-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe differences in clinical, radiological and treatment modalities of cervical intramedullary arachnoid cysts and cervical syringomyelia – report of 12 cases
Rozdíly v klinických, radiologických a léčebných modalitách intramedulárních arachnoidálních cyst a syringomyelie v oblasti krční páteře – 12 případů
Úvod:
Intramedulární arachnoidální cysty jsou vzácné cystické útvary, které jsou často zaměňovány za syringomyelii. Nemusí nutně představovat komplikaci patologického stavu v páteři a/ nebo mozku. Etiologie a léčba tohoto stavu však zůstávají nejasné. Syringomyelie je spojena s abnormalitou mozku a ve většině případů se objevuje jako komplikace jiných patologií. Cílem této studie bylo určit klinické a radiologické charakteristiky intramedulární arachnoidální cysty a diskutovat rozdíly mezi intramedulární arachnoidální cystou a syringomyelií.Soubor pacientů a metodika:
Od roku 2002 do roku 2012 bylo retrospektivně hodnoceno celkem 77 pacientů s intramedulárními cystickými útvary, z toho 12 s intramedulární arachnoidální cystou bylo vyhodnoceno statisticky. Všichni pacienti měli vstupní snímky a v rámci sledování byli klinicky vyšetřováni a podstupovali MR vyšetření a neuroelektrofyziologické testy.Výsledky:
Průměrná doba sledování byla 50 měsíců (rozmezí 18 – 120 měsíců). Střední rozměr intramedulární arachnoidální cysty v axiální rovině byl 13,26 ± 4,76 mm a střední rozměr v sagitální rovině byl 23,29 ± 7,95 mm. Neurologické vyšetření bylo normální u všech pacientů kromě jednoho, u kterého se po nedávném traumatu v cervikální oblasti objevila drobná ztráta čití. U pacientů nebyly nalezeny žádné motorické neurologické deficity a pacienti zůstali asymptomatičtí nebo stabilní. Velikost a tvar intramedulární arachnoidální cysty byly radiologicky neměnné, vyšetření motorickými evokovanými potenciály a somatosenzorickými evokovanými potenciály na začátku studie a na konci sledování pacientů ukázala normální odpovědi nervus medianus a nervus tibialis.Závěr:
Je pravděpodobné, že radiologické nálezy a klinické známky/ příznaky intramedulární arachnoidální cysty se liší od těch spojených se syringomyelií, neboť se liší primární příčinou. Dále se u intramedulární arachnoidální cysty liší léčebné modality a modality následného sledováníKlíčová slova:
intramedulární arachnoidální cysta – syringomyelie – magnetická rezonanceAutoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors: H. I. Secer 1; S. Kahraman 2; N. C. Ören 3; M. A. Dirik 3; N. Bulakbaşı 3
Authors place of work: Department of Neurosurgery, Faculty of Medicine, University of Kyrenia, Kyrenia, Cyprus 1; Department of Neurosurgery, Anadolu Medical Center, Istanbul, Turkey 2; Department of Radiology, Faculty of Medicine, University of Kyrenia, Kyrenia, Cyprus 3
Published in the journal: Cesk Slov Neurol N 2018; 81(4): 427-434
Category: Původní práce
doi: https://doi.org/10.14735/amcsnn2018427Summary
Background:
Intramedullary arachnoid cysts are rare cystic masses that are usually mixed up with syringomyelia. It is not necessarily a complication of any pathologic condition in the spine and/or brain but the etiology and management of this entity remains unclear. Syringomyelia is a disorder that is related to an abnormality of the brain and in most cases, occurs as a complication of other pathologies. The aim of this study was to establish the clinical and radiological characteristics of this intramedullary arachnoid cyst and discuss the differences between intramedullary arachnoid cyst and the syringomyelia.Patients and methods:
A total of 77 patients with intramedullary cystic masses were retrospectively assessed between 2002 and 2012 and 12 of them with intramedullary arachnoid cyst were analysed statistically. All patients had initial scans and followed up with clinical examinations and MRI images and neuro-electrophysiological tests.Results:
The average follow-up duration was 50 months (range 18 – 120 months). The mean axial intramedullary arachnoid cyst size was 13.26 ± 4.76mm and the mean sagittal size was 23.29 ± 7.95mm. Neurological examinations were normal in all except one patient who had minor sensory loss due to recent cervical trauma. There were no motor neurological deficits, and all patients remained asymptomatic or stable. The intramedullary arachnoid cyst size and shape remained unchanged radiologically, motor evoked potentials and somatosensory evoked potentials studies revealed normal median and tibial responses initially and at the end of the follow-up in all patients.Conclusion:
It is probable that intramedullary arachnoid cyst has different radiologic findings and clinical signs/symptoms from syringomyelia that are associated with different primary causes. And also, the treatment and follow-up modalities are different in intramedullary arachnoid cyst.Key words:
intramedullary arachnoid cyst – syringomyelia – magnetic resonance imagingIntroduction
Spinal arachnoid cysts are very rare nonspecific and usually asymptomatic benign lesions. The incidence of spinal arachnoid cysts is low, with most of the cases having an incidental diagnosis by MRI [1 – 2]. Several classification systems have been designed for description of the spinal arachnoid cysts based on anatomical locations and characteristics of the lesions [3 – 4]. But the last classification system was reported by Qi et al, as intramedullary, subdural extramedullary, subdural/ epidural, intraspinal epidural, or intraspinal/ extraspinal, based on the anatomical location and abnormalities observed by MRI, in order to evaluate a large case series and long follow-up [5].
Many authors put forward several theories, such as congenital, idiopathic, and secondary to bleeding, inflammation, infections, or puncture-related traumas [6 – 7]. Especially the main theory for formation of intramedullary arachnoid cyst is the misplaced cellular remnants that compose the cyst [8]. But the certain etiology of spinal arachnoid cysts is unclear yet.
MRI is the excellent imaging modality for evaluating and delineating these lesions [5,9 – 11]. On conventional T1-weighted and T2-weighted images, intramedullary arachnid cysts can have generally similar characteristics as syringomyelia and rarely epidermoid tumours [12 – 14]. The intramedullary arachnoid cysts are mostly reported as syringomyelia.
In many cases, follow-up with MRI and examination is enough. Spinal arachnoid cysts are only treated when symptoms emerge. Intramedullary arachnoid cysts are very rare and only 31 cases have been reported and 10 of them were localized in cervical region [5,8,15 – 17]. Literature review shows that these lesions are commonly seen in the paediatric population more commonly in the first decade. They are more common in females as compared to males and are located more commonly in the thoracic region than in the cervical region.
