-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaARTERIALIZED VENOUS FREE FLAPS – A RECONSTRUCTIVE ALTERNATIVE FOR LARGE DORSAL DIGITAL DEFECTS
Autoři: P. Hýža 1; J. Veselý 1; P. Novák 1; I. Stupka 1; J. Sekáč 2; U. Choudry 3
Působiště autorů: Clinic of Plastic and Aesthetic Surgery, St. Anna University Hospital, Brno, Czech Republic 1; Department of Orthopaedics and Traumatology, Medical Faculty, University Hospital, Košice, Slovak Republic, and 2; Division of Plastic Surgery, Department of Surgery, University of Minnesota, Minneapolis, USA 3
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 50, 2, 2008, pp. 43-50
INTRODUCTION
Reconstruction of large dorsal digital defects is simple if the peritenon is preserved. These defects can be easily reconstructed with skin grafts. In the presence of exposed bone, joint and/or tendon, however, a flap becomes necessary. A variety of local or regional flaps are available to cover small defects in the dorsal digital region. The difficulty arises for larger defects or in cases of major regional injury. In these instances, distant pedicled flaps or conventional free flaps are required. These flaps are usually bulky and are not optimal for the area. Recently, venous free flaps have been employed because of their thin and supple characteristics that are ideally suited for the dorsal digital region.
Nakayama described the first venous free flap in 1981 (32) as an experimental flap. Subsequently, Yoshimura described the first clinical use of this flap in the reconstruction of finger defects (58). In 1991, Chen et al. (8) divided venous flaps into four distinct types, which have recently been modified to include an additional fifth type (Table 1) (28, 35, 52, 57). In 1993, Thatte and Thatte similarly categorized venous free flaps into three types (42, 43). Both classifications are based on the description of the vessels that enter and leave the flap.
Tab. 1. Classification of venous free flaps (8) 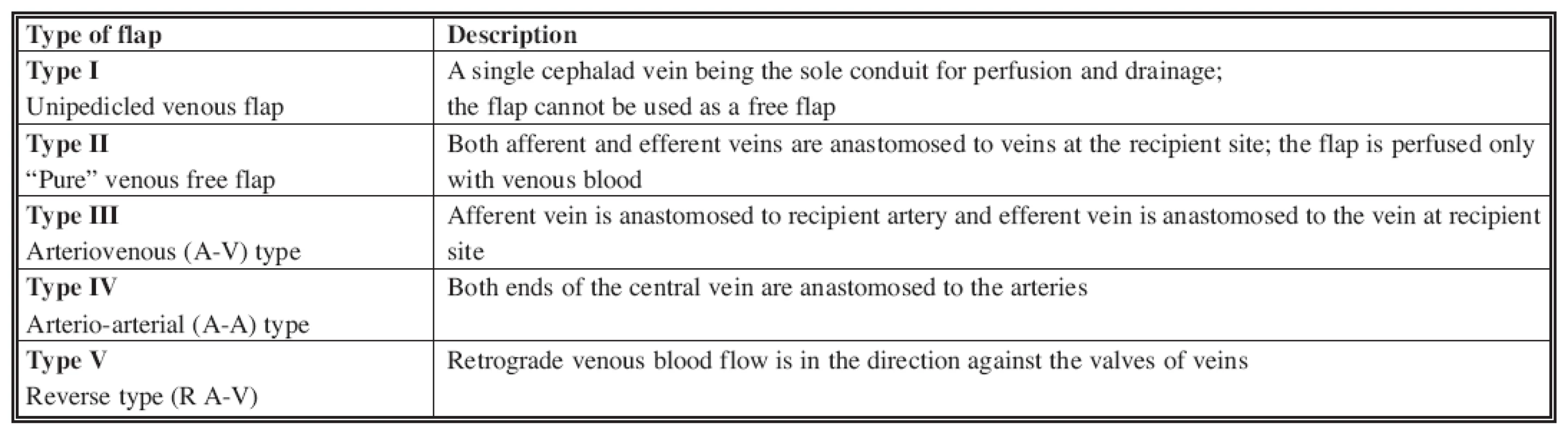
The flow of blood through a flap is secondary to the pressure gradient between the inflow (arterial) and outflow (venous) vessels. The same concept is true in venous flaps; the difference being that both ends are venous. The blood flow is established either between two ends of a central vein or between two veins interconnected through a venous network. There are many examples of venous free flaps having been used for different reconstructive indications. They are technically easy to harvest and have low donor site morbidity (4, 19, 21, 25, 31, 44, 57). The arterialization of the venous network has also proved to be useful in cases of replantation (3, 9, 27, 41, 45, 47,48) where conventional arterial anastomosis is not possible, as well as in the salvage of cutaneous free flaps (38).
There are several potential donor sites for venous free flaps. Flaps can be harvested from the volar forearm based on the cephalic or basilic veins. On the lower extremity, saphenous venous flap and dorsalis pedis venous flaps have been described (12, 23, 43). Glabrous, thin and durable skin for finger pulp reconstruction can be obtained from the thenar and hypothenar regions of the hand (26, 20).
MATERIAL AND METHODS
A retrospective chart review from January 1993 to January 2007 was conducted. A total of 13 arterialized venous free flaps were utilized in 12 patients. The general indication for using this flap was a large dorsal digital defect(s) with exposed bone, joint and/or extensor tendon and the unavailability or inadequacy of local flaps. We describe our surgical technique, complications and outcome, types of venous flaps used, and review the published literature on this useful free flap.
