-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Why Mitochondria are Excellent Targets for Cancer Therapy
Prečo sú mitochondrie vhodné ciele pre liečbu rakoviny
Nové trendy v liečbe rakoviny sa spájajú s rozvojom presne cielených terapeutík, s účinkom na rakovinové bunky a zameraním na špecifické biologické dráhy. Úloha onkoproteínov a tumor-supresorových proteínov v proliferačnej signalizácii, regulácii bunkového cyklu a pozmenenej adhézii je už dobre preskúmaná. Chemické látky, vírusy a žiarenie sú tiež všeobecne prijímanými faktormi, ktoré vyvolávajú mutácie v génoch kódujúcich proteíny súvisiace s tvorbou rakoviny. Nedávne experimenty ukázali, že existujú dva nové kľúčové faktory pôsobiace na proliferujúce bunky – hypoxia a nedostatok glukózy. Tieto môžu iniciovať a podporovať proces malígnej transformácie v malom množstve buniek, ktorým sa podarilo uniknúť bunkovému starnutiu. Neregulovaná bunková proliferácia vedie k tvorbe bunkovej masy presahujúcej svoje rezervy, čo znižuje množstvo kyslíka a živín. Vzniknutý stav hypoxie iniciuje ďalšie kľúčové úpravy, ktoré umožňujú prežitie nádorových buniek. Proces apoptózy je potlačený a metabolizmus glukózy pozmenený. Nedávne experimenty naznačili, že vyčerpanie zásob kyslíka stimuluje mitochondrie, aby spracovávali väčšie množstvá reaktívnych foriem kyslíka (ROS). Aktivujú sa tak signálne dráhy, ako je hypoxiu-indukujúci faktor 1, ktoré podporujú prežívanie nádorových buniek a rast nádorov. Mitochondrie sú čoraz častejšie považované za kľúčové organely podieľajúce sa na chemoterapii, a preto je dôležité nájsť spôsob ako aktivovať apoptózu v mitochondriách za podmienok hypoxie, určiť vzťah medzi mitochondriami, ROS signalizáciou a procesmi aktivujúcimi prežívanie buniek. Každé nové zistenie môže otvoriť cestu pre pochopenie a odhalenie podstaty rakoviny a následné vytvorenie na mieru šitej terapie.
Kľúčové slová:
mitochondria – bunková smrť – energetický metabolizmus – bunková transformácia
Authors: Z. Tatarkova; S. Kuka; M. Petras; P. Racay; J. Lehotský; D. Dobrota; P. Kaplán
Authors place of work: Department of Medical Biochemistry, Jessenius Faculty of Medicine, Comenius University, Martin, Slovak Republic
Published in the journal: Klin Onkol 2012; 25(6): 421-426
Category: Přehledy
Táto práca bola podporená projektom „Centrum excelentnosti pre výskum v personalizovanej terapii“ (CEVYPET), ITMS: 2622012053, spolufinancovaným zo zdrojov EÚ a Európskeho fondu regionálneho rozvoja, a grantom VEGA 1/0028/11 Ministerstva školstva a vedy Slovenskej republiky.
Obdrženo: 2. 12. 2011
Přijato: 31. 5. 2012Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 2. 12. 2011
Přijato: 31. 5. 2012Summary
New insights into cancer cells – specific biological pathways are urgently needed to promote development of exactly targeted therapeutics. The role of oncoproteins and tumor suppressor proteins in proliferative signaling, cell cycle regulation and altered adhesion is well established. Chemicals, viruses and radiation are also generally accepted as agents that commonly induce mutations in genes encoding these cancer-inducing proteins, thereby giving rise to cancer. More recent evidence indicates the importance of two additional key factors imposed on proliferating cells – hypoxia and/or lack of glucose. These two additional triggers can initiate and promote the process of malignant transformation, when a low percentage of cells escape cellular senescence. Disregulated cell proliferation leads to formation of cellular masses that extend beyond the resting vasculature, resulting in oxygen and nutrient deprivation. Resulting hypoxia triggers a number of critical adaptations that enable cancer cell survival. The process of apoptosis is suppressed and glucose metabolism is altered. Recent investigations suggest that oxygen depletion stimulates mitochondria to compensate increased reactive oxygen species (ROS). It activates signaling pathways, such as hypoxia-inducible factor 1, that promote cancer cell survival and tumor growth. During the last decade, mitochondria have become key organelles involved in chemotherapy-induced apoptosis. Therefore, the relationship between mitochondria, ROS signaling and activation of survival pathways under hypoxic conditions has been the subject of increased study. Insights into mechanisms involved in ROS signaling may offer novel ways to facilitate discovery of cancer-specific therapies.