Causes of formation, shapes and radiologic, clinical findings and treatment strategy of the intramedullary arachnoid cysts and the syringomyelia are different. Our experience with intramedullary arachnoid cysts defined as such in a retrospective case series is presented in association with a comprehensive review. Differences between these two conditions are discussed with the current base of evidence.
Patients and methods
A total of 77 patients with cervical intramed - ullary cysts were retrospectively analysed between 2002 and 2012. Clinically; patient’s symptoms, neurologic signs, neuro-electrophysiological tests, underlying etiologies were assessed. Radiologically; the shape, location, size, signal intensities on T1-weighted and T2-weighted images were evaluated.
Intramedullary arachnoid cyst was defined as follows:
- a) A well-defined, ovoid, cystic lesion, which is isointense to CSF without contrast enhancement and without any associated septae, nodularity or signal intensity changes in adjacent spinal cord.
- b) No additional pathology, for example, Chiari malformation, spinal cord tumours, any compressive lesions, vascular malformations, a tethered cord or any other congenital spinal disraphysm, any history of central nervous system infection, any history of spinal cord trauma (vertebral fracture, penetrating injury), hydrocephalus, or previous spinal surgery.
- c) Normal neuro-electrophysiological tests (tibial and median nerve somatosensory evoked potentials [SSEP] and motor evoked potentials [MEP]) at the time of the first diagnosis and in their follow-up studies.
- d) No clinical or radiologic progression in at least their three follow-up examinations in a frequency of six months.
All medical records of these patients were reviewed and confirmed by two neurosurgeons who have 20 years and 19 years of experience regarding the spine. Changes in clinical symptoms, physical exams and neuro-electrophysiological tests were identified and documented.
MRI was performed using a 1.5 T MRI system (Gyroscan Intera, Philips Medical Systems, Best, The Netherlands). MRI sequences and parameters were sagittal T1--weighted TSE (FOV: 180, slice thickness [ST]: 3 mm, TR: 400 ms, TE: 7.4 ms, ACQ matrix: 196 × 145, FA: 90), sagittal T2-weighted TSE (FOV: 180, ST: 3 mm, TR: 3,500 ms, TE: 120 ms, ACQ matrix: 196 × 140, FA: 90), axial T2-weighted TSE (FOV: 150, ST: 4 mm, TR: 5,200, TE: 120, ACQ matrix: 176 × 120, FA: 90), sagittal T2-weighted TSE SPAIR (FOV: 180, ST: 3 mm, TR: 3,700 ms, TE: 120 ms, ACQ matrix: 184 × 136, FA: 90, fat suppression +) and axial T1-weighted TSE SPIR (FOV: 180, ST; 3 mm, TR: 2,154 ms, TE: 7.8 ms, ACQ matrix: 176 × 121, FA: 90, fat suppression +), sagittal T1-weighted TSE SPIR (FOV: 180, ST: 3 mm, TR: 400 ms, TE: 7.4 ms, ACQ matrix: 196 × 145, FA: 90, fat suppression +) after gadolinium injection. Two radiologists in consensus who have 17 years and 6 years of experience in diagnostic imaging assessed all the MRI images including the follow-up images. Synapse (PACS) Workstation, Version 4.4 (Fujifilm Medical Systems, Stamford, USA) was used in the assessment of MRI images.
Intramedullary arachnoid cysts were defined as a well-demarcated, non-enhancing cavity within the spinal cord that had the same signal intensity with CSF on T1-weighted and T2-weighted images without any septa, nodule and/ or abnormal signal intensity in the adjacent cord.
Changes in the shape of the intramedullary arachnoid cyst, size of the intramedullary arachnoid cyst in millimetres in the axial and sagittal planes and the number of cervical vertebral levels involved in the sagittal plane were assessed and recorded from the MRI studies at presentation and follow-up. Axial and sagittal T2-weighted images were used to determine the maximum dimensions of intramedullary arachnoid cyst.
The first three MRI scans and the last one (a total of four MRI scans for each patients) were included in the statistical analyses. An increase of 20% in the axial and/ or sagittal maximum diameter of intramedullary arachnoid cyst in any time was accepted as a progressive disease. Statistical analysis was performed using SPSS statistics 21 for Windows (SPSS, Chicago, IL, USA). Descriptive analyses were presented as proportions for count data and as means with standard deviations (SD) for continuous data and the one-way Anova test was used to compare the means of three or more independent samples. A p value less than 0.05 was considered statistically significant.
Neurologic examinations, imaging studies and neuro-electrophysiological tests were performed at regular intervals (ranging from 6 months to 1 year for MRI and neurological examinations, and 1 year for neuro-electrophysiological tests).
Results
A total of 77 patients were included in the study. Thirty-four (44%) of them were Chiari malformations type 1 or 2, 6 (7.7%) of them were posttraumatic, 7 (9%) of them were tumours (1 of them subependymoma, 3 of them ependymomas and 5 of them were astrocytomas), 8 (10%) of them were secondary to scoliosis and kyphosis, 3 (3.8%) were due to severe intervertebral disc herniation, 3 (3.8%) were due to severe cervical spinal stenosis, 2 (2.5%) were due to post-meningitis arachnoiditis, 1 (1.2%) were due to arachnoid cyst in posterior fossa, 1 (1.2%) were due to rheumatoid pannus.
Twelve (15.5%) of them were defined as intramedullary arachnoid cyst according to the inclusion criterion and they were analysed statistically. The patients’ ages ranged between 21 and 45 years with an average of 33.5 years. Four were males, and 8 were females. The most common presenting symptom was neck pain. Neurological examinations were normal but one patient who described sensory loss at the right C6 dermatome after recent minor cervical trauma. At the end of the follow-up, there were no neurological abnormalities in any patient but the pain without any significant changes continued even though a variety of conservative interventions including medications, counselling, physiotherapy and exercises. MEP and SSEP studies revealed normal median and tibial responses initially and at the end of the follow-up in all patients (Tab. 1). The follow-up period ranged from 18 to 120 months (with a mean ± SD of 50 ± 28.06 months).