Surgical Technique
A tourniquet is inflated over the upper arm without draining the limb. It is inflated just sufficiently to overcome venous blood pressure and highlight the subcutaneous veins. The appropriately dimensioned flap is then marked either on the proximal forearm centered over a central vein (usually cephalic or basilic vein), or on the distal forearm centered over a venous network. If the flap is harvested distally on the limb, usually a smaller caliber vein is used for inflow and at least two veins are used for outflow. The dense venous network is left in the central part of the flap. The tourniquet is then deflated, the arm exsanguinated and then re-inflated to ensure a bloodless field. A superficial incision is then made around the marked out flap and the veins are meticulously dissected out to at least a 1 cm to 2 cm length. The flap is then harvested through the suprafascial plane. The “inflow vessel” is then anastomosed in a retrograde fashion with the distal part of the digital artery. The “outflow vessel(s)” are then anastomosed to one or more dorsal digital veins. Then the flap is sutured loosely with interrupted stitches in order to prevent flap constriction from significant postoperative edema associated with these free flaps.
RESULTS
A total of 13 venous free flaps were utilized in 12 patients. Five (38%) flaps were harvested from the proximal forearm, based on the central cephalic or basilica vein. Eight (62%) flaps were designed distally on the forearm, based over a venous network. In one case, the flap was used as a bilobed arterialized venous flap for two separate defects of the middle finger. In another case, two separate venous free flaps were used in the reconstruction of dorsal defects of two fingers (Table 2). Eleven flaps in 10 cases (85%) were used within 24 hours of the injury, while 2 flaps (15%) were done within 7 days of the injury. The total flap survival rate (complete flap survival, n=10) was 77% (see Table 2). Three flaps (23%) had partial necrosis of the skin. They required debridement of the necrotic tissue and were salvaged with skin grafts. There was no complete flap loss, thus the overall flap survival rate was 100%. In 6 patients (7 flaps – 54%), superficial ischemic bulae developed between the second and fourth post-operative day. The bulae resolved spontaneously without any detrimental effects to the flaps. We also noticed that temporary hyperemia developed in most of the flaps. The hyperemia was stronger in the “inflow part” of the flap and gradually diminished towards the “outflow part”. The ratio of number of veins used for inflow versus outflow was 1 : 1 (or 2 : 2) in 6 flaps (46%), 1 : 2 in 4 flaps (31%), and 2 : 1 in 3 flaps (23%). The donor sites were closed primarily and healed uneventfully in all cases.
Tab. 2. Overview of the cases treated from 1993 till 2007 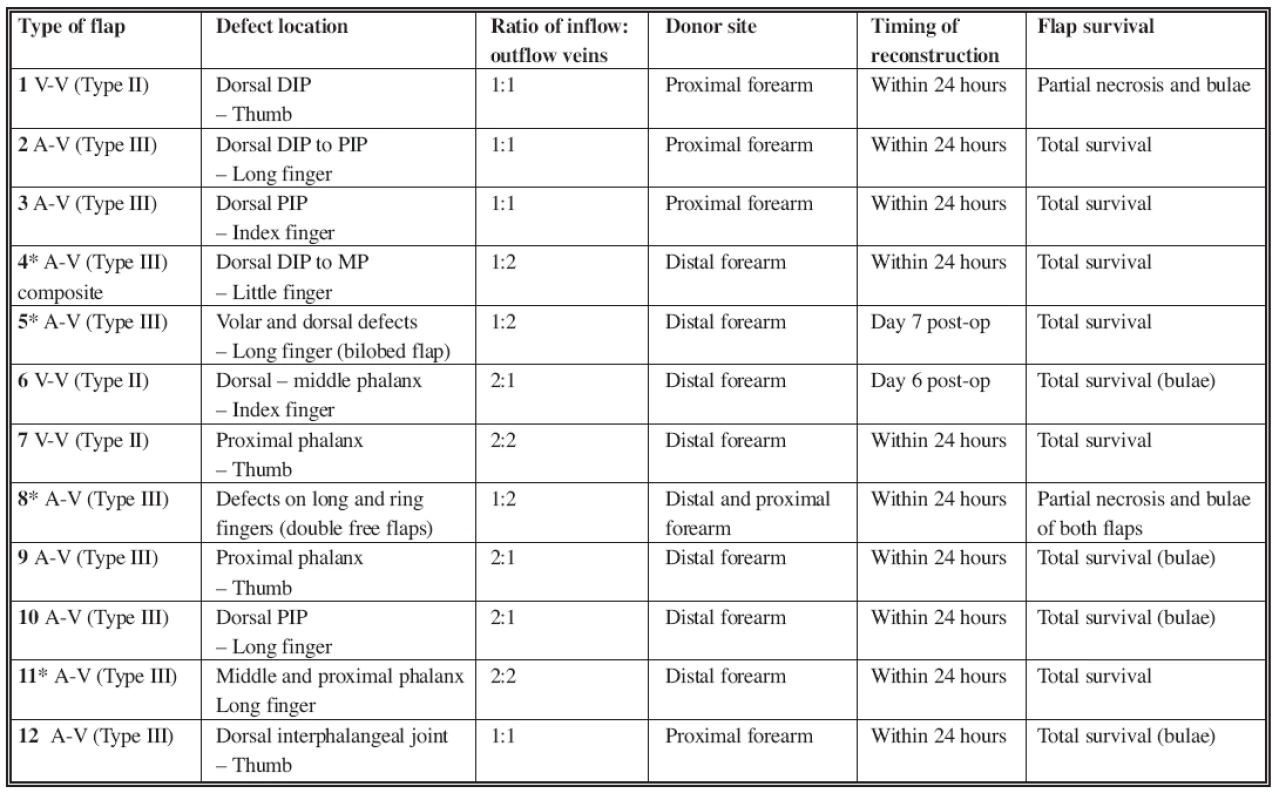
DIP: Distal Interphalangeal Joint; PIP: Proximal Interphalangeal Joint *Cases described in the text
Case 1
A 42 year-old, right-hand dominant male presented acutely with an avulsion injury to the dorsum of his right fifth finger sustained in a motorcycle accident (Fig. 1a). He was an active smoker. The defect involved the loss of the extensor tendon with exposure of the proximal phalanx and proximal interphalangeal (PIP) joint. X-rays did not show any fracture. After careful debridement, we utilized an arterialized tendo-cutaneous venous free flap from the volar aspect of the distal forearm (Fig. 1b). The palmaris longus tendon was incorporated into the skin paddle of the flap, and was used to reconstruct the extensor tendon of the finger. The afferent vein of the flap (inflow vessel) was anastomosed to the distal stump of the radial digital artery of the little finger. The two efferent veins of the flap (outflow vessels) were anastomosed to the dorsal digital veins. The donor site was closed primarily. After initial hyperemia (Fig. 1c), the flap healed without necrosis or bulae formation. The functional result was satisfactory, with 80-degrees of the flexion of the PIP joint and 30-degrees extensor lag. The patient refused to wear his night extension splint to improve finger extension and was happy with his result (Fig. 1d).
