-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Relapsing-remitting Multiple Sclerosis and Oligoclonal Band Pattern During Disease Modifying Drug Therapy
Relabující-remitující roztroušená skleróza a oligoklonální pruhy v průběhu léčby modifikující průběh choroby
Cílem studie bylo vyhodnotit oligoklonální pásy (OCB) v mozkomíšním moku (CSF) u pacientů s relapsující remitující roztroušenou sklerózou (RRMS) léčených nemoc modifikujícími léky (DMD). Autoři vyšetřili skupinu 22 pacientů, z nichž bylo 5 mužů (ve věku 19–44 let, průměrný věk 29,8 ± 6,5 let) a 17 žen (ve věku 26–51 let, průměrný věk 37,8 ± 6,7 let). Vzorky CSF byly odebrány 0–42 měsíců před a 1–16 měsíců po zahájení DMD léčby. Počet OCB v CSF byl stanoven metodou izoelektrické fokusace. K vyhodnocení statistické významnosti byl použit párový t-test a Wilcoxonův jednovýběrový test. V pacientské skupině se počet OCB při sledování významně snížil (průměrný pokles byl 6,2, medián 3,5, p = 0,001, párový t-test). Tyto výsledky prokazují změny vzorců OCB, respektive podporují hypotézu o možném imunomodulačním účinku DMD léčby.
Klíčová slova:
oligoklonální pásy – mozkomíšní mok – interferon beta – roztroušená skleróza
Authors: J. Mareš 1; R. Herzig 1; K. Urbánek 1
; J. Podivínský 5; V. Bekárek 2; J. Sklenářová 2; J. Zapletalová 3; P. Hluštík 1; V. Sládková 1; D. Doležil 4; P. Kaňovský 1
Authors place of work: Department of Neurology, LF UP a FN Olomouc 1; Department of Clinical Biochemistry, LF UP a FN Olomouc 2; Department of Biometrics, LF UP a FN Olomouc 3; Department of Neurology, University Hospital Ostrava-Poruba 4; Neurology Geriatric Medical Institution, Moravský Beroun 5
Published in the journal: Cesk Slov Neurol N 2007; 70/103(6): 674-677
Category: Krátké sdělení
Summary
The aim of this study was to assess oligoclonal bands (OCB) in the cerebrospinal fluid (CSF) in patients with relapsing-remitting multiple sclerosis (RRMS) treated with disease modifying drug (DMD) therapy. The authors examined a group of 22 patients, 5 males (aged 19–44, mean 29.8 ± 6.5 years) and 17 females (aged 26–51, mean 37.8 ± 6.7 years). CSF samples were taken 0–42 months before and 1–16 months after the initiation of DMD therapy. The number of OCB in the CSF was assessed by isoelectric focusing. Paired sample t-test and Wilcoxon signed-rank test were applied when assessing statistical significance. In the patient group, the number of OCB at follow-up decreased significantly (mean decrease 6.2, median 3.5, p = 0.001, paired t-test). These results demonstrate changes in OCB patterns, respectively support the hypothesis about possible immunomodulation effect of DMD therapy.
Key words:
oligoclonal bands – cerebrospinal fluid – interferon beta – multiple sclerosisIntroduction
The detailed assessment of the CSF is currently a part of multiple sclerosis (MS) diagnostics. In all stages of this disease, we can find increased IgG levels in CSF which after correction for the function of the blood-brain barrier show its intrathecal synthesis. It is possible to demonstrate it as OCB. OCB represent immunoglobulin fractions participating in myelin destruction. OCB detection by isoelectric focusing is the most specific CSF test for MS [1] - it provides a significant support for MS diagnostics because they are not usually found in the serum of these patients and are therefore a proof of antibody production directly in central nervous system (CNS). OCB in the alkaline area are by CSF assessment positive (2 or more bands) in 95 - 100% of patients with MS [2, 3, 4]. In 40% of MS patients, OCB may be found in the serum as well.
OCB can be present even when the CSF IgG level is normal. Finally, these bands are not specific only for MS - they are also found in various inflammatory disorders as well as in chronic infections of the CNS, in acute disseminated encephalomyelitis [5], Guillain-Barré syndrome [6] or neurodegenerative dementia [7], vascular, toxic, metabolic, traumatic or psychiatric disorders, radicular syndromes or in most peripheral neuropathies [8]. The finding of oligoclonal bands in the CSF almost doubles the risk of develop in clinically definite MS [9]. OCB have a predictive value in the case of negative MRI but there is no direct correlation between OCB in the CSF and the demyelinating process as assessed by the MRI.
