-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Remodelling Surgery in Craniosynostosis
Léčba kraniosynostóz remodelační technikou
Cíl:
Autoři popisují komplexní diagnostický postup, individuální předoperační přípravu a pooperační sledování, které zavedli na svém pracovišti, včetně individuální předoperační hematologické přípravy a remodelační operační techniky u pacientů s kraniosynostózou.Soubor a metodika:
Soubor 14 pacientů operovaných remodelační technikou je srovnáván se souborem pacientů operovaných metodou strip kraniektomie z hlediska kosmetického efektu, potřeby krevní transfuze, doby trvání operačního výkonu a komplikací.Výsledky:
Pacienti operovaní remodelační technikou vykazovali signifikantní zlepšení cefalického indexu a lepší kosmetický efekt ve srovnání s pacienty operovanými metodou strip kraniektomie. Autoři neshledali statisticky významný rozdíl v délce trvání operace u obou srovnávaných skupin. Individuální předoperační hematologická příprava eliminovala zvýšenou potřebu krevních transfuzí u velmi malých pacientů.Závěry:
Remodelační operační technika poskytuje lepší kosmetické a léčebné výsledky ve srovnání s technikou strip kraniektomie. Remodelační operační technika spolu s komplexní, individuální předoperační přípravou představuje bezpečnou a účinnou metodu v léčbě kraniosynostóz i u velmi malých dětí.Klíčová slova:
kraniosynostóza – diagnostický algoritmus – genetika – remodelace klenby lební – časná operace – hematologická příprava
Authors: E. Brichtová; Z. Mackerle
Authors place of work: Pediatric Surgery, Orthopaedics and Traumatology Clinic, Brno Faculty Hospital, Brno, Czech Republic
Published in the journal: Cesk Slov Neurol N 2011; 74/107(2): 168-174
Category: Původní práce
Summary
Aims:
The authors describe a comprehensive diagnostic algorithm, individual pre-operative care and postoperative follow-up procedures, established at the author’s workplace, to address tailored pre-operative haematological preparation and cranial vault remodelling surgery in craniosynostosis patients.
Materials and methodology:
A set of 14 patients newly operated upon using the remodelling technique is presented and compared to a set of patients operated upon using strip craniectomy, in terms of cosmetic effects, the need for transfusion, surgery time and complications.Results:
Remodellation technique surgery patients showed significant improvement in cephalic index and a better cosmetic effect compared with the strip craniectomy patient group. There was no significant difference in surgery time between the operational techniques. Pre-operative haematological preparation was sufficient to eliminate the higher transfusion requirements of very young patients.Conclusions:
The remodelling surgery technique was found to provide better cosmetic and therapeutic effects compared with strip craniectomy. Cranial vault remodelling surgery combined with comprehensive, tailored pre-operative care is a safe and efficient procedure in craniosynostosis treatment even in very young children.Key words:
craniosynostosis – diagnostic algorithm – genetics – cranial vault remodellation – early surgery – haematology preparationIntroduction
Craniosynostosis is defined as premature closure of one or more of the cranial sutures in a child leading to secondary changes in the shape and/or volume of the skull. Its incidence is generally reported at around one in 2,500 live births [1]. Primary or simple, non-syndromic craniosynostosis involves premature closure of one or more cranial sutures in otherwise healthy children. Craniosynostosis that occurs as part of a complex craniofacial syndrome or congenital malformation is known as congenital or complex. More than 90 syndromes involving craniosynostosis and associated abnormalities have been identified and about 27 different chromosomal aberrations connected with craniosynostosis have been described, with more added every year [2]. Craniosynostosis diagnosis is based on clinical examination, anthropometric examination and especially on proper imaging techniques. The cephalic index (CI), derived from craniometric data, is commonly used, but it has obvious limitations. Skull 3D computer tomography (CT) provides precise information about the state of cranial sutures; its also informs of intracranial circumstances. Sophisticated stereoscopic cameras and recently-developed 3D morphometric methods appear to be the most promising assessment and follow-up tools. Regular neurological examination, together with diligent monitoring of psychomotor development and neurological status are necessary in craniosynostosis-diagnosed patients. Craniosynostosis surgery has been through considerable and extended development, from simple suture resection to complex skull remodelling. All the surgical approaches aim for total craniosynostosis elimination and optimal skull shape remodeling to achieve long-term physiological intracranial volume, normal further brain development and neurocognitive functions, together with optimum cosmetic effect. Classical strip craniectomy is effective in young children, without developed compensatory changes in skull vault shape and skull base abnormalities. For children with such deformities, more demanding remodelling surgery techniques have been developed. Most of these complex remodelling procedures take far longer and nearly always require blood transfusion. Estimated blood loss may range from 25% to 500% of circulation blood volume [3–8]. Specific haematology protocols have been designed to prepare child patients for such major operations.