Obr. 1. a. Defect with exposure of the proximal interphalangeal joint and loss of extensor tendon b. Design of the venous flap on the distal forearm c. Venous flap 2nd day post-op. Please note the degree of hyperemia is higher on the inflow (distal) part of the flap d. Flap completely healed 1 month post-op 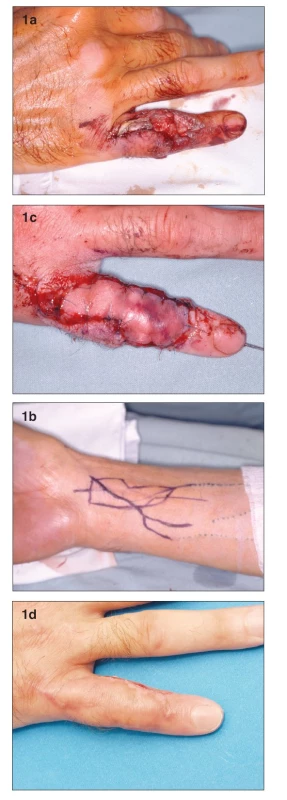
Case 2
A 19-year old female presented with multiple finger contusions of her left hand after it got caught in a kneading machine. Debridement was performed four days later. She had small tissue defects over the dorsum of her index and ring fingers, which were covered by local flaps. The largest defect was on the volar and dorsal aspect of her long finger. The volar defect was at the PIP joint level and measured 2 cm in largest diameter, with exposed flexor tendons. The dorsal defect was 1 cm wide and involved the entire length of the dorsal aspect of the proximal phalanx, with exposure of the extensor tendon. On post-injury day 7, a two-island (bilobed) venous free flap from the volar aspect of the left forearm was used to cover both defects (Fig. 2a). The donor site was closed primarily. One inflow vessel and two outflow vessels were used. During the postoperative course, significant venous congestion developed in the volar skin flap, which was treated expectantly. No necrosis or bulae formation resulted. The functional result was excellent, with full range of motion restored at the PIP joint (7) (Fig. 2b, c).
Obr. 2. a. Bilobed arterialized venous flap. Flap harvested on the distal volar forearm (7) A– Dorsal skin paddle. B – Volar skin paddle. C – subcutaneous bridge between the two skin paddles b. Early post-operative result at 1 month. A – Dorsal skin paddle c. Early post-operative result at 1 month. B – Volar skin paddl 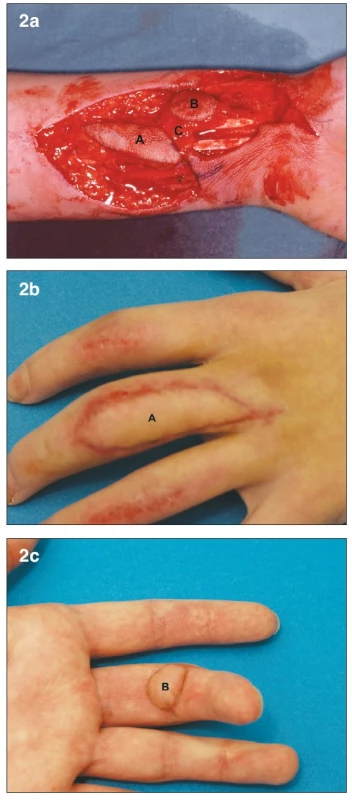
Case 3
A 53-year old male presented acutely after an avulsion injury from a planer machine to his left hand. This patient was a manual laborer by profession and smoked heavily. He had dorsal lacerations of the distal phalanges of his index and little fingers, and dorsal avulsion injuries of the middle and distal phalanges of his long and ring fingers. The soft tissue loss on both fingers involved skin and dorsal extensor tendon, with exposure of bone and PIP joints. Due to injury of the adjacent fingers, cross-finger flaps were not available. We decided to use two venous free flaps, one for each digit. The flaps were designed on the volar side of the left forearm (Fig. 3a). Each flap was based on one afferent vein and two efferent veins. The donor site was closed primarily. The digital arteries and dorsal digital veins were used as recipient vessels. On post-operative day 3, severe venous congestion was apparent in both flaps (Fig. 3b). Although venous congestion is commonly seen in venous flaps, we decided to explore the anastomoses due to the severity of the congestion. All the anastomoses were patent, except for the “arterial” anastomosis of the ring finger flap, which was revised. Despite this, superficial desquamation and partial flap necrosis resulted in both flaps. Conservative wound therapy was instituted for this complication. The patient began physiotherapy three weeks after the surgery. Five weeks later we debrided the necrosed skin, and skin-grafted the resulting defects. The patient returned to work four months after the surgery with satisfactory hand function. He was later lost to follow-up.