Therapy of RRMS with interferons leads to a significant decrease in the number of relapses and to a shortening of their duration as well to marked improvement of MRI findings. There are only limited data concerning immunological CSF findings during the interferon therapy in the current literature and changes of OCB patterns mostly have not been described [10].
The aim of the study, which has been realized at the Department of Neurology, University Hospital in Olomouc, Czech Republic, was to find out whether OCB number changes during the DMD therapy. We tried to investigate whether there are changes in the OCB patterns during the DMD therapy.
Subjects and Methods
The studied patient group (N = 22) consisted of 5 males (aged 19 - 44, mean 29.8 ± 6.5 years), and 17 females (aged 26 - 51 years, mean 37.8 ± 6.7 years). The diagnosis of RRMS was established based on the McDonald’s criteria [11].
The CSF was collected by a routine lumbar puncture as part of the standard diagnostic process prior to the start of the DMD therapy (0 - 42 months before, mean 13,5 + 8,2) and for a second time (after receiving informed consent) 1 - 16 (mean 6,2 + 2,1) months after the beginning of the DMD therapy. During the study, some patients were treated with immunosupressive therapy (Imuran p.o.) or chronic steroid therapy (Medrol p.o., Prednison p.o.). In the case of relaps was used bolus of steroids (5g Solu-Medrol i.v.) - see Table 1.
Tab. 1. Changes of OCB by patients with DMD and steroids/immunosupressive therapy. 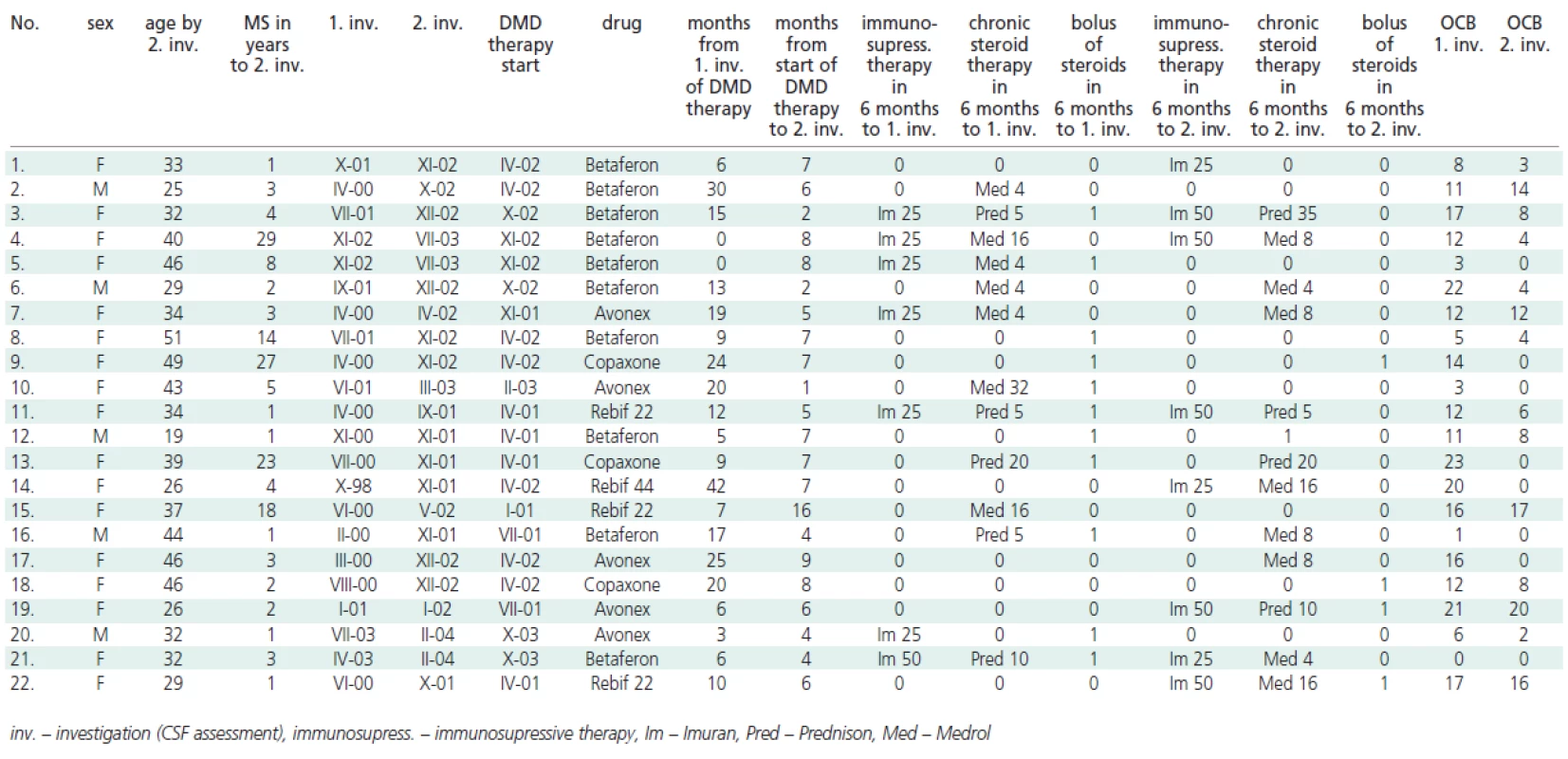
The method of Pharmacia Biotech modified for using of acrylamid gel PhastGel ICF 3-9 and by isoelectric focusing (IEF) with successive affinity immunoblot was used. The number of OCB in the CSF was assessed by the method of isoelectric focusing in a laboratory with the certificate KB/0079, which was blinded to the aim of this study and to the patient's therapy.
Paired-sample t-test (parametric) and Wilcoxon signed-ranks test (nonparametric) were applied when assessing statistical significance, using SPSS-10 software package (SPSS, Chicago, USA).
The whole study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 1983) and it was approved by local ethics committee of University Hospital in Olomouc, Czech Republic.
Results
The numbers of OCB before immunomodulation treatment and after the initiation of DMD therapy are summarized in Table 1 and Figure 1.