Key words:
mitochondria – cell death – energy metabolism – cell transformationIntroduction
Cells in the body that retain normal growth control will eventually undergo the process of cellular senescence leading to cellular turnover by their death and replacement. By contrast, cells undergoing the process of oncogenic transformation continue to survive as immortalized cells, leading to uncontrolled proliferation that is associated with tumor formation. More recently, a relationship between changes in mitochondrial function, associated ROS production and its involvement in the process of cellular senescence have become increasingly clear [1]. Interplay between mitochondrial ROS production and the role of oncoproteins and tumor suppressors in modulating mitochondrial function to promote malignant cell transformation and avoid senescence has also become apparent [2]. Numerous studies focused their attention to the physiological process of cell aging [3,4], which is accompanied by elevated ROS production. On the other side, ROS production is increased in malignant cells in part as result of oncogene signaling via the NADPH oxidase complex and by hypoxia-related mitochondrial ROS. Increased oxidant levels contribute to enhanced cell proliferation and apoptosis suppression. Two independent therapeutic strategies targeting these pathways are possible. One target of attack would be to increase ROS scavenging, thereby dampening hydrogen peroxide signaling and depressing tumor growth. An opposite approach would be to treat cells with agents that interfere with ROS scavenging, resulting in excess reactive products that would trigger apoptosis [5,6].
It has become evident that telomere and telomerase are main components of the stem cell ignition mechanism, providing a way to restrain cancer [7]. With aging, oxidative stress accelerates vascular endothelial cell telomere shortening [8]. This process is referred to as accelerated replicative senescence, when mild repeated oxidative stress induces telomeric DNA damage [9]. On the other hand, senescence can be telomere-independent, the so-called stress induced senescence, when it is triggered prematurely by sublethal oxidative stress inducing non-telomeric DNA damages and growth arrest signals [10].
Tumor Cells and Glycolysis
Tumor cells and normal cells metabolize oxygen differentially. Because the activation of glycolysis in tumor cells is essential to prevent cell death induced by ATP depletion and H2O2 accumulation, the attenuation of glycolysis in tumor cells can induce their death. Normal cells would be less affected by this because they do not have increased glycolytic rates to ensure their survival [11,12]. Tumor cells exhibit profound genetic, biochemical and histological differences with respect to the original, nontransformed cellular types. In early studies on energy metabolism of tumor cells, it was proposed that enhanced glycolysis was induced by decreased oxidative phosphorylation. Since then, it has been indiscriminately applied to all types of tumor cells without an appropriate experimental evaluation and different findings [13]. The most notorious and well--known energy metabolism alteration in tumor cells is increased glycolytic capacity, even in the presence of high oxygen concentration [14]. It has been proposed that this increase in the glycolytic flux is a metabolic strategy of tumor cells to ensure survival and growth in environments with low oxygen concentrations [15]. The main mechanism responsible for the constant glycolytic flux is enhanced transcription of genes of several or all pathway enzymes and transporters, which is accompanied by an enhanced protein synthesis [16]. Experimental data have shown that in comparison to normal rat hepatocytes, all glycolytic enzymes are over-expressed by two - to four-fold while pyruvate kinase is over-expressed by ten-fold, hexokinase and phosphofructokinase type 1 are over-expressed up to 17 - to 300-fold [17]. For human cervix HeLa cells, all enzymes are over-expressed by two - to seven-fold, with the exception of lactate dehydrogenase, which is expressed at a level sevenfold lower than in rat hepatocytes. However, for this last case, a more rigorous comparison should be made with normal uterine cervix epithelial cells [17]. One of the alternative approaches, in addition to building up a complex set of DNA changes, evidence suggests that the development of any cancer requires an alteration in oxygen metabolism of tumor cells. Interestingly, this alteration in oxygen metabolism can make cancer cells vulnerable to therapeutic intervention. Their increased hydrogen peroxide level and higher dependence on glycolysis for their survival make tumor cells more susceptible than normal cells to treatment with prooxidant agents or glycolysis inhibitors [12]. Several mechanisms for the enhanced glycolysis in human and rodent fast-growing tumor cells have been advanced and documented [13]. It is:
- increase in the isoform expression of glycolytic enzymes and glucose transporters,
- decreased expression of mitochondrial oxidative enzymes and transporters,
- lowering in the amount of mitochondria per cell,
- inhibition of oxidative phosphorylation by glycolysis activation (Crabtree effect),
- increased amount in the natural inhibitor protein of the mitochondrial ATP synthase,
- higher sensitivity of mitochondrial DNA to oxidative stress.