Tab. 1. Summary of the patients with cervical intramedullary arachnoid cyst. 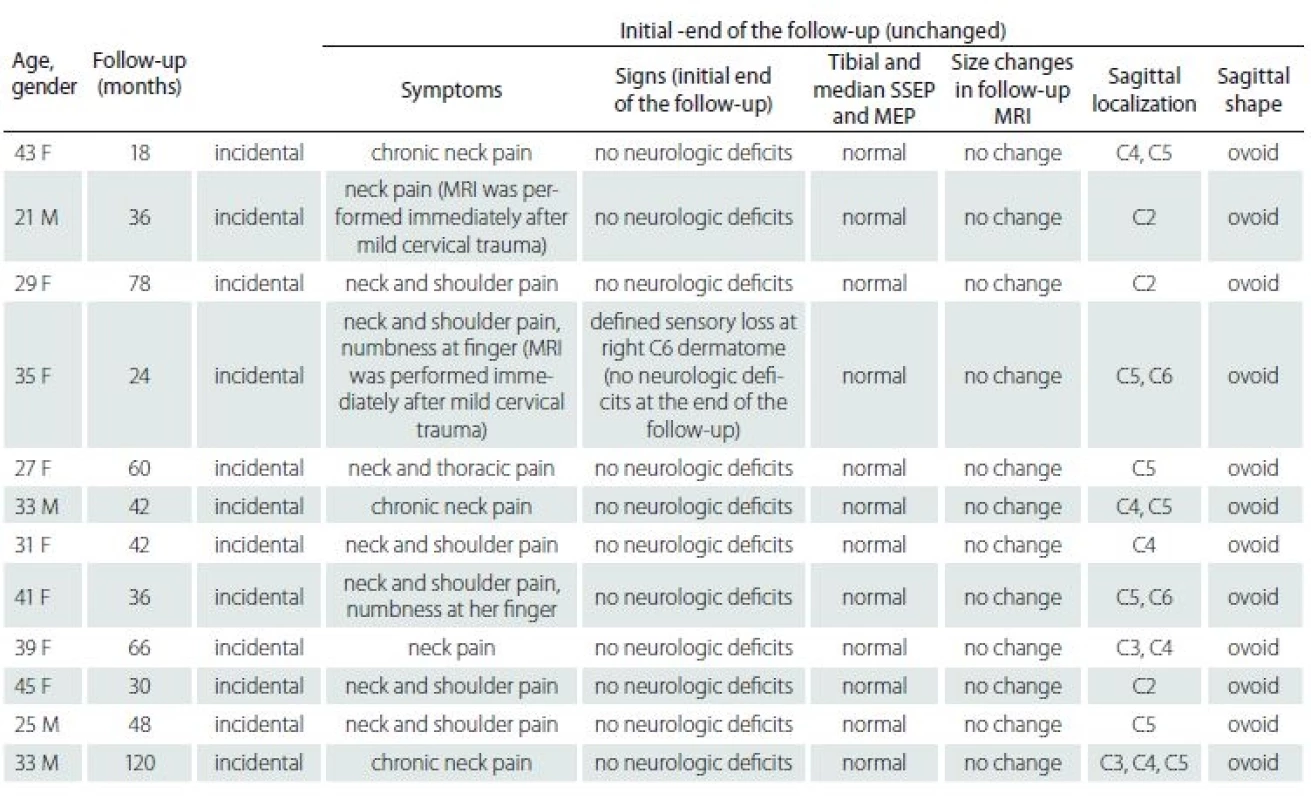
F – female; M – male; MEP – motor evoked potentials; SSEP – somatosensory evoked potentials The first MRI revealed a mean ± SD axial diameter of 13.46 ± 4.94 (range 5.32 – 21.0) mm and a mean ± SD sagittal diameter of 23.49 ± 8.30 (range 13.30 – 39.20) mm. The second MRI revealed a mean ± SD axial diameter of 13.15 ± 4.75 (range 5.32 – 22.4) mm and a mean ± SD sagittal diameter of 23.41 ± 8.41 (range 13.40 – 39.80) mm. The third MRI revealed a mean ± SD axial diameter of 13.30 ± 4.95 (range 5.32 – 23.0) mm and a mean ± SD sagittal diameter of 23.21 ± 8.13 (range 13.1 – 38.7) mm. The last MRI revealed that a mean ± SD axial diame-ter of 13.13 ± 5.02 (range 5.32–23.0) mm and a mean ± SD sagittal diameter of 23.06 ± 8.09 (range 13 – 38.0) mm (Tab. 2). There were not any significant differences of the axial and sagittal diameters between MRI studies (p = 0.99, > 0.05).
Tab. 2. The results of intramedullary arachnoid cysts size in axial and sagittal MRI images during follow-ups. 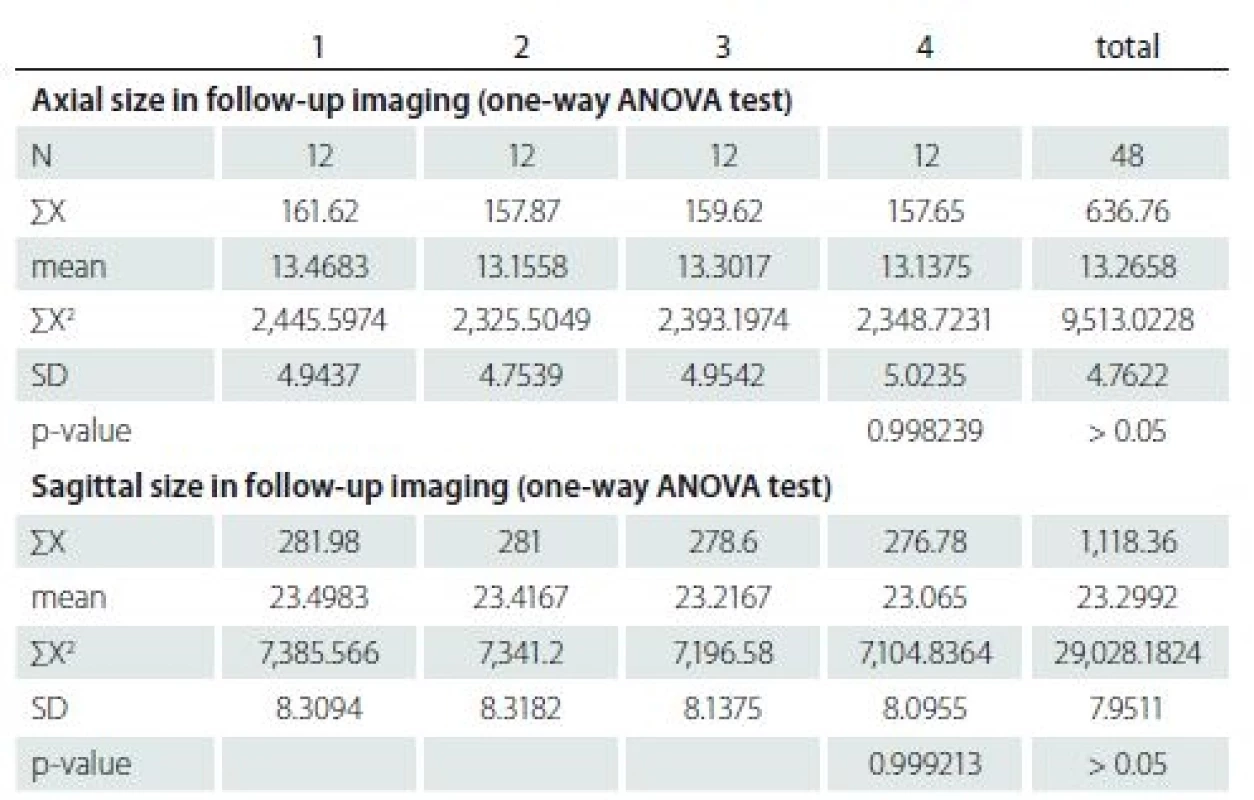
SD – standard deviation Discussion
The pathophysiology of the syringomyelia remains controversial. In general, all theories focused on three mechanisms:
- CSF entrance from the 4th ventricle;
- CSF entrance from the subarachnoid space;
- extracellular fluid accumulation [18,19].