Figure 1. Changes of OCB from between the 1<sup>st</sup> and 2<sup>nd</sup> examinations. 
Only OCB bands present in the CSF and absent in serum were considered. In 18 patients, a reduction in the number of OCB was observed (mean 7.8 ± 5.9), in 2 patients the number of OCB increased by 1 and 3 (mean 2 ± 1) and in 2 patients there were no changes in the number of OCB. In several patients, a regression from 8 to 1 OCB was observed. Overall, the number of OCB between 1st and 2nd examination at follow up decreased significantly (mean 6.2, median 3.5; p = 0.001, paired t-test), see Figure 1.
Considering that the distribution of OCB counts is asymmetrical with lower bound of 0, statistical significance was confirmed using a nonparametric Wilcoxon signed-ranks test (p = 0.0004). Sensitivity for MS prediction was 90.9, specificity 76.1 and negative predictive value 99,3.
Discussion
OCB represent the set of antibodies against yet unknown antigens of the CNS, probably of "nonsense" nature. The dynamics of the appearance and the development of OCB during the course of MS is not generally known yet [12]. In the study by Kaiser et al. [13], only two cases (1%) out of the 185 CSF samples obtained from patients with MS demonstrated the specificity of OCB antibodies against known CNS antigens. Rudick et al. [14] did not observe any changes in the IgG index, light kappa chains nor OCB patterns in 137 repeated samples of CSF before and 2 years after the beginning of Avonex therapy. Administration of interferon beta intrathecally (i.t.) for duration of 2 months did not lead in study by Confavreux et al. [15] in 11 patients after 6 months to OCB change. On the contrary, a characteristic individual "fingerprint" of the OCB in the CSF was preserved, allowing recognition of the patient to whom the CSF sample belonged. Saiz et al. made similar observations in MS patients who underwent an autologous hematopoietic stem cell transplantation - the baseline CSF OCB persisted for 1 year following transplantation [16]. Kinnunen et al. [17] have found that in 3 out of the 6 patients with progressive MS, alfa-interferon therapy lead to increased i.t. synthesis and production of OCB.
Other studies [18-20] described the changes of OCB during the steroid and Cladribin therapy where the changes observed in the CSF banding pattern were not significant.