Perhaps the prime driving mechanism for the enhanced glycolysis is activation, via the hypoxia inducible factor 1 (HIF-1), of the transcription and translation of glycolytic genes in tumor cells. HIF-1 is a transcription factor constituted by two subunits, HIF-1α and HIF-1β. Factor stability mostly depends on HIF-1α. Under aerobic conditions, an active process of HIF-1α degradation is promoted, whereas, in anaerobic conditions, this subunit becomes highly stable [18]. In addition to hypoxia, HIF-1α may be induced under aerobic conditions by cytokines, growth factors, reactive oxygen species and nitric oxide; or by the energy metabolism intermediates: pyruvate, lactate and oxaloacetate [19]. HIF-1α might be detected only in malignant tumors but not in normal, healthy tissues and benign tumors. The reason for this hypothesis is change on the level of the von Hippel-Lindau protein, a tumor suppressor. Von Hippel-Lindau protein binds to HIF-1α and induces its degradation, but in some aggressive tumors it is mutated, thus becoming ineffective in promoting HIF-1α degradation [20,21]. Regardless of the oxygen level, metastatic tumor cell lines (breast MDA, DU145 prostate, renal RCC4) show high levels of HIF-1α, over-expression of glycolytic enzymes and high glycolysis rate, whereas non-metastatic tumor cells (breast MCF-7, BX-PC3 prostate, A549 lung) increase HIF-1α over-expression and glycolysis only under hypoxia [21]. Simon [22] has shown direct association between HIF-1α and increasing generation of lactate from pyruvate due to pyruvate dehydrogenase complex inhibition (phosphorylation by pyruvate dehydrogenase kinase). Further association of HIF-1α with expression of other mitochondrial proteins has not yet been found.
Tumor Cells Energy Metabolism – Glycolysis or Oxidative Phosphorylation
Pioneering studies with solid and ascites tumor cells led to a proposal of universal mechanism that all tumor cell types were energetically dependent, mainly or only, on glycolysis. In particular, glycolysis seems to be the main energy pathway in slow-growing solid tumors, such asmammary adenocarcinoma, human melanomas [23] and rat rhabdomyosarcomas [24], as oxidative phosphorylation is apparently limited by the low oxygen availability inside the tumor [25]. It should also be considered that, in addition to lower oxygen availability in solid tumors, especially in the initial and avascular developmental stages under which a poor vascularization occurs, glucose supply can be similarly affected, thus inducing a severe decrease in the generation of glycolytic ATP [26]. Some authors [26,27] have determined a normal oxygen concentration (8–57 µM depends on tissue) in the center of glioma, carcinoma and in the hypoxic regions of human tumors. If the oxygen concentration surrounding mitochondria does not fall below 1 µM, mitochondria will work normally. Therefore, tumor mitochondrial metabolism would not be affected by hypoxia level found in tumors, unless there was prolonged exposure to weeks or months. Simultaneously, in a hypoxic microenvironment, the expression of mitochondrial enzymes alters somehow, perhaps through a p53-mediated mechanism [28]. It is assumed, but not experimentally determined, that oxidative phosphorylation in mitochondria is negligible under hypoxic conditions. Enhanced glycolysis of tumor cells is usually considered to be a sufficiently good reason for proposing that ATP supply only or mainly depends on glycolysis [19,28–30] but the quantitative contribution of each energy supply has rarely been determined. It also remains to be analyzed whether the accelerated glycolysis under hypoxia indeed serves only for ATP supply or its role is the supply of intermediates for biosynthesis of polysaccharides, precursors for lipids, amino and nucleic acids [25], which are required for active angiogenesis in solid tumors.
Initial studies proposed that a high glycolytic rate in tumor cells was the result of a damaged respiratory chain. It was shown later [30] that respiration of tumor mitochondria was as efficient as that of normal mitochondria and diminished oxidative phosphorylation observed in tumor cells was the result of a lower proportion of mitochondria (20–50%). However, significant respiratory deficiencies have been identified in some respiratory chain components – cytochrome C oxidase, iron-sulphur center, cytochrome C reductase, but an increase in activity of cytochrome C reductase has also been determined in the same brain tumors [31,32]. In contrast, no differences in oxidative enzyme activities with normal cells have been detected for Morris and Novikoff hepatomas. It is important to emphasize that a decrease of one enzyme or transporter does not automatically lead to diminution in the pathway flux or metabolite concentration. Unfortunately, detection of protein levels by Western blot, or of gene expression by Northern blot, provides information with little functional meaning, unless these measurements are accompanied by determination of enzyme activity and pathway flux.