Obr. 3. a. Design of both of the free flaps on the distal volar forearm b. Postoperative swelling and bulae on both flaps on the 3rd day post-op 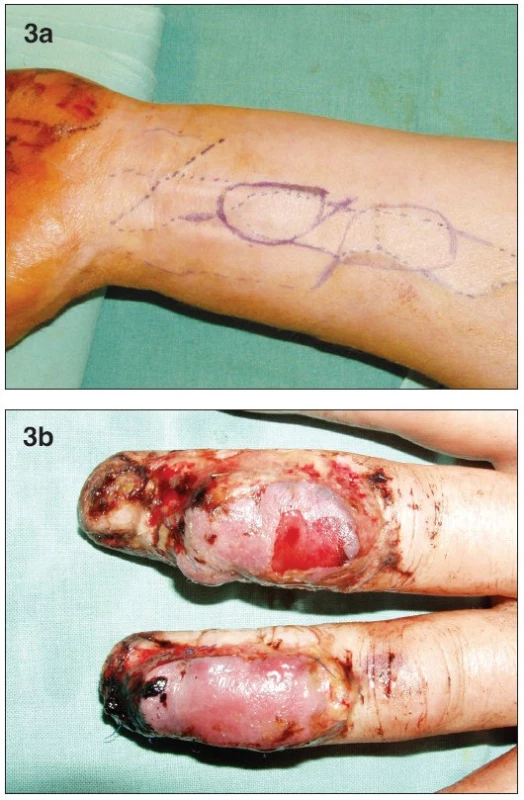
DISCUSSION
Venous free flaps have evoked much debate in the literature. The main concerns are the unconventional blood flow, the high incidence of venous congestion, and the unreliable survival rates of these flaps. They do have certain advantages over conventional flaps. They can be harvested quickly and very thin. They do not require the need to sacrifice a major artery of the limb and the donor site morbidity is very minimal. Nonetheless, venous free flaps have not been widely used for reconstruction.
The physiology of the circulation in venous flaps is still not completely understood. Retrograde blood flow through the venous network, early neovascularization, and plasma imbibition are some theories behind flap survival (2, 18). The type of venous flap used also seems to have an impact on survival. Wolff et al. investigated this by isotope perfusion and thermography on a rat model. The average area of flap survival was 93% for type III, 69% for type II and only 30% for type I flaps (49). The microcirculation and survival of type II (pure venous) flaps has been investigated by direct capillaroscopy (55). During the initial 72 hours, solely the venous blood nourishes the flaps; this was termed the “venous nourishing stage”. The “arterial nourishing stage and vascular reconstructive stage” follows from 72 hours to 6 weeks. During this period, neovascularization of the flap margins takes place, which takes over the blood supply of the flap from the 4th day. The stimuli for neovascularization seem to be the low perfusion of the flap and hypoxia. By the 7th day, the flaps are seen to have normal antegrade blood flow and velocity in the pre-capillary, capillary and post-capillary vessels.
The survival of type III (arterialized) venous flaps has been shown to be most likely dependent on the richness of the venous network and blood circulation (39, 40, 50). Similarly, the importance of neovascularization has been established (36). The inflow vein (arterial end) goes through progressive arterialization. The vascular wall thickens and the lumen of the vessel narrows due to proliferation of smooth-muscle cells and elastic fibers of the media (50, 34). Exfoliation of the intima produces spots in the vascular wall, which serve as niduses for formation of new vessels – neovascularization. Moreover, neovascularization may also originate from vasa-vasorum (6, 33, 42). Neovascularization is promoted by ischemic conditions in the tissue and can be demonstrated by histological examination after four weeks (36, 50). A higher tendency of veins to form neo-vessels and re-canalize was shown compared to arteries in an experiment at eight weeks after the separation and ligature of the vessels from their bed (6).
Isotope perfusion studies have shown that there is not a remarkable difference in the perfusion between type II and type III venous flaps. However, studies have shown that arterialized flaps (type III) have better oxygenation. Moreover, pressure gradients are higher and venous thromboses are probably less frequent in arterialized flaps than in pure venous flaps (49). It has also been shown that survival of arterialized venous free flaps depends more on the number of veins that drain the flap than on the inflow (13, 37, 50, 52). With greater number of draining veins, venous channels are wider, deoxygenated hemoglobin does not accumulate and oxygen utilization is superior (37). However, we did not see this in our study. In our opinion, the number of draining veins is not as important as the diameter of the draining veins (Poisseulle’s Law). In addition, the location of the veins inside the flap is, in our opinion, very significant. The arterialized vein is able to vascularize about 1 cm of its surrounding tissue and if properly placed, the blood supply of the flap is made superior. The more veins inside the flap, with a rich venous network, might then sustain better blood supply than a single large-caliber vein.
In our study, we did not utilize venous flaps types I (1, 14, 29), IV and V (see Table 2). The overall complete flap survival rate in the literature is about 74% (Table 3). This includes all those flaps that had no partial necrosis. Our rate (77%) is comparable to published literature. Improved venous flap survival has been shown by using surgical or chemical delays and/or by pre-expansion of the flaps (5, 10, 11, 12, 30, 52) (Table 4). These methods were not used in our cases. Increased flap survival rates may be also achieved by pre-arterialization (i.e. arteriovenous fistula formation at the pedicle) for at least two weeks before the flap harvest (53, 54). In two to three weeks after arterialization, the venous system dilates, the valves are made incompetent and retrograde vascular resistance decreases. Despite evidence of improved flap survival following pre-arterialization, venous flaps have still not reached the level of reliability of conventional free flaps (11, 54, 46).