On the basis of the above-mentioned findings, it is possible to note that during DMD therapy, the number of OCB in the CSF may change – in our group of patients we found a tendency to decrease or disappeareance of OCB – similar findings was desribed in study of Andersonn et al. [14] who found disappeareance of OCB during methylprednisolone treatment of MS patients in 3 patients out of the 45. The disappearance of OCB was described also by patients with other autoimunne neurological diseases – in study of Bergamaschi et al. [21] OCB have been found in 3 of 11 patients with Devic’s neuromyelitis optica and by repeated CSF assessment always disappeared. The results in our study showed significantly decreased numbers of OCB, but this study has some limitations: firstly, the group of patients was relative small with absence of control group (patients with RRMS without DMD therapy constitute an ethic probleme). Secondly, the possible influences of bolus of steroids in relaps of MS and immunosupressive therapy – can accelerate the supression of inflammatory process and oligoclonal syntesis. Thirdly, the pathogenesis of OCB in MS is still obscure and the final picture of oligoclonal syntesis is based on manifestation the different participations both of T-cells and B-cells on autoimmune response with a variability in intrathecal syntesis. The emphasis on T cells has derived from the detection of activated T cells in MS plaques. Currently the role of B cells, plasma cells and immunoglobulins in MS have been re-examined, and findings indicate that humoral immunity also plays a major role in MS pathogenesis. According to Coreale et al.[3], B cells and their products could exert several potential effects during the course of MS - autoantibodies against specific myelin antigens could mediate damage to myelin membranes, some studies suggest that natural autoantibodies could enhance remyelination, antibodies directed against myelin components can participate in anti-idiotypic networks, which may regulate the course of MS. Therefore, a critical task is to clearly desribe this issue and follow-up studies are necessary considering the patophysiological aspects of OCB changes in MS.
Přijato k recenzi: 19. 12. 2007
Přijato do tisku: 10. 5. 2007
MUDr. Jan Mareš
Centrum pro diagnostiku a léčbu demyelinizačních onemocnění
Neurologická klinika LF UP a FN
I. P. Pavlova 6, 775 20 Olomouc
e-mail: maresja@seznam.cz
Zdroje
1. Andersson M, Alvarez-Carmeno J, Bernardi G, Cogato I, Fredman P, Frederiksen J et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J Neurol Neurosurg Psychiatry 1994; 57 : 897-902.
2. Caudie C, Allauzen O, Bancel J, Later R. Diagnostic usefullness of oligoclonal immunoglobulin G bands in cerebro-spinal fluid using isoelectric focusing in early diagnosis of multiple sclerosis. Annales de Biologie Clinique 2000; 2(58): 187-193.
3. Correale J, de los Milagros Bassani Molinas M. Oligoclonal bands and antibody responses in multiple sclerosis. J Neurol 2002; 249 : 375-389.
4. Miller JR, Burke AM, Bever CT. Occurence of oligoclonal bands in multiple sclerosis and other CNS diseases. Ann Neurol 1983; 13(1): 53-58.
5. Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG.
Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 2000; 123 : 2407-2422.
6. Kruger H, Englert D, Pflughaupt KW. Demonstration of oligoclonal immunoglobulin G in Guillain-Barre syndrome and lymphocytic meningoradiculitis by isoelectric focusing. J Neurol 1981; 226(1): 15-24.
7. Janssen JC. The prevalence of oligoclonal bands in the CSF of patients with primary neurodegenerative dementia. J Neurol 2004; 251(2): 184-188.
8. Sindic CHJM, Van Antwerpen MP, Goffette S. The Intrathecal Humoral Immune Response: Laboratory Analysis and Clinical Relevance. Clin Chem Lab Med 2001; 39(4): 333–340.
9. Barry A, Singer MD. Diagnosis and Monitoring of Multiple Sclerosis: Focus on Cerebrospinal Fluid Analysis and Brain Imaging. [online] Ectrims 2006. Dostupné z URL: http://doctor.medscape.com/viearticle/548062
10. Rudick RA, Cookfair DL, Simonian NA, Ransohoff RM, Richert JR, Jacobs LD el al. Cerebrospinal fluid abnormalities in a phase III trial of Avonex (IFNbeta-1a) for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. J Neuroimmunol 1999; 93(1-2): 8-14.
11. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50 : 121-179.
12. Antel JP, Birnbaum G, Hartung HP. Clinical neuroimunology. London: Blackwell Science 1998.
13. Kaiser R, Obert M, Kaufmann R, Czygan M. IgG-antibodies to CNS proteins in patients with multiple sclerosis. Eur J Med Res 1997; 2(4): 169-172.