The exact mechanism however, is still debated. Syringomyelia may herald the presence of several primary conditions such as Chiari malformations, spinal cord compression and tethering due to previous spinal trauma, infections, spinal cord tumours and vascular malformations. If the syringo-myelia is secondary to a cause, treatment of the underlying disorder is principal. Successful treatment of the primary problem will often reduce or resolve the syringomyelia. The embryogenetic model of syringomyelia postulates pathologic alterations in neurogenesis as the basis of the structural abnormalities that lead to syringomyelia.
Syringomyelia is widely used as a term describing the presence of a fluid-filled cavity oriented in a rostral-caudal axis within the tissue of the spinal cord. This cyst expands and elongates over time, destroying a portion of the spinal cord at its centre and it expands outwardly. The inner floor of the syringomyelia is covered with ependymal or glial cells and is presumed to be filled with a derivative of CSF [20]. Syringomyelia is located at the centre of the spinal cord probably in connection with the central canal that has an ependymal wall [21].
The origin of intramedullary arachnoid cysts is not yet well defined. Aithala et al described the first intramedullary arachnoid cyst [22]. The relations with dysraphic anomalies of spinal cord with arachnoid cysts were defined later [23]. Gelabert et al described the occurrence of intramedullary cyst without neural tube defects [24]. According to the hypothesis of Fortua and Mercuri, the intramedullary arachnoid cysts arise as secondary cystic development of the atypical intramedullary arachnoid granulations [25]. The other alternative hypothesis postulated describes the anatom - ical communication between the cyst and subarachnoid space as a one-way valve allowing the CSF to seep into the cyst and cause the consequent expansion of the cyst [24]. There was no obvious communication between the cyst and the spinal canal that was evident due to no CSF leak on Valsalva manoeuvre in our cases.
Syringomyelia is associated with symptoms and findings such as cervical pain, stiffness in the back, shoulders, arms, or legs; arm and/ or leg weakness, headaches, and a loss of the ability to feel extremes of hot or cold [26]. Postural changes or Valsalva-like manoeuvres may exacerbate symptoms, a phenomenon that is consistent with the hydrodynamic theories of the genesis of syringomyelia. These symptoms may vary with the extent and the location of the syringomyelia within the spinal cord and are progressive.
On the other hand, all cases of intramedullary arachnoid cysts are recognized incidentally. They are usually asymptomatic, but rarely become symptomatic once the cyst starts compressing the cord or nerve roots. The most common presenting symptom is slowly progressive weakness in the limbs because of gradual and continuous enlargement of the cyst. If it becomes symptomatic, several surgical techniques were described such as decompressive laminectomy, percutaneous drainage, shunting procedures, communicating the cyst to the subarachnoid space, myelotomy and partial or total cyst wall excision for intramedullary arachnoid cyst [5,9 – 11,27]. Neck pain was the most common and usually the only presenting symptom in our cases. In almost all cases, the cause of this neck and shoulder pain is myofascial pain syndrome, which is amenable to treatment with physiotherapy and exercises. We did not perform any surgery for intramedullary arachnoid cyst in our cases.
The syringomyelia is frequently found in the cervical region at the vertebral column, but sometimes it can extend to the thoracic spine, however, the intramedullary arachnoid cysts are frequently limited to only the cervical spine.
MRI is an excellent modality for identification of the syringomyelia and accompanying pathologies [28]. MRI allows the demonstration of any cystic lesion in a normal or atrophic spinal cord. Additionally, it permits determination of the lower and upper borders of the cyst, determination of any septa in the cysts, detection of associated disorders such as cysts in the posterior fossa, tethered cord, Chiari malformation etc., detection the complications of treatments and trauma, studying the fluid circulation within the subarachnoid space and in the cyst by using flow imaging techniques [29]. T1 and T2 relaxation characteristics of the syringomyelia suggest a non-enhancing CSF-like fluid [30]. Syringomyelia usually extends to more than two or three levels, whereas intramedullary arachnoid cysts are almost usually limited in three or less cervical vertebrae levels on the sagittal images. Syringomyelia was recognized as a lopsided round or fusiform shape [30] in contrast to the intramedullary arachnoid cyst, which has an ovoid shape like an American football ball (Fig. 1). The shape of the syringomyelia may be complex with septations (unlike the syrinx) and generally involves a portion of the central canal at some level. It has been reported recently that unenhanced MRI including sagittal and axial T2-weighted imaging has been demonstrated to differentiate syringomyelia from intramedullary arachnoid cyst associated mass with a 100% sensitivity and negative predictive value by using imaging features such as nodularity, septations, a spinal cord signal intensity abnormality or a mass adjacent to the intramedullary arachnoid cyst [31]. None of these MRI features were recognized in our patients with intramedullary arachnoid cyst and this also supports our consideration that the intramedullary arachnoid cyst and syringomyelia are different entities (Fig. 2, 3).