It is intriguing that despite accelerated glycolysis in many fast-growing tumor cells, the total contribution to the ATP supply only reaches 10% [33] (Tab. 1). In contrast to other fast growing tumor cell lines, glycolysis indeed covers 50–70% of the ATP demand. Some human and rodent gliomas exhibit high or moderate susceptibility to respiratory chain inhibitors, indicating the presence of fully functional mitochondria and dependency on oxidative phosphorylation [34]. On the contrary, many brain tumors in comparison with other tissues have lower succinyl-CoA acetoacetyl transferase activity than normal neurons or glia, and are unable to metabolize ketone bodies. Then, as fatty acids do not pass the blood-brain barrier, brain tumors seem to be dependent on glucose and glycolysis for ATP supply [35]. Therefore, the generalized statement that glycolysis predominates over oxidative phosphorylation for ATP supply in tumor cells should be experimentally determined for each particular types of tumor cell. Thus, the main thermodynamic reason for increased glycolysis in tumor cells (associated with damaged or unaltered oxidative phosphorylation) might rather be energy deficiency induced by highly ATP-dependent processes, such as an accelerated cell proliferation or stimulated nucleic acid, protein and cholesterol synthesis.
Tab. 1. Oxidative phosphorylation/Glycolysis – dependent energy metabolism in different tumor cell types (modified from [14]). ![Oxidative phosphorylation/Glycolysis – dependent energy metabolism in different tumor cell types (modified from [14]).](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/80ed0fe695a98080babcf33d322ea31e.png)
Mitochondria as Excellent Targets for Cancer Therapy
On the one hand, mitochondria are important for normal functioning and cell survival, but on the other hand, they are key regulators of intrinsic apoptotic cascade. The major nuclear encoded oncogenic proteins (MYC, p53, STAT-3, RAS) act either alone or in an integrated fashion to modulate gene expression involved in mitochondrial function, regulate the expression of gene encoding mitochondrial proteins by directly altering mitochondrial function inside cancer cells to promote cancer development [2]. Recent research established that mitochondrial associated gene expression is significantly different in cancer cells in comparison to normal cells. Most of the common features of cancer cells [36–38], can be found (either in a direct or indirect fashion) in mitochondria. Since mitochondria occupy a strategic position between bioenergetic/biosynthetic metabolism and cell death regulation, they are emerging as privileged targets for the development of novel chemotherapeutic agents [39]. During the last decade, numerous approaches that selectively target cancer cells by virtue of their mitochondrial defects have been shown to exert antitumor effects [40]. These include:
- mitochondriotoxic agents that preferentially accumulate in cancer cells due to mitochondrial hyperpolarization (e.g., F16) [41],
- pharmacological modulators of the Bcl-2 protein family (e.g., ABT-737) [42],
- compounds that bind to putative permeability transition pore complex (PTPC) subunits (e.g., PK11195) [43],
- redox active agents that trigger cell death by provoking futile redox cycles in mitochondria (e.g., arsenic trioxide, phenethyl isothiocyanate) [44],
- retinoid-related molecules that induce mitochondrial permeability transition independently of retinoid receptors (e.g., CD437, ST1926) [45].
One of the most extensively studied redox active agents is dietary phenethyl isothiocyanate (PEITC). PEITC can induce glutathione S-transferase and quinone reductase that inactivate carcinogens and promote their excretion. More recently, it has been found that PEITC could induce cell cycle arrest and apoptosis [46], and [47] have shown that PEITC treatment of the human MCF7 breast cancer cells produced significant alterations in some genes involved in tumor suppression and cellular apoptosis. Phenethyl isothiocyanate is also an effective inhibitor of HIF-1. The ability of PEITC to inhibit its activity was independent of the activity of the von Hippel-Landau protein and the proteasome, which are required for the normal turnover of HIF-1α in normoxia. Decreased expression of HIF-1α in PEITC treated human MCF7 breast cancer cells was not associated with HIF-1α RNA levels suggesting that PEITC may inhibit HIF activity by decreasing translation of the HIF-1α RNA. These results may contribute to the anti-angiogenic and anti-cancer effects of PEITC [48]. Currently, there is an ongoing phase II clinical trial for preventing lung cancer with phenethyl isothiocyanate in a group of heavy smokers [49].
Hypothetically, the most efficient mitochondrial therapies would be those that affect processes in mitochondria linked to several features of the neoplastic phenotype. As an example, compounds that disrupt the interaction between hexokinase and the voltage-dependent anion channel might display a consistent dual antitumor effect: uncoupling of aerobic glycolysis from residual ATP synthesis in mitochondria and sensitization to PTPC-dependent cell death [50].