Tab. 3. The venous flap survival in literature 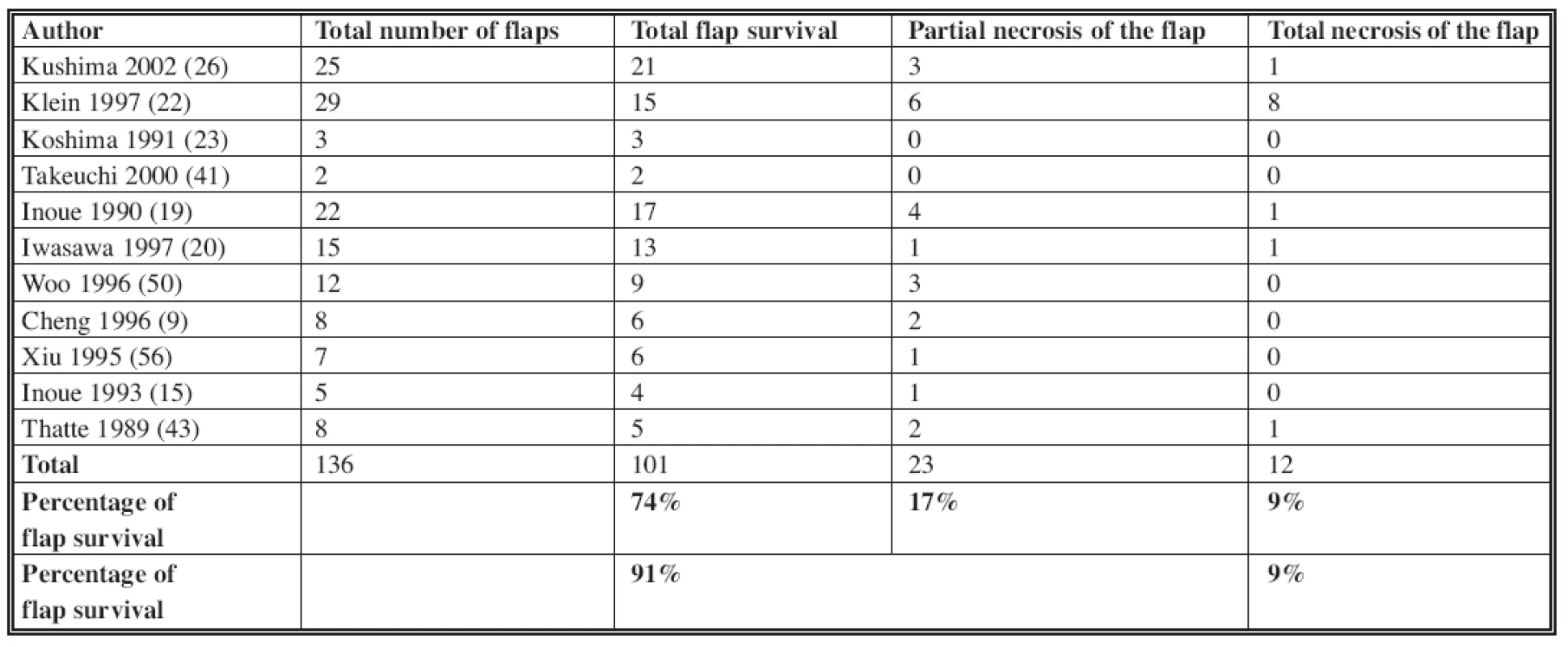
Tab. 4. The venous flap survival after delay phenomenon in literature 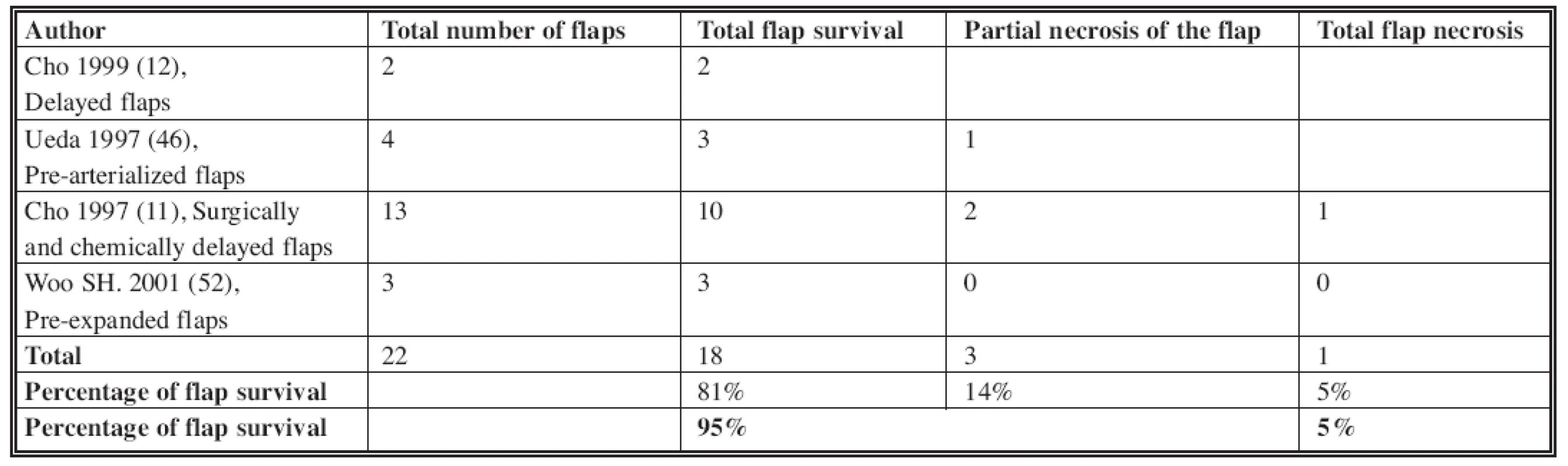
In our experience, we found flaps harvested from the distal third of the forearm took good advantage of the abundant venous network that exists there. In addition, the vessels are about 1x1.5 mm in diameter, a good size-match with the digital vessels. The retrograde flow from the distal part of the finger proximally (reverse blood flow) in the recipient digital artery is also advantageous. This may reduce the typical phenomenon of venous congestion of the flap by lowering the arterial pressure at the inflow and increasing the diameter of the veins for the outflow. We feel that one should not be too concerned about the postoperative swelling and venous congestion, which is commonly seen during healing of venous flaps. This should be taken into consideration at the time of flap planning and the flap should be designed a little larger to fit into the defect loosely, and should be sutured loosely. Additionally, the flap should be monitored with hourly acoustic Doppler checks of the recipient vein, rather than by monitoring capillary refill and color of the flap because of the same reason.