Fig. 1. An ovoid shaped C2 level intramedullary arachnoid cyst is demonstrated on T2-weighted sagittal (A), T1-weighted sagittal (B) and T2-weighted axial (C) MRI images.
Obr. 1. Intramedulární arachnoidální cysta ovoidního tvaru v úrovni C2 je prokázaná v T2 váženém obraze v sagitální rovině (A), T1 váženém obraze v sagitální rovině (B) a v T2 váženém obraze v axiální rovině (C) pomocí MR.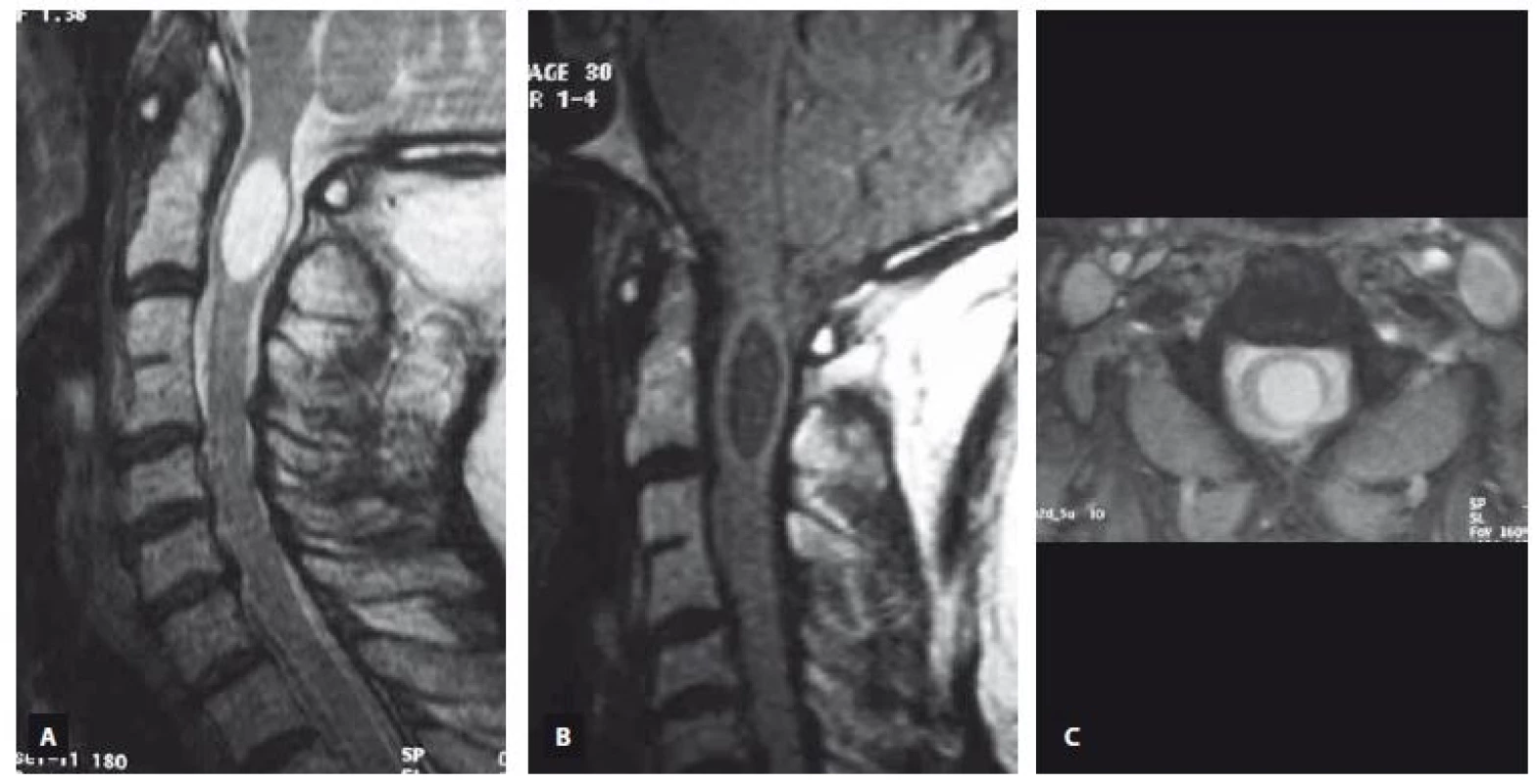
Fig. 2. MRI scan demonstrating the intramedullary arachnoid cyst extending from C3 to C5. T2-weighted image (A – sagittal view, C – axial view), T1-weighted image (B – sagittal view, D – axial view).
Obr. 2. MR snímek ukazující intramedulární arachnoidální cystu sahající od C3 k C5. T2 vážený obraz (A – sagitální rovina, C – axiální rovina), T1 vážený obraz (B – sagitální rovina, D – axiální rovina).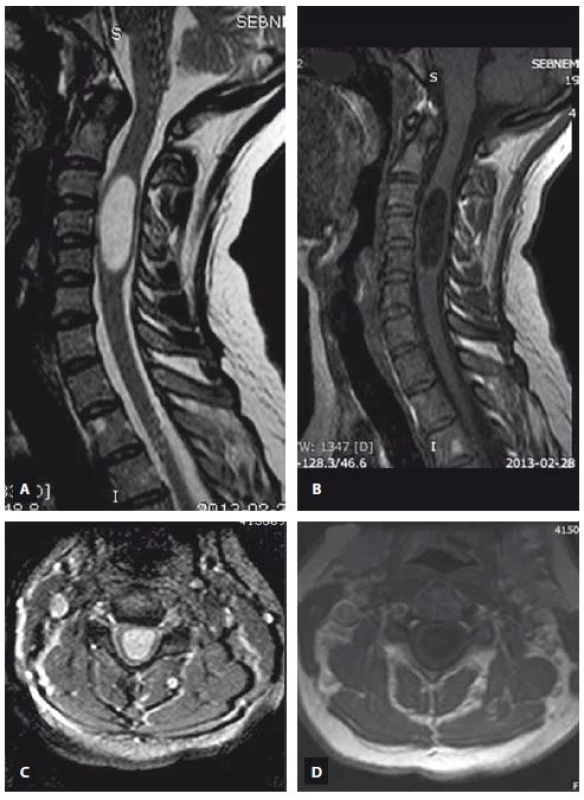
Fig. 3. MRI scan demonstrating the syringomyelia extending from C5 to C7. T2-weighted image (A – sagittal view, C – axial view), T1-weighted image (B – sagittal view, D – axial view).