The biochemical strategy – simultaneous blockage of both ATP generating pathways for suppressing the accelerated tumor proliferation was originally proposed by [51]. For example, blockage of glycolysis (NAD+ dependent enzymes) by the drug gossypol (AT-101) in diverse fast-growth tumor cells, together with oxidative phosphorylation inhibitor rhodamine 123 (Tab. 2), decreased tumor cell proliferation by 60% [52]. Gossypol alone simultaneously inhibits anti-apoptotic proteins of Bcl-2 family [Bcl-2, Bcl-XL, Bcl-W) and demonstrates clinical activity in a phase I trial against prostate cancer [53]. Treatment of several human and rodent tumors by rhodamine 123 and 2-deoxyglucose together induced almost full blockage of growth [54], but 2-deoxyglucose alone significantly increased the cytotoxicity of cisplatin in neck and head tumor cells [55]. There are ongoing phase I/II clinical trials in patients with advanced solid tumors or prostate cancer.
Tab. 2. Examples of compounds targeting energy metabolism in fast-growing tumor cells. 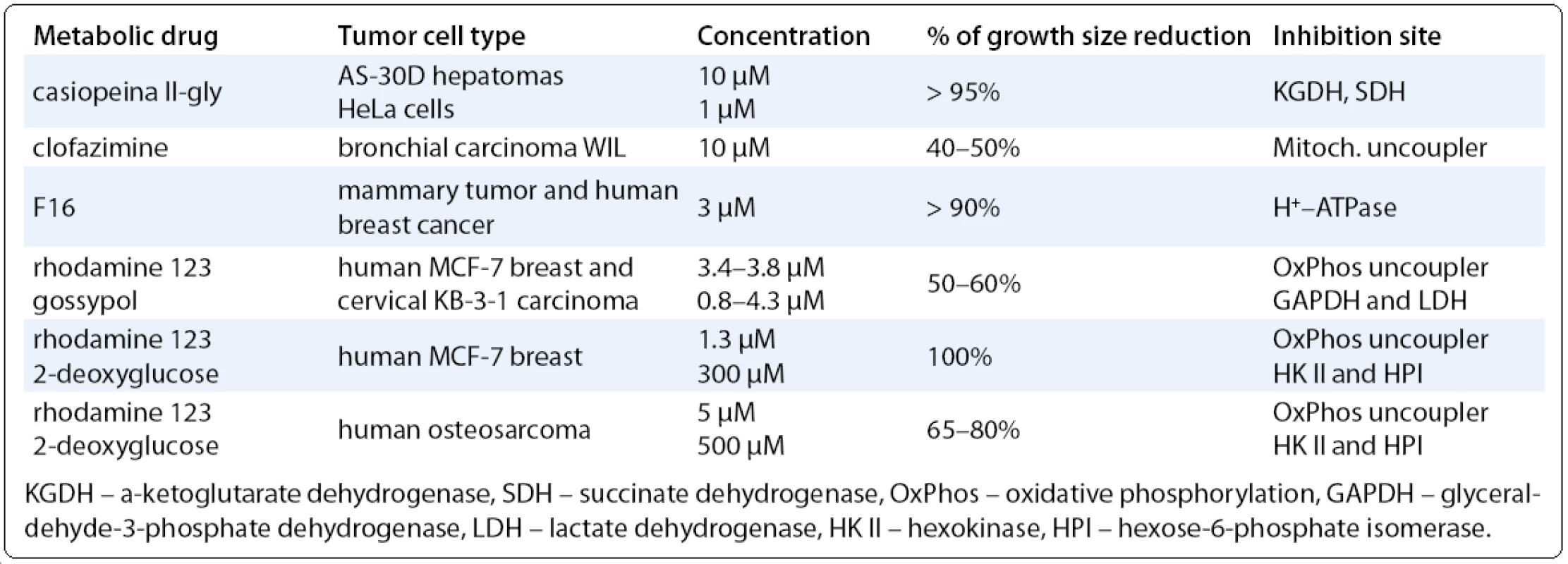
In the search for the drugs that are more specific for tumor cells, some authors have used the typical mitochondrial inhibitors, rotenone and oligomycin, for blocking tumor cell proliferation. Oligomycin at low doses (0.06–0.7 µM) does not affect normal cells but it stops cell cycle progression from G1 to S phase in human leukemia cells (HL-60) [56]. At a higher concentration (3–6 µM), oligomycin has arrested over 50% of HL-60 cells in the G2/M phase, but this concentration has an impact on normal cells. Rotenon, typical inhibitor of respiratory complex I, arrests the cell cycle in G2/M phase with strong inhibition (50–90%) of cell proliferation in human lymphoma WP and 134B osteosarcoma at concentration (0.1–1 µM) [57]. This effect is related to a severe diminution of the proton gradient across the inner mitochondrial membrane, but also to an increase in the membrane fluidity and activation of apoptosis [58]. Still, there are a lot of drugs with side effects to normal cells. Therefore, therapies that are able to specifically target the respiratory chain to further elevate ROS production in cancer cells should selectively precipitate these cells into apoptosis. An example of such agents targeting mitochondria as anti-cancer drugs (mitocans, like vitamin E succinate or vitamin K3) have been recently reviewed [59–61]. For example, [62] have shown specific mitochondrial inhibitors of succinate-quinone reductase/complex II, which regulate production of reactive oxygen species in mitochondria and protect normal cells from ischemic damage but induce specific cancer cell death.