The presented clinical cases show that venous flaps are particularly useful in specific situations. In cases where there is deficient extensor tendon, the palmaris longus tendon can be included in the flap. However, the result of this type of reconstruction is still far from optimal. Similar functional result might be reached by including the deep forearm fascia, which is a flat structure more similar to the extensor tendon aponeurosis (12, 16, 17). The bilobed arterialized venous free flap (7) may be particularly useful if two separate defects of a digit are present. This procedure allows for simultaneous reconstruction of both defects using only one free flap and three venous anastomoses (7). In cases of complex hand injuries or multiple digital defects, where local tissue is insufficient for reconstruction, venous free flaps may be ideal options. In general, they are more comfortable for the patient than distant pedicled flaps. Moreover, venous flaps from the volar side of the forearm have excellent color, thickness and texture match with the dorsal digital skin.
CONCLUSION
Venous free flaps are versatile, easy to perform reconstructive options for large defects of the digits, particularly in complex hand injuries and multiple finger defects. The tendo-cutaneous venous free flap, the bilobed venous free flap and double venous free flap transfers are some of the different ways in which they can be used to reconstruct difficult defects. Some of the advantages of using these flaps are the preservation of major extremity arteries during harvest, very minimal donor site morbidity, and excellent match to the dorsal digital skin in color, thickness and texture. They may also serve as a lifeboat in conventional free flap failures due to arterial problems. However, these flaps are less reliable from a survival standpoint and do suffer from venous congestion. These disadvantages make them second choice flaps in reconstruction of dorsal defects of the fingers. Nevertheless, venous free flaps should always be considered in a reconstructive surgeon’s armamentarium.
Address for correspondence:
Petr Hýža, M.D.
Clinic of Plastic and Aesthetic Surgery,
St. Anna University Hospital
Berkova 34
612 00 Brno
Czech Republic
E-mail: petrhyza@hotmail.com
Zdroje
1. Baek SM, Weinberg H., Song Y., Park CG, Biller HF. Experimental studies in the survival of venous island flaps without arterial inflow. Plast. Reconstr. Surg., 75, 1985, p. 88.
2. Fukui A., Maeda M., Mine T., Tamai S., Inada Y. Proof of plasmatic imbibition by using enzymatic study. Jpn. J. Plast. Reconstr. Surg., 34, 1991, p. 521.
3. Fukui A., Maeda M., Inada Y., Tamai S., Sempuku T. Arteriovenous shunt in digit replantation. J. Hand. Surg., 15A, 1990, p. 160.
4. Fukui A., Inada Y., Maeda M., Tamai S., Mizumoto S., Yajima H., Sempuku T. Pedicled and flow through venous flaps: clinical applications. J. Reconstr. Microsurg., 5, 1989, p. 235.
5. Fulsi A., Inada Y., Murata K., Ueda Y., Tamai S. A method for prevention of arterialized venous flap necrosis. J. Reconstr. Microsurg., 14, 1998, p. 67-74.
6. Hýža P., Ponížilová J. Changes in ligated vessels after 8 weeks – an experimental study. Skripta Medica (Brno), 71(8), 1998, p. 423-437.
7. Hyza P., Vesely J., Stupka I., Cigna E., Monni N. The bilobed arterialized venous free flap for reconstruction for 2 simultaneous defects of a digit. Ann. Plast. Surg., 55(6), 2005, p. 679-683.
8. Chen HC., Tang YB., Nordhoff MS. Four types of venous flaps for wound coverage: a clinical appraisal. J. Trauma, 31(9), 1991, p. 1286-1293.
9. Cheng SL., Wong SS. Salvage of superficial palmar avulsion. J. Trauma, 40(1), 1996, p. 22.
10. Cho BC., Lee MS., Lee LK., Byun JS., Baik BS. The effects of surgical and chemical delay procedures on the survival of arterialized venous flaps in rabbits. Plast. Reconstr. Surg., 102, 1998, p. 1134-1143.
11. Cho BC., Lee JH., Byun JS., Baik BS. Clinical applications of the delayed arterialized venous flap. Ann. Plast. Surg., 39(2), 1997, p. 145.