Obr. 3. MR snímek ukazující syringomyelii sahající od C5 k C7. T2 vážený obraz (A – sagitální rovina, C – axiální rovina), T1 vážený obraz (B – sagitální rovina, D – axiální rovina).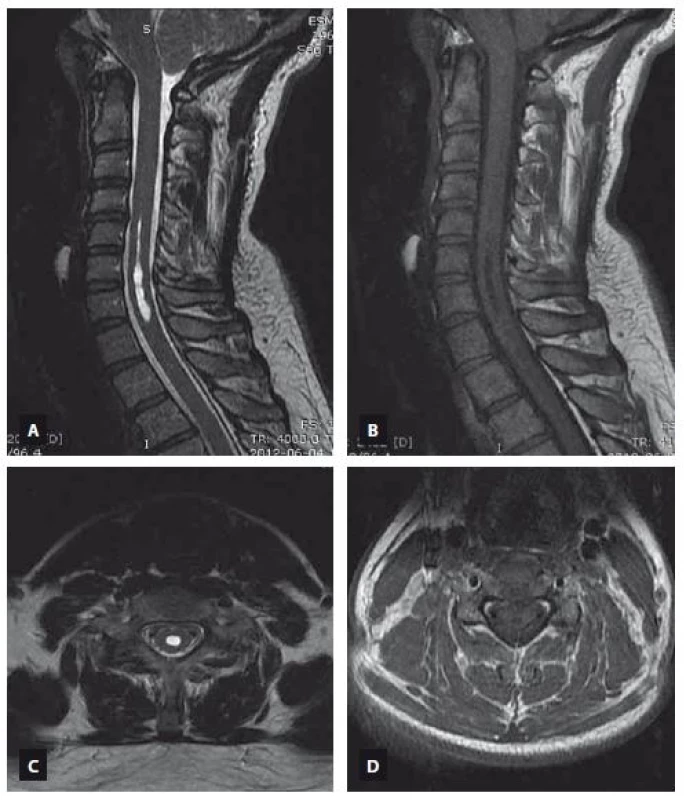
An intramedullary arachnoid cyst is usually a stagnant pathology, whereas syringomyelia is a dynamic pathology that progresses over time and can be associated with T2-weighted hyperintensity within the adjacent cord tissue, which is probably due to myelin degeneration, edema or gliosis [29]. The size of the syringomyelia usually increases in the sagittal and axial planes unless surgical intervention is performed [20,21]. Increasing widespread use of Cine-MR flow studies provide additional information about CSF flow velocity and patterns [32,33]. Application of other techniques, such as diffusion-weighted imaging and diffusion tensor imaging, may eventually provide useful additional data on the intrinsic properties of the neuroaxis.
Electrodiagnostic findings associated with syringomyelia may play an important role in the quantification of deficits induced by the spinal cord cavity and they are essential components of clinical diagnostics [34]. SSEPs and MEPs obtained after non-invasive electrical or magnetic stimulation of the motor cortex are sensitive tools for the detection of morphological and functional lesions of the spinal cord pathways [35,36]. SSEP sensitivity for centromedullary symptoms such as hypalgesia or hypesthe-sia is reported to be 54% and 81%, resp. [37]. MEP recordings have been shown to have good correlation with motor deficits [38]. In spite of all these SSEP and MEP abnormalities described in patients with syringomyelia [36,37,39], there were no abnormalities of the SSEP and MEP records at the time of presentation and during the follow-up in our cases with intramedullary arachnoid cyst.
Also, the mean limitation that the small sample size which is limited to only twelve intramedullary arachnoid cyst cases, should be taken into account when interpreting the results of this study. Despite the limitations mentioned, the strengths of this study included a relatively long duration of follow-up.
The etiological causes were different between syringomyelia and idiopathic intramedullary arachnoid cysts. The syringomyelia occurs secondary to a primary pathology, but the etiology of intramedullary arachnoid cyst is unknown. Also, the clinical signs and symptoms are different in these two conditions. The syringomyelia usually has progressive clinical symptoms and signs whereas the intramedullary arachnoid cyst is recognized incidentally without any related symptom. The intramedullary arachnoid cyst has well-defined borders within homogeneous internal signal intensity on MRI images. The intramedullary arachnoid cyst shows no progression in size or signal intensity changes over time in contrast with the dynamic mechanism of syringomyelia of which progression is demonstrated in most cases due to myelin degeneration with accompanying gliosis. We created a summary table according to the results of our study to help the understanding of this topic by showing the differences between intramedullary arachnoid cyst and syringomyelia (Tab. 3).
