.
41. FrancisRC, VaporidiK, BlochKD, IchinoseF, ZapolWM (2011) Protective and Detrimental Effects of Sodium Sulfide and Hydrogen Sulfide in Murine Ventilator-induced Lung Injury. Anesthesiology 115 : 1012–1021.
42. ZeldinDC, PlitmanJD, KobayashiJ, MillerRF, SnapperJR, et al. (1995) The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest 95 : 2150–2160.
43. CapdevilaJH, FalckJR, DishmanE, KararaA (1990) Cytochrome P-450 arachidonate oxygenase. Methods Enzymol 187 : 385–394.
44. FoxT, GotlingerKH, DunnMW, LeeOL, MilmanT, et al. (2013) Dysregulated heme oxygenase-ferritin system in pterygium pathogenesis. Cornea 32 : 1276–1282.
Štítky
Genetika Reprodukční medicína
Článek Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis inČlánek Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 11- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Akutní intermitentní porfyrie
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Molecular Recognition by a Polymorphic Cell Surface Receptor Governs Cooperative Behaviors in Bacteria
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- Ribosome Synthesis and MAPK Activity Modulate Ionizing Radiation-Induced Germ Cell Apoptosis in
- Retrotransposon Silencing During Embryogenesis: Cuts in LINE
- Roles of XRCC2, RAD51B and RAD51D in RAD51-Independent SSA Recombination
- Parallel Evolution of Chordate Regulatory Code for Development
- A Genetic Approach to the Recruitment of PRC2 at the Locus
- Deletion of the Murine Cytochrome P450 Locus by Fused BAC-Mediated Recombination Identifies a Role for in the Pulmonary Vascular Response to Hypoxia
- Elevated Mutagenesis Does Not Explain the Increased Frequency of Antibiotic Resistant Mutants in Starved Aging Colonies
- Deletion of an X-Inactivation Boundary Disrupts Adjacent Gene Silencing
- Interplay between Active Chromatin Marks and RNA-Directed DNA Methylation in
- Recombinogenic Conditions Influence Partner Choice in Spontaneous Mitotic Recombination
- Crosstalk between NSL Histone Acetyltransferase and MLL/SET Complexes: NSL Complex Functions in Promoting Histone H3K4 Di-Methylation Activity by MLL/SET Complexes
- A New Role for the GARP Complex in MicroRNA-Mediated Gene Regulation
- RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Loss of DNMT1o Disrupts Imprinted X Chromosome Inactivation and Accentuates Placental Defects in Females
- Inhibition of the Smc5/6 Complex during Meiosis Perturbs Joint Molecule Formation and Resolution without Significantly Changing Crossover or Non-crossover Levels
- Disruption of Lipid Metabolism Genes Causes Tissue Overgrowth Associated with Altered Developmental Signaling
- Translation Initiation Factors eIF3 and HCR1 Control Translation Termination and Stop Codon Read-Through in Yeast Cells
- Recruitment of TREX to the Transcription Machinery by Its Direct Binding to the Phospho-CTD of RNA Polymerase II
- MYB97, MYB101 and MYB120 Function as Male Factors That Control Pollen Tube-Synergid Interaction in Fertilization
- Oct4 Is Required ∼E7.5 for Proliferation in the Primitive Streak
- Contrasted Patterns of Crossover and Non-crossover at Meiotic Recombination Hotspots
- Transposable Prophage Mu Is Organized as a Stable Chromosomal Domain of
- Ash1l Methylates Lys36 of Histone H3 Independently of Transcriptional Elongation to Counteract Polycomb Silencing
- Fine-Mapping the Genetic Association of the Major Histocompatibility Complex in Multiple Sclerosis: HLA and Non-HLA Effects
- Genomic Mechanisms Accounting for the Adaptation to Parasitism in Nematode-Trapping Fungi
- Decoding a Signature-Based Model of Transcription Cofactor Recruitment Dictated by Cardinal Cis-Regulatory Elements in Proximal Promoter Regions
- Removal of Misincorporated Ribonucleotides from Prokaryotic Genomes: An Unexpected Role for Nucleotide Excision Repair
- Fission Yeast Shelterin Regulates DNA Polymerases and Rad3 Kinase to Limit Telomere Extension
- Activin Signaling Targeted by Insulin/dFOXO Regulates Aging and Muscle Proteostasis in
- Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans
- Demographic Divergence History of Pied Flycatcher and Collared Flycatcher Inferred from Whole-Genome Re-sequencing Data
- Recurrent Tissue-Specific mtDNA Mutations Are Common in Humans
- The Histone Variant His2Av is Required for Adult Stem Cell Maintenance in the Testis
- The Maternal-to-Zygotic Transition Targets Actin to Promote Robustness during Morphogenesis
- Reconstructing the Population Genetic History of the Caribbean
- and Are Required for Growth under Iron-Limiting Conditions
- Whole Genome, Whole Population Sequencing Reveals That Loss of Signaling Networks Is the Major Adaptive Strategy in a Constant Environment
- Neuron-Specific Feeding RNAi in and Its Use in a Screen for Essential Genes Required for GABA Neuron Function
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
- Mouse BAZ1A (ACF1) Is Dispensable for Double-Strand Break Repair but Is Essential for Averting Improper Gene Expression during Spermatogenesis
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- DUX4 Binding to Retroelements Creates Promoters That Are Active in FSHD Muscle and Testis
- Pathways-Driven Sparse Regression Identifies Pathways and Genes Associated with High-Density Lipoprotein Cholesterol in Two Asian Cohorts
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Are Required for Growth under Iron-Limiting Conditions
- Genetic and Functional Studies Implicate Synaptic Overgrowth and Ring Gland cAMP/PKA Signaling Defects in the Neurofibromatosis-1 Growth Deficiency
- The Light Skin Allele of in South Asians and Europeans Shares Identity by Descent
- RNA∶DNA Hybrids Initiate Quasi-Palindrome-Associated Mutations in Highly Transcribed Yeast DNA
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