Conclusion
Cancer kills more than six million people worldwide every year [63]. The small decrease in some types of cancer is not attributed only to better therapies but also to the implementation of prevention and early detection campaigns. Despite these campaigns, the low efficiency of chemotherapy in patients with advanced cancers is reflected in the low, five year survival rates observed in these patients [64]. A novel therapeutic approach has emerged during the last decade. This approach seeks to attack the tumor cells selectively and is based on understanding differences between tumor cells and nonmalignant cells, particularly the intracellular organelles, such as mitochondria.
Damage to the mitochondria is at the crossroad between normal metabolism and the regulation of cell death, which is a promising direction for the development of new therapies. Molecules that can reverse malignant cells from hyperglycolytic state and, simultaneously, increase their sensitivity to induction of apoptosis appear to be very effective anticancer agents. Recent analyses of human cancers have revealed, however, that the genetic defects of tumor cells are much more numerous and unstable than expected. It varies by type of cancer of the patient, which means that two people with the same type of cancer do not have the same changes in the level of genes. Despite the complexity of the cancer genome, much effort is devoted to characterize the genetic profile of tumors with the aim of rationalization and personalization of cancer therapy.
Ing. Zuzana Tatarkova, PhD.
Department of Medical Biochemistry
Jessenius Faculty of Medicine, Comenius University
Mala Hora 4
036 01 Martin
Slovak Republic
e-mail: tatarkova@jfmed.uniba.sk
Submitted: 2. 12. 2011
Accepted: 31. 5. 2012
Zdroje
1. Passos JF, Simillion C, Hallinan J et al. Cellular senescence: unravelling complexity. Age 2009; 31(4): 353–363.
2. Ralph SJ, Rodríguez-Enríquez S, Neuzil J et al. The causes of cancer revisited: mitochondrial malignancy and ROS-induced oncogenic transformation – why mitochondria are targets for cancer therapy. Mol Aspects Med 2010; 31(2): 145–170.
3. Muller FL, Lustgarten MS, Jang Y et al. Trends in oxidative aging theories. Free Radic Biol Med 2007; 43(4): 477–503.
4. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005; 120(4): 483–495.
5. Fleury C, Mignotte B, Vaysière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002; 84(2–3): 131–141.
6. Le Bras M, Clément MV, Pervaiz S et al. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol 2005; 20(1): 205–219.
7. Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett 2010; 584(17): 3826–3830.
8. Voghel G, Thorin-Trescases N, Mamarbachi AM et al. Endogenous oxidative stress prevents telomerase-dependent immortalization of human endothelial cells. Mech Ageing Dev 2010; 131(5): 354–363.
9. Houben JM, Moonen HJ, van Schooten FJ et al. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008; 44(3): 235–246.
10. Klimova TA, Bell EL, Shroff EH et al. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J 2009; 23(3): 783–794.
11. Aykin-Burns N, Ahmad IM, Zhu Y et al. Increased levels of superoxide and hydrogen peroxide mediate the differential susceptibility of cancer cells vs. normal cells to glucose deprivation. Biochem J 2009; 418(1): 29–37.
12. López-Lázaro M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol Med 2010; 16(3–4): 144–153.
13. Moreno-Sánchez R, Rodríguez-Enríguez S, Marín-Hernández A et al. Energy metabolism in tumor cells. FEBS J 2007; 274(6): 1393–1418.
14. Rodríguez-Enríguez S, Torres-Márquez ME, Moreno-Sánchez R. Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch Biochem Biophys 2000; 375(1): 21–30.
15. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4(11): 891–899.
16. Dang CV, Lewis BC, Dolde C et al. Oncogenes in tumor metabolism, tumorigenesis and apoptosis. J Bioenerg Biomembr 1997; 29(4): 345–354.
17. Marín-Hernández A, Rodríguez-Enríquez S, Vital-González PA et al. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J 2006; 273(9): 1975–1988.
18. Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today 2007; 12(19–20): 853–859.
19. Thomas DD, Espey MG, Ridnour LA et al. Hypoxic inducible factor 1, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA 2004; 101(24): 8894–8899.
20. Guppy M. The hypoxic core: a possible answer to the cancer paradox. Biochem Biophys Res Commun 2002; 299(4): 676–680.
21. Robey IF, Lien AD, Welsh SJ et al. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 2005; 7(4): 324–330.
22. Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab 2006; 3(3): 150–151.
23. Eskey CJ, Koretsky AP, Domach MM et al. Role of oxygen versus glucose in energy metabolism in a mammary carcinoma perfused ex vivo: direct measurement by 31P NMR. Proc Natl Acad Sci USA 1993; 90(7): 2646–2650.
24. Thews O, Kelleher DK, Lecher B et al. Blood flow, oxygenation, metabolic and energetic status in different clonal subpopulations of a rat rhabdomyosarcoma. Int J Oncol 1998; 13(2): 205–211.
25. Rofstad EK, Halsør EF. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res 2000; 60(17): 4932–4938.
26. Schroeder T, Yuan H, Viglianti BL et al. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res 2005; 65(12): 5163–5171.
27. Sutherland RM. Tumor hypoxia and gene expression – implications for malignant progression and therapy. Acta Oncol 1998; 37(6): 567–574.
28. Matoba S, Kang JG, Patino WD et al. p53 regulates mitochondrial respiration. Science 2006; 312(5780): 1650–1653.
29. Stubbs M, Bashford CL, Griffith JR. Understanding the tumor metabolic phenotype in the genomic era. Curr Mol Med 2003; 3(1): 49–59.
30. Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res 1978; 22 : 190–274.
31. LaNoue KF, Hemington JG, Ohnishi T et al. Defects in anion and electron transport in Morris hepatoma mitochondria. In: Hormone and Cancer. Mc Kerns KW (ed). New York: Academic Press 1974 : 131–167.
32. Lichtor T, Dohrmann GJ. Oxidative metabolism and glycolysis in benign brain tumors. J Neurosurg 1987; 67(3): 336–340.
33. Zhu XL, Guppy M. Cancer metabolism: facts, fantasy and fiction. Biochem Biophys Res Commun 2004; 313(3): 459–465.
34. Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol 2005; 74(2): 123–133.
35. Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr Metab 2005; 2 : 30.
36. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57–70.
37. Zitvogel L, Apetoh L, Ghiringhelli F et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8(1): 59–73.
38. Morselli E, Galluzzi L, Kepp O et al. Nutlin kills cancer cells via mitochondrial p53. Cell Cycle 2009; 8(11): 1647–1648.
39. Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 2008; 18(4): 165–173.
40. Galluzi L, Morselli E, Kepp O et al. Mitochondrial gateways to cancer. Mol Aspects Med 2010; 31(1): 1–20.
41. Fantin VR, Berardi MJ, Scorrano L et al. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell 2002; 2(1): 29–42.
42. Mason AR, Drummond MF. Public funding of new cancer drugs: Is NICE getting nastier? Eur J Cancer 2008; 45(7): 1188–1192.
43. Decaudin D, Castedo M, Nemati F et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res 2002; 62(5): 1388–1393.
44. Toogood PL. Mitochondrial drugs. Curr Opin Chem Biol 2008; 12(4): 457–463.
45. Garattini R, Gianni M, Terao M. Retinoid related molecules an emerging class of apoptotic agents with promising therapeutic potential in oncology: pharmacological activity and mechanisms of action. Curr Pharm Des 2004; 10(4): 433–448.
46. Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-α36. J Cell Mol Med 2010; 14(6B): 1485–1493.
47. Moon YJ, Brazeau DA, Morris ME. Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells. Evid Based Complement Alternat Med 2011; 2011 : 1–8.
48. Wang XH, Cavell BE, Syed Alwi SS et al. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem Pharmacol 2009; 78(3): 261–272.
49. Yuan JM. Masonic Cancer Center at University of Minnesota. National Cancer Institute at the National Institutes of Health. Available from: http://cancer.gov/clinicaltrials/UMN-2007NT127.