12. Cho BC., Byun JS., Baik BS. Dorsalis pedis tendocutaneous delayed arterialized venous flap in hand reconstruction. Plast. Reconstr. Surg., 104, 1999, p. 2138.
13. Inada Y., Fukui A., Tamai S., Mizumoto S. The arterialized venous flap: experimental studies and clinical case. Br. J. Plast. Surg., 46, 1993, p. 61.
14. Inada Y., Fukui A., Tamai S., Kahikana T., Maeda M. The sliding venous flap for covering skin defects with poor blood supply on the lateral aspects of fingers. Br. J. Plast. Surg., 44, 1991, p. 368.
15. Inoue G., Suzuki K. Arterialized venous flap for treating multiple skin defects of the hand. Plast. Reconstr. Surg., 91(2), 1993, p. 299, discussion p. 303.
16. Inoue G., Tamura Y., Suzuki K. One-stage repair of skin and tendon digital defects using the arterialized venous flap with palmaris longus tendon: an additional four cases. J. Reconstr. Microsurg., 12(2), 1996, p. 93-97.
17. Inoue G., Tamura Y. One-stage repair of both skin and tendon digital defects using the arterialized venous flap with palmaris longus tendon. J. Reconstr. Microsurg., 7(4), 1991, p. 339.
18. Inoue G., Nakamura R., Maeda M., Suzuki K. Arterialized venous flap coverage of a big toe defect resulting from a wrap around flap transfer. Jpn. J. Reconstr. Surg., 32, 1989, p. 1013.
19. Inoue G., Maeda N., Suzuki K. Resurfacing of skin defects of the hand using the arterialized venous flap. Br. J. Plast. Surg., 43, 1990, p. 135.
20. Iwasawa M., Ohtsuka Y., Kushima H., Kiyono M. Arterialized venous flaps from the thenar and hypothenar regions for repairing finger pulp tissue losses. Plast.Reconstr. Surg,. 99, 1997, p. 1765.
21. Kantarci U., Cepel S., Gurbuz C. Venous free flaps for reconstruction of skin defects of the hand. Microsurgery, 18(3), 1998, p. 166.
22. Klein C,. Kovács A., Stuckensen T. Free arterialized venous forearm flaps for intraoral reconstruction. Br. J. Plast. Surg., 50, 1997, p.166.
23. Koshima I., Soeda S., Nakayama Y., Fukuda H., Tanaka J. An arterialized venous flap using long saphenous vein. Br. J. Plast. Surg., 44, 1991, p. 23.
24. Krishnan KG. The venous flaps: an experimental study of the microvascular architecture, the area of perfusion and their correlation. Br. J. Plast. Surg., 55, 2002, p. 340.
25. Krishnan KG., Stützle H., Stock W. The venous flap. Eur. J. Plast. Surg., 23, 2000, p. 64.
26. Kushima H. Iwasawa, M., Maruyama Y. Recovery of sensitivity in the hand after reconstruction with arterialized venous flaps. Scand. J. Plast. Reconstr .Surg. Hand Surg., 36(6), 2002, p. 362.
27. Morris SF., Mac Gill KA., Taylor IG. Scalp replantation by arterialized venous network flow through. Br. J. Plast. Surg., 45, 1992, p. 187.
28. Murata K., Inada Y., Fukui A., Tamai S., Takakura Y. Clinical application of the reversed pedicled venous flap containing perivenous areolar tissue and/or nerve in the hand. Br. J. Plast. Surg., 54(7), 2001, p. 615-620.
29. Murata K., Tamai S., Inada Y., Fukui A., Miamoto S. Transfer of pedicled venous flap containing perivenous areolar tissue and nerve: an experimental study. Br. J. Plast. Surg., 52, 1999, p. 223.
30. Mutaf M., Tasaki Y., Fujii T. Expansion of venous flaps: an experimental study in rats. Bt. J. Plast. Surg., 51, 1998, p. 393.
31. Nakayama Y., Iino T., Uchida A., Kiosawa T. Soeda S. Vascularized free nail grafts nourished by arterial inflow from the venous system. Plast. Reconstr. Surg., 85, 1900, p. 239.
32. NakayamaY., Soeda S., Kasai Y. Flaps nourished by arterial inflow through the venous system: an experimental investigation. Plast. Reconstr. Surg., 67, 1981, p. 328.
33. Noreldin AA., Fukata K., Jackson IT. Role of perivenous areolar tissue in the viability of venous flaps: an experimental study on the inferior epigastric venous flap of the rat. Br. J. Plast. Surg., 45, 1992, p. 18.
34. Ozec C., Zbang R., Lineaweaver WC., Chin BT., Newlin L., Eiman T., Buncke HJ. Arterialization of the venous system in a rat lower limb model. Br. J. Plast. Surg., 50, 1997, p. 402-407.
35. Oshammer HE., Schwarzl FX., Haas FM., Maechler H., Pierer G., Wiltgen M, Koch H. Retrograde arterialized venous flap: an experimental study. Microsurgery, 23(2), 2003, p. 130-134.
36. Pittet B., Chang P., Cederna P., Cohen MB., Blair WF., Cram AE. The role of neovascularization in the survival of an arterialized venous flap. Plast. Reconstr. Surg., 97(3), 1996, p. 621-629.
37. Roberts AP., Cohen LL., Cook TA. The rat ventral island flap: a comparison of the effects of reduction in arterial inflow and venous outflow. Plast. Reconstr. Surg., 97, 1996, p. 610-615.