14. Anderson TJ, Donaldson IM, Sheat JM, George PM. Methylprednisolone in multiple sclerosis exacerbation: changes in CSF parameters. Aust N Z J Med 1990; 20(6): 794-797.
15. Confavreux C, Chapuis-Cellier C, Arnaud P, Robert O, Aimard G, Devic M. (1986) Oligoclonal "fingerprint“ of CSF IgG in multiple sclerosis patients is not modified following intrathecal administration of natural beta-interferon. J Neurol Neurosurg Psychiatry 1986; 49(11): 1308-1312.
16. Saiz A, Carreras E, Berenguer J, Yague J, Martinez C, Marin P et al. MRI and CSF oligoclonal bands after autologous hematopoietic stem cell transplantation in MS. Neurology 2001; 56(8):1084-1089.
17. Kinnunen E, Timonen T, Pirttila T, Kalliomaki P, Ketonen L, Matikainen E et al. Effects of recombinant alpha-2b-interferon therapy in patients with progressive MS. Acta Neurol Scand 1993; 87(6): 457-460.
18. Caputo D, Zaffaroni M, Ghezzi A, Cazzullo CL. Azathioprine reduces intrathecal IgG synthesis in multiple sclerosis. Acta Neurol Scand 1987; 75(2): 84-86.
19. Durelli L, Cocito D, Riccio A, Barile C, Bergamasco B, Baggio GF et al. High-dose intravenous methyprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology 1986; 36(2): 238-243.
20. Sipe JC, Romine JS, Koziol JA, McMillan R, Zyroff J, Beutler E. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet 1994; 344 : 9-13.
21. Bergamaschi R, Tonietti S, Franciotta D, Candeloro E, Tavazzi E, Piccolo G et al. Oligoclonal bands in Devic’s neuromyelitis optica and multiple sclerosis: differences in repeated cerebrospinal fluid examinations. Multiple Sclerosis 2004; 10(1): 2-4.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2007 Číslo 6- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Facial Palsy
- Electrophysiological Examination of the Facial Nerve
- Antibodies Against Glycoconjugates in the Diagnosis of Autoimmune Neuropathies
- 5 Years of Activities of the National Reference Laboratory for Human Prion Diseases Attached to the Department of Pathology and Molecular Medicine of FTNsP: Our Experience and an Overview of Literature
- A Clinical Approach to Computed Tomography in Acute Cerebral Ischemia
- Ultrasound Evaluation of Substantia Nigra in Patients with Parkinsonian Syndromes
- Comparison of Results of Measurement of Visual Evoked Potentials in Patients with Multiple Sclerosis and Neuroborreliosis
- Cognitive Evoked Potentials – the P300 Wave in Patients with Sclerosis Multiplex: Relation to the Form of the Disease, Somatic Affection and Quality of Life
- Relapsing-remitting Multiple Sclerosis and Oligoclonal Band Pattern During Disease Modifying Drug Therapy
- Cerebral Venous Thrombosis in the Users of Hormonal Contraceptives
- Successful Use of a Single Question in Restless Legs Syndrome Screening in the Czech Republic
- Does the Development of Urinary Dysfunction in Multiple Sclerosis Depend on the Type of Neurological Treatment?
- Swallowing Disorders Related to Vertebrogenic Dysfunctions
- Central Neurocytoma: Case Report and Review of the Literature
- Gelastic Seizures in Hypothalamic Hamartoma: a Case Study
- Dercum’s Disease (Lipomatosis Dolorosa) – a Rarely Diagnosed Disease: a Case Study
- Post-traumatic Olfactory Disorders: Case Studies
- K šedesátinám doc. MUDr. Martina Bojara, CSc.
-
Směruje použití mediánu ke statistickým neparametrickým postupům zpracování dat?
Okénko statistika – Analýza dat v neurologii – III.
Nebojme se mediánu a robustních statistik - Webové okénko
-
Analýza dat v neurologii VI.
Přesnost, spolehlivost a reprodukovatelnost měření u diskrétních dat
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Facial Palsy
- Swallowing Disorders Related to Vertebrogenic Dysfunctions
- Antibodies Against Glycoconjugates in the Diagnosis of Autoimmune Neuropathies
- Dercum’s Disease (Lipomatosis Dolorosa) – a Rarely Diagnosed Disease: a Case Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