Tab. 3. The differences between the intramedullary arachnoid cyst and syringomyelia. 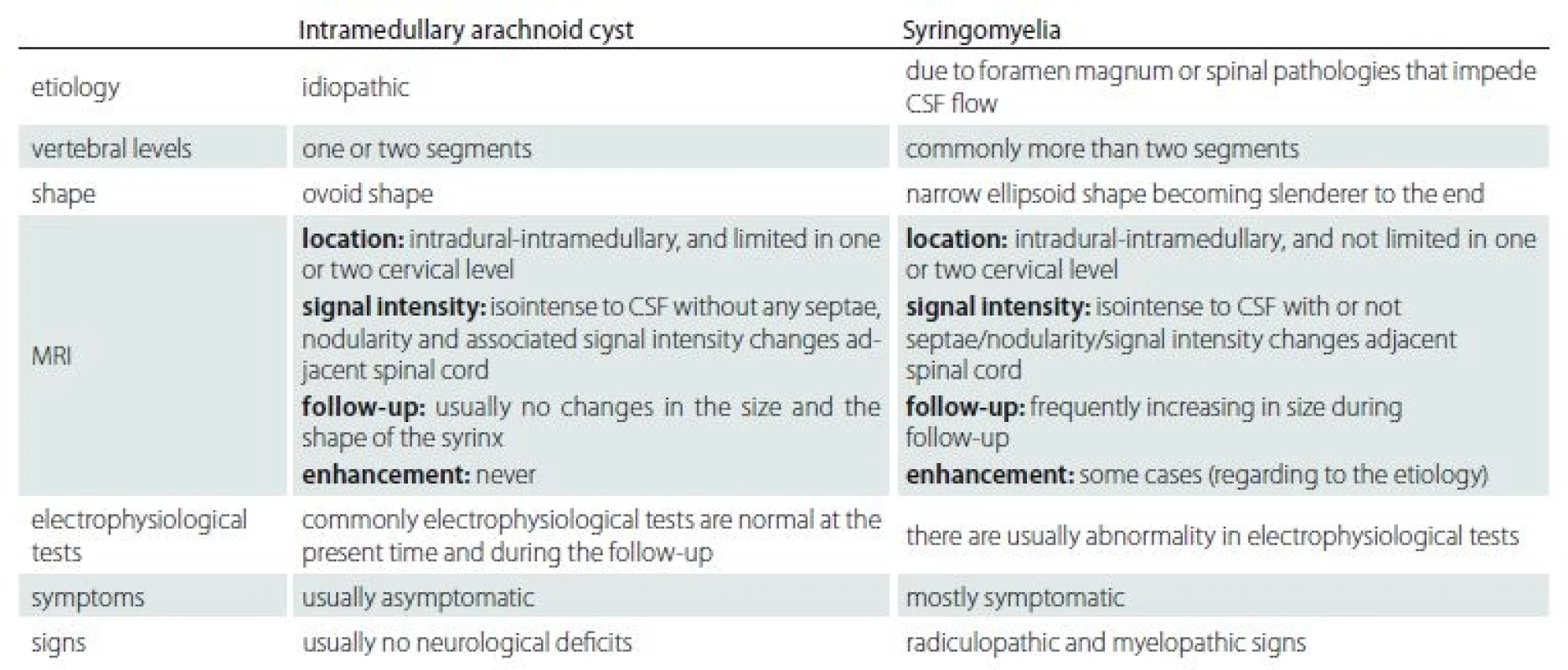
CSF – cerebrospinal fluid Conclusion
It is very important to be able to differentiate between the intramedullary arachnoid cyst and syringomyelia during MRI reporting because cases of intramedullary arachnoid cyst can be managed successfully without surgery by using conservative approaches.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manu script met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 21. 10. 2017
Accepted for print: 13. 3. 2018
Halil İbrahim Secer, MD
Department of Neurosurgery
Faculty of Medicine
University of Kyrenia Şehit
Yahya Bakır Sokak
Karakum Kyrenia
Cyprus
e-mail: hisecer@yahoo.com
Zdroje
1. Pradilla G, Jallo G. Arachnoid cysts: case series and review of the literature. Neurosurg Focus 2007; 22(2): E7.
2. Bitaraf MA, Zeinalizadeh M, Meybodi AT et al. Multiple extradural spinal arachnoid cysts: a case report and review of the literature. Cases J 2009; 2 : 7531. doi: 10.1186/ 1757-1626-2-7531.
3. Hughes G, Ugokwe K, Benzel EC. A review of spinal arachnoid cysts. Cleve Clin J Med 2008; 75(4): 311 – 315.
4. Nabors MW, Pait TG, Byrd EB et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68(3): 366 – 377. doi: 10.3171/ jns.1988.68.3.0366.
5. Qi J, Yang J, Wang G. A novel ve-category multimodal T1-weighted and T2 weighted magnetic resonance imaging-based strati cation system for the selection of spinal arachnoid cyst treatment: a 15-year experience of 81 cases. Neuropsychiatr Dis Treat 2014 : 10 : 499 – 506. doi: 10.2147/ NDT.S52517.
6. Brant WE, Helms CA. The Brant and Helms Solution: fundamentals of diagnostic radiology. 3rd ed. Philadelphia: Lippincott Williams and Wilkins 2006.
7. Medved F, Seiz M, Baur MO et al. Thoracic intramedullary arachnoid cyst in an infant. J Neurosurg Pediatr 2009; 3(2): 132 – 136. doi: 10.3171/ 2008.10.PEDS08202.
8. Sharma A, Sayal P, Badhe P et al. Spinal intramedullary arachnoid cyst. Indian J Pediatr 2004; 71(12): e65 – e67.
9. Zekai E, Saleh C, Servello D. Intramedullary cyst formation after removal of multiple intradural spinal arachnoid cysts: a case report. Surg Neurol Int 2016; 7 (Suppl 17): S473 – S474. doi: 10.4103/ 2152-7806.185779.
10. Kataria R, Sinha VD, Chopra S. Intramedullary arachnoid cyst: report of two cases. Neurol India 2012; 60(1): 123 – 124. doi: 10.4103/ 0028-3886.93618.
11. Alugolu R, Arradi V, Sahu BP. Intramedullary arachnoid cyst in an adult: case report and review. Asian J Neurosurg 2016; 11(1): 70. doi: 10.4103/ 1793-5482.145054.
12. Kukreja K, Manzano G, Ragheb J et al. Differentiation between pediatric spinal arachnoid and epidermoid - -dermoid cysts: is diffusion-weighted MRI useful? Pediatr Radiol 2007; 37(6): 556 – 560. doi: 10.1007/ s00247-007-0463-8.
13. Heinz R, Curnes J, Friedman A et al. Exophytic syrinx, an extreme form of syringomyelia: CT, myelographic, and MR imaging features. Radiology 1992; 183(1): 243 – 246. doi: 10.1148/ radiology.183.1.1549680.
14. Willems PW, van den Bergh WM, Vandertop WP. An arachnoid cyst presenting as an intramedullary tumour. J Neurol Neurosurg Psychiatry 2000; 68(4): 508 – 510.
15. Rahimizadeh A, Soufiani H. Intramedullary arachnoid cyst in association with cervical spondylosis: case report. Spine J 2013; 13(10): e21 – e25. doi: 10.1016/ j.spinee.2013.05.014.
16. Guzel A, Tatli M, Yilmaz F et al. Unusual presentation of cervical spinal intramedullary arachnoid cyst in childhood: case report and review of the literature. Pediatr Neurosurg 2007; 43(1): 50 – 53. doi: 10.1159/ 000097527.
17. Sharma A, Karande S, Sayal P et al. Spinal intramedullary arachnoid cyst in a 4-year-old girl: a rare cause of treatable acute quadriparesis: case report. J Neurosurg 2005; 102 (4 Suppl): 403 – 406. doi: 10.3171/ ped.2005.102.4.0403.
18. Klekamp J. The pathophysiology of syringomyelia – historical overview and current concept. Acta Neurochir (Wien) 2002; 144(7): 649 – 664. doi: 10.1007/ s00701-002-0944-3.