50. Goldin J. Working the program. Moving through cancer: strength and fitness training improves outlook for cancer patients. Rehab Manag 2008; 134(2): 358–367.
51. Müller M, Siems W, Buttgereit F et al. Quantification of ATP-producing and consuming processes of Ehrlich ascites tumour cells. Eur J Biochem 1986; 161(3): 701–705.
52. Jaroszewski JW, Kaplan O, Cohen JS. Action of gossypol and rhodamine 123 on wild-type and multidrug-resistant MCF-7 human breast cancer cells: 31P nuclear magnetic resonance and toxicity studies. Cancer Res 1990; 50(21): 6936–6943.
53. Liu G, Kelly WK, Wilding G et al. An open-label, multicenter, phase I/II study of single-agent AT 101 in men with castrate-resistant prostate cancer. Clin Cancer Res 2009; 15(9): 3172–3176.
54. Liu H, Hu YP, Savaraj N et al. Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry 2001; 40(18): 5542–5547.
55. Simons AL, Ahmad IM, Mattson DM et al. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 2007; 67(7): 3364–3370.
56. Sweet S, Singh G. Accumulation of human promyelocytic leukemic (HL-60) cells at two energetic cell cycle checkpoints. Cancer Res 1995; 55(22): 5164–5167.
57. Armstrong JS, Hornung B, Lecane P et al. Rotenone-induced G2/M cell cycle arrest and apoptosis in a human B lymphoma cell line PW. Biochem Biophys Res Commun 2001; 289(5): 973–978.
58. Barrientos A, Moraes CT. Titrating the effects of complex I mitochondrial impairment in the cell physiology. J Biol Chem 1999; 274(23): 16188–16197.
59. Biasutto L, Dong LF, Zoratti M et al. Mitochondrially targeted anti-cancer agents. Mitochondrion 2010; 10(6): 670–681.
60. Rohlena J, Dong LF, Ralph SJ et al. Anticancer drugs targeting the mitochondrial electron transport chain. Antioxid Redox Signal 2011; 15(12): 2951–2974.
61. Dong LF, Jameson VJ, Tilly D et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem 2011; 286(5): 3717–3728.
62. Ralph SJ, Moreno-Sánchez R, Neuzil J et al. Inhibitors of succinate: quinone reductase/Complex II regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm Res 2011; 28(11): 2695–2730.
63. Parkin DM, Bray F, Ferlay J et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55(2): 74–108.
64. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2009. CA Cancer J Clin 2009; 59(4): 225–249.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2012 Číslo 6- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Nejasný stín na plicích – kazuistika
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
-
Všechny články tohoto čísla
- Klinická onkologie slaví 25. výročí
- Molecular Basis of Waldenström Macroglobulinemia
- Why Mitochondria are Excellent Targets for Cancer Therapy
- Liver Function Assessment in Oncology Practice
- EML4-ALK Fusion Gene in Patients with Lung Carcinoma: Biology, Diagnostics and Targeted Therapy
- Cost Analysis of XELOX and FOLFOX-4 Chemotherapy Regimens for Colorectal Carcinoma
- Therapeutic Results of the Treatment Brain Tumors Using Radiosurgery and Stereotactic Radiotherapy
- Profile of Cancer Patients Treated at the Emergency Room of a Tertiary Cancer Care Centre in Southern Brazil
- Proteins of Resistence and Drug Resistence in Ovarian Carcinoma Patients
- A Case Report: Patient with Advanced Ovarial Tumour and Supporting Care
- Incidentally Discovered White Subcupsular Liver Nodules during Laparoscopic Surgery: Biliary Hamartoma and Peribiliary Gland Hamartoma
- Paraneoplastic Neurological Syndrome in 64-year-old Patient in Association with a Small Cell Lung Carcinoma
- Vzpomínka na MUDr. Vladimíra Spurného, CSc.
- Prof. MUDr. Rostislav Vyzula, CSc., již a teprve šedesátiletý
- Klinický registr CORECT
- Možnosti překonání rezistence k hormonální léčbě u pacientek s hormonálně dependentním metastatickým karcinomem prsu
- Aprepitant a pruritus – komentář k článku
- Informace z České onkologické společnosti
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Liver Function Assessment in Oncology Practice
- Cost Analysis of XELOX and FOLFOX-4 Chemotherapy Regimens for Colorectal Carcinoma
- Incidentally Discovered White Subcupsular Liver Nodules during Laparoscopic Surgery: Biliary Hamartoma and Peribiliary Gland Hamartoma
- A Case Report: Patient with Advanced Ovarial Tumour and Supporting Care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