38. Sakai S. Arterialized venous groin flap: case report. Br. J. Plast. Surg., 49, 1996, p. 90.
39. Shalaby HA., Saad MA. The venous flap: is it purely venous? Br. J. Plast. Surg., 46, 1993, p. 285.
40. Suzuki Y., Isshiki K., Ishikawa K, Koyama H. Viability and quantitative dermofluorometry of experimental arterialized and non arterialized venous flaps. Br. J. Plast. Surg., 46, 1993, p. 273.
41. Takeuchi M., Sakurai H., Kenji S., Motohiro N. Treatment of finger avulsion injuries with innervated arterialized venous flaps. Plast. Reconstr. Surg., 106, 2000, p. 881.
42. Thatte RL., Thatte MR. Venous flaps (letter). Br. J. Plast. Surg., 45, 1992, p. 404.
43. Thatte RL., Thatte MR. The saphenous venous flap. Br. J. Plast. Surg., 42, 1989, p. 399.
44. Townsend PLG., Taylor GI. Vascularized nerve grafts using composite arterialized neuro-venous systems. Br. J. Plast. Surg., 37, 1984, p. 1.
45. Tsai TM., Matiko JD., Breidenbach W., Kutz JE. Venous flaps in digital revascularization and replantation. J. Reconstr. Microsurg., 3, 1987, p. 113.
46. Ueda Y, Mizumoto S., Hirai T., Doi Y., Fukui A., Tamai S. Two-stage arterialized flow-through venous flap transfer for third-degree burn defects on the dorsum of the hand. J. Reconstr. Microsurg., 13(7), 1997, p. 489.
47. Veselý J., Kučera J. Immediate free flap reconstruction of traumatic defects. Acta Chir. Plast., 37(1), 1995, p. 7-11.
48. Veselý J., Smrčka V. Replantation by arterialization of the venous system of amputated parts. Acta Chir. Plast., 37, 1995, p. 67.
49. Wolff KD., Telzrow T., Rudolph KH., Franke J., Wartenberg E. Isotope perfusion and infrared thermography of arterialized, venous flow through and pedicled venous flaps. Br. J. Plast. Surg., 48, 1995, p. 61.
50. Woo SH., Kim SE., Lee TH., Jeong JH., Seul JH. Effects of blood flow and venous network on the survival of the arterialized venous flap. Plast. Rec. Surg., 101, 1998, p. 1280.
51. Woo SH., Jeong JH., Seul JH. Resurfacing relatively large skin defects of the hand using arterialized venous flaps. J. Hand Surg. [Br], 21(2), 1996, p. 222.
52. Woo SH., Seul JH. Pre-expanded arterialized venous free flaps for burn contracture of the cervicofacial region. Br. J. Plast. Surg., 54(5), 2001, p. 390.
53. Wungcharoen B., Pradidarcheep W., Santidhananaon Y., Chongchet V. Pre arterialization of the arterialized venous flap: an experimental study in rat. Br. J. Plast. Surg., 54, 2001, p. 621.
54. Wungcharoen B., Santidhananon Y., Chongchet V. Pre-arterialization of an arterialized venous flap: clinical cases. Br. J. Plast. Surg., 54, 2001, p. 112.
55. Xiu ZF., Chen ZJ. The microcirculation and survival of experimental flow through venous flaps. Br. J. Plast. Surg., 49, 1996, p. 41.
56. Xiu ZF., Chen ZJ. Clinical applications of venous flaps. Ann. Plast. Surg., 34(5), 1995, p. 518.
57. Yilmaz M., Menderes A., Karatas Ö., Karaca C., Barutcu A. Free arterialized venous forearm flaps for limb reconstruction. Br. J. Plast. Surg., 49, 1996, p. 396.
58. Yoshimura M., Shimada T., Imura S., Shimamura K., Yamauchi S. The venous skin graft method for repairing skin defects of the fingers. Plast. Reconstr. Surg., 79, 1987, p. 243-248.
Štítky
Chirurgie plastická Ortopedie Popáleninová medicína Traumatologie
Článek vyšel v časopiseActa chirurgiae plasticae
Nejčtenější tento týden
2008 Číslo 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
- Neodolpasse je bezpečný přípravek v krátkodobé léčbě bolesti
- Léčba akutní pooperační bolesti z pohledu ortopeda
-
Všechny články tohoto čísla
- ARTERIALIZED VENOUS FREE FLAPS – A RECONSTRUCTIVE ALTERNATIVE FOR LARGE DORSAL DIGITAL DEFECTS
- RECONSTRUCTION OF LARGE UPPER LIP DEFECTS BY FREE TISSUE TRANSFER
- THE VALUE OF CLINICAL DIAGNOSIS OF DIGITAL GLOMUS TUMORS
- GIANT CELL REPARATIVE GRANULOMA IN THE MANDIBLE – CASE REPORT AND REVIEW OF THE LITERATURE
- IPSILATERAL FIBULAR TRANSFER: A VALUABLE OPTION FOR TREATMENT OF LARGE TIBIAL DEFECTS IN CHILDREN
- ČESKÉ A SLOVENSKÉ SOUHRNY
- Acta chirurgiae plasticae
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- ARTERIALIZED VENOUS FREE FLAPS – A RECONSTRUCTIVE ALTERNATIVE FOR LARGE DORSAL DIGITAL DEFECTS
- GIANT CELL REPARATIVE GRANULOMA IN THE MANDIBLE – CASE REPORT AND REVIEW OF THE LITERATURE
- THE VALUE OF CLINICAL DIAGNOSIS OF DIGITAL GLOMUS TUMORS
- RECONSTRUCTION OF LARGE UPPER LIP DEFECTS BY FREE TISSUE TRANSFER
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