19. Koyanagi I, Houkin K. Pathogenesis of syringomyelia associated with Chiari type 1 malformation: review of evidences and proposal of a new hypothesis. Neurosurg Rev 2010; 33(3): 271 – 284. doi: 10.1007/ s10143-010-0266-5.
20. Heiss JD, Patronas N, DeVroom HL et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg 1999; 91(4): 553 – 562. doi: 10.3171/ jns.1999.91.4.0553.
21. Williams B. On the pathogenesis of syringomyelia: a review. J R Soc Med 1980; 73(11): 798 – 806.
22. Aithala GR, Sztriha L, Amirlak I et al. Spinal arachnoid cyst with weakness in the limbs and abdominal pain. Pediatr Neurol 1999; 20(2): 155 – 156.
23. Rabb CH, McComb JG, Raffel C et al. Spinal arachnoid cysts in the pediatric age group: An association with neural tube defects. J Neurosurg 1992; 77(3): 369 – 372. doi: 10.3171/ jns.1992.77.3.0369.
24. Gelabert-González M, Cutrín-Prieto JM, García--Allut A. Spinal arachnoid cyst without neural tube defects. Childs Nerv Syst 2001; 17(3): 179 – 181. doi: 10.1007/ s003810000367.
25. Fortuna A, Mercuri S. Intradural spinal cysts. Acta Neurochir (Wien) 1983; 68(3 – 4): 289 – 314.
26. Rossier AB, Foo D, Shillito J et al. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain 1985; 108(2): 439 – 461.
27. Diyora B, Kamble H, Nayak N et al. Thoracic intramedullary arachnoid cyst. Neurol India 2010; 58(6): 964 – 966. doi: 10.4103/ 0028-3886.73774.
28. Tanghe HL. Magnetic resonance imaging (MRI) in syringomyelia. Acta Neurochirurgica 1995; 134(1 – 2): 93 – 99.
29. Barkovich AJ, Sherman JL, Citrin CM et al. MR of postoperative syringomyelia. AJNR 1987; 8(2): 319 – 327.
30. Jinkins JR, Reddy S, Leite CC et al. MR of parenchymal spinal cord signal change as a sign of active advancement in clinically progressive posttraumatic syringomyelia. Am J Neuroradiol 1998; 19(1): 177 – 182.
31. Timpone VM, Patel SH. MRI of a syrinx: is contrast material always necessary? AJR Am J Roentgenol 2015; 204(5): 1082 – 1085. doi: 10.2214/ AJR.14.13310.
32. Hofmann E, Warmuth-Metz M, Bendszus M et al. Phase-contrast MR imaging of the cervical CSF and spinal cord: volumetric motion analysis in patients with Chiari I malformation. AJNR Am J Neuroradiol 2000; 21(1): 151 – 158.
33. Koç K, Anık Y, Anık I et al. Chiari 1 malformation with syringomyelia: correlation of phase-contrast cine MR imaging and outcome. Turk Neurosurg 2007; 17(3): 183 – 192.
34. Smith HC, Savic G, Frankel HL et al. Corticospinal function studied over time following incomplete spinal cord injury. Spinal Cord 2000; 38(5): 292 – 300.
35. Masur H, Oberwittler C. SEPs and CNS magnetic stimulation in syringomyelia. Muscle Nerve 1993; 16(6): 681 – 682.
36. Restuccia D, Mauguière F. The contribution of median nerve SEPs in the functional assessment of the cervical spinal cord in syringomyelia. A study of 24 patients. Brain 1991; 114(1B): 361 – 379.
37. Morioka T, Kurita-Tashima S, Fujii K et al. Somatosensory and spinal evoked potentials in patients with cervical syringomyelia. Neurosurgery 1992; 30(2): 218 – 222.
38. Emery E, Hort-Legrand C, Hurth M et al. Correlations between clinical deficits, motor and sensory evoked potentials and radiologic aspects of MRI in malformative syringomyelia. 27 cases. Neurophysiol Clin 1998; 28(1): 56 – 72.
39. Roser F, Ebner FH, Liebsch M et al. A new concept in the electrophysiological evaluation of syringomyelia. J Neurosurg Spine 2008; 8(6): 517 – 523. doi: 10.3171/ SPI/ 2008/ 8/ 6/ 517
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek EditorialČlánek Treatment targeted for B lymphocytes – significant progress in the treatment of multiple sclerosisČlánek Possibilities of regulation of neuroimmune and neuroendocrine processes using physiotherapy
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2018 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Editorial
- Detection of unstable carotid plaque in ischemic stroke prevention
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - YES
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - NO
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - COMMENT
- Treatment targeted for B lymphocytes – significant progress in the treatment of multiple sclerosis
- Biomarkery progrese onemocnění a prognózy u pacientů s roztroušenou sklerózou
- Possibilities of regulation of neuroimmune and neuroendocrine processes using physiotherapy
- Parietal atrophy score on magnetic resonance imaging of the brain in normally aging people
- Imaging of peripheral nerves using diffusion tensor imaging and MR tractography
- The differences in clinical, radiological and treatment modalities of cervical intramedullary arachnoid cysts and cervical syringomyelia – report of 12 cases
- The relationship between tinnitus intensity and the degree of sensorineural hearing loss from the aspect of contribution of hyperbaric oxygen therapy
- Vzťah medzi intenzitou tinnitu a mierou senzorineurálnej straty sluchu z aspektu prínosu hyperbarickej oxygenoterapie
- Antiplatelet and anticoagulant therapy in carotid endarterectomies
- Early postoperative complications after elective degenerative lumbar spine surgery in elderly patients
- A comparison of efficacy of subcutaneous interferon β-1a 44 μg, dimethyl fumarate and fingolimod in the real-life clinical practise – a multicenter observational study
- A set of pictures with the opposite difficulty of naming
- Pharyngo-cervico-brachial variant of Guillain-Barré syndroma
- Orolingual bradykinin angioedema after tissue plasminogen activator in acute stroke – treatment with or without C1-esterase inhibitor
- Bilateral abducens nerve palsy after head and cervical spinal injury
- Late-onset Huntington’s disease – an overlooked diagnosis
- Analýza dat v neurologii - LXX. Kovariance
- Výroční setkání věnované novinkám v léčbě roztroušené sklerózy
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antiplatelet and anticoagulant therapy in carotid endarterectomies
- Bilateral abducens nerve palsy after head and cervical spinal injury
- Imaging of peripheral nerves using diffusion tensor imaging and MR tractography
- Late-onset Huntington’s disease – an overlooked diagnosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