Tab. 1. Patients operated for craniosynostosis in the years 2003–2009. 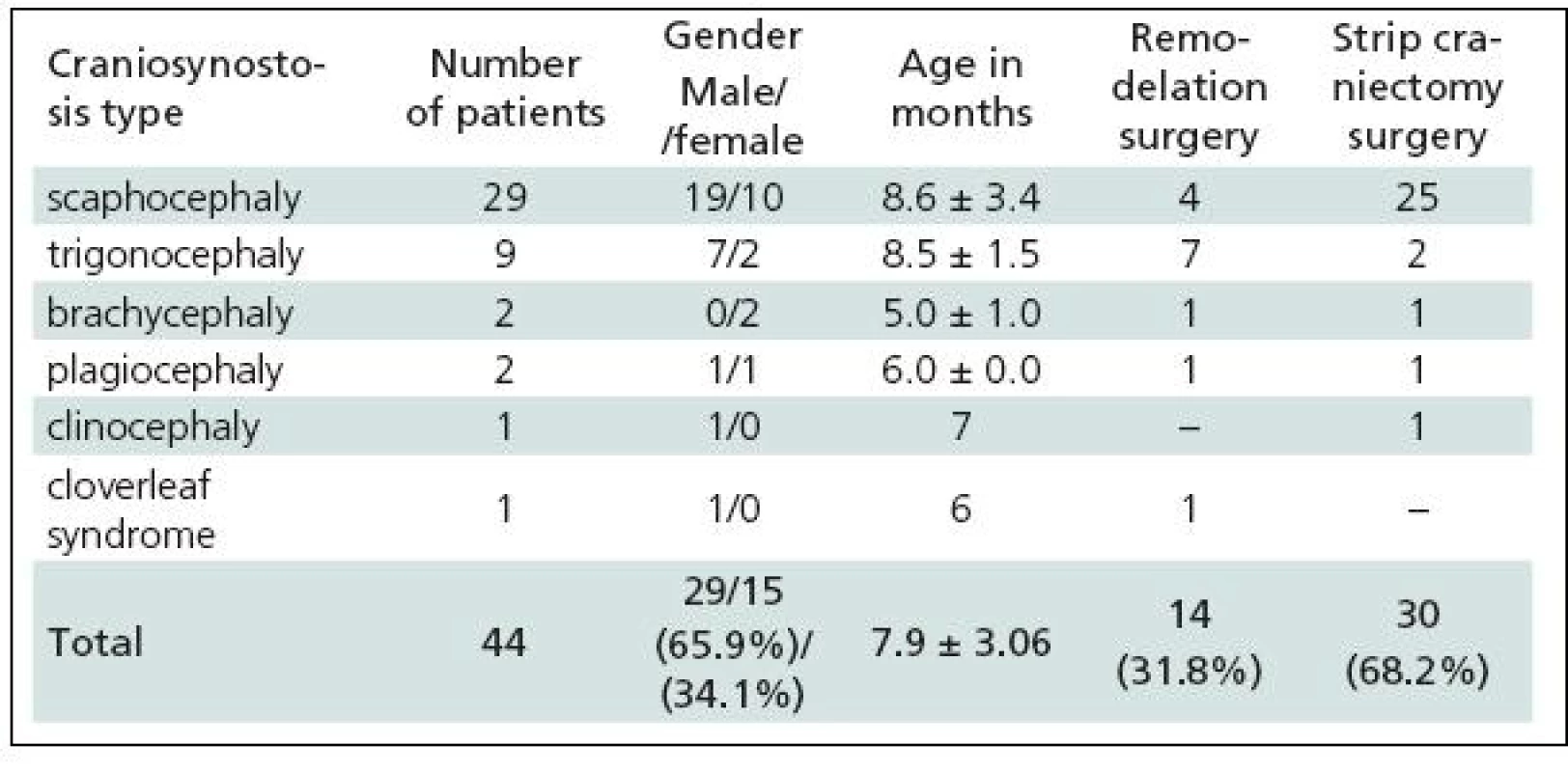
Tab. 2. Remodelation technique surgery for craniosynostis – list of patients 2007–2009. 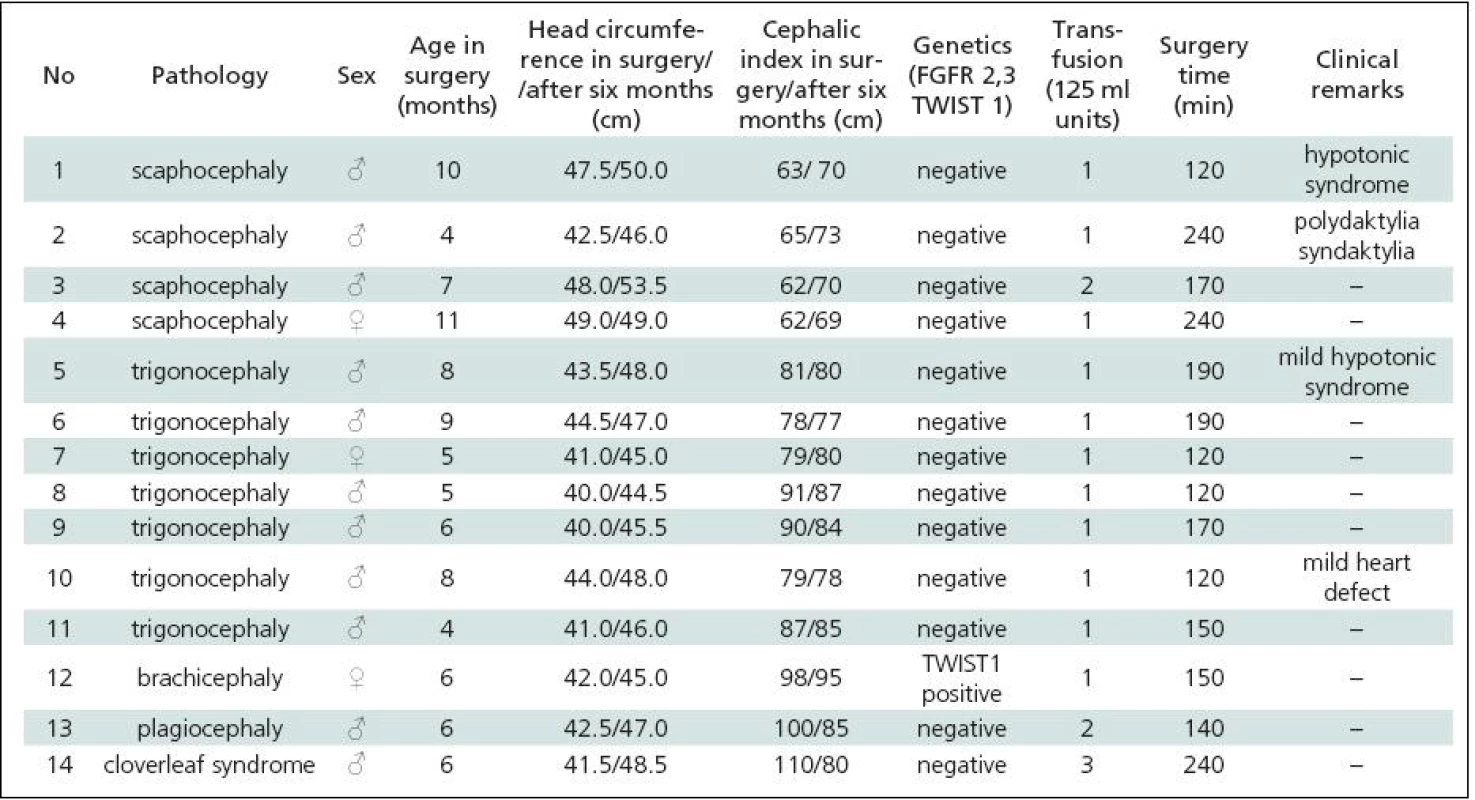
Tab. 3. Strip technique surgery for craniosynostis – list of patients 2003–2009. 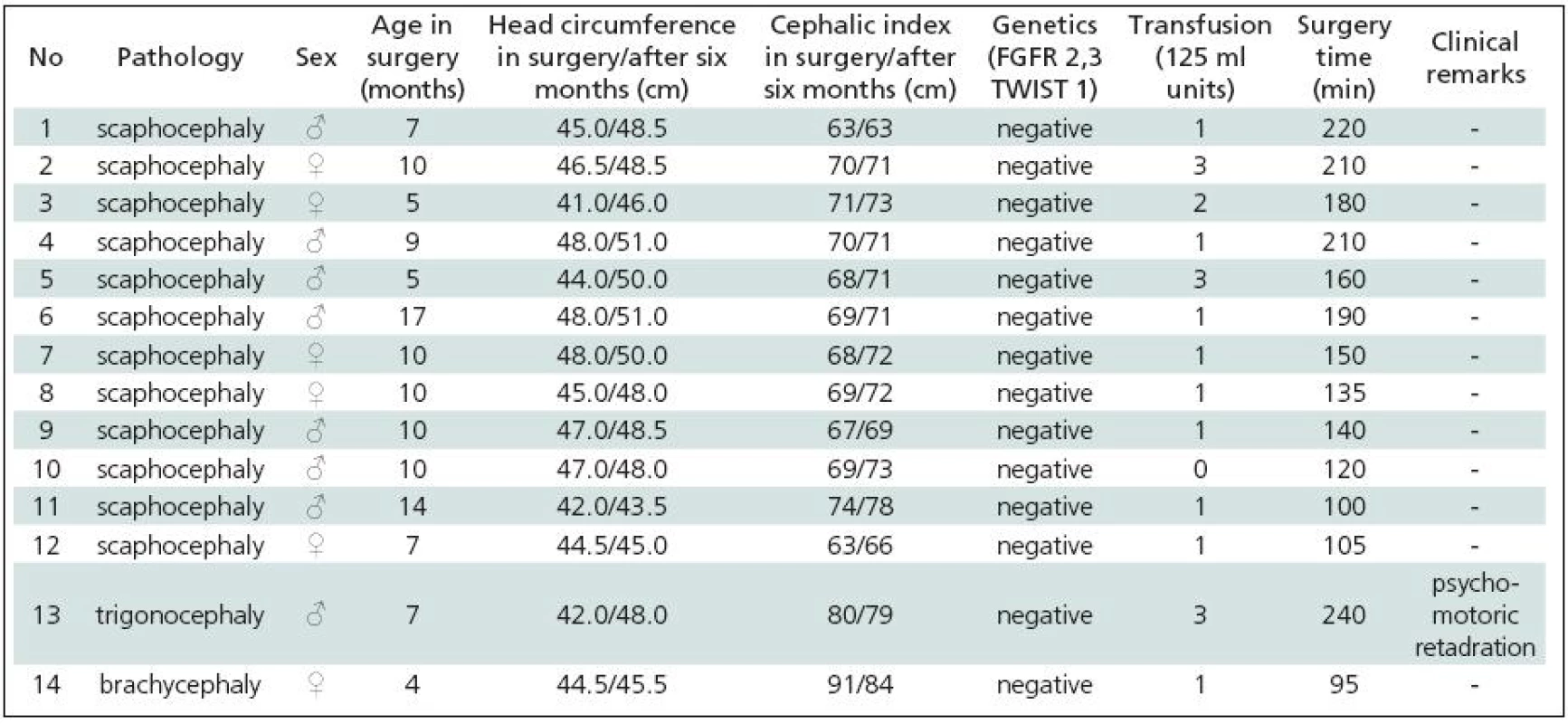
Materials and methodology
Pediatric patients suffering from craniosynostosis and operated upon in the period 2003–2009 at the Department of Pediatric Surgery, Orthopedics and Traumatology, Faculty Hospital Brno, were included in the evaluation. Beginning in 2007, the authors created a new algorithm for early assesment, diagnostics and early operative treatment of craniosynostosis patients, making good use of collaboration between various medical specialities. The group of patients operated upon in accord with the new diagnostic and treatment protocols, using remodelling and comprehensive pre-operative care. was then compared with patients who had undergone standard strip craniectomy without such pre-operative procedures. Age, blood transfusions, surgery time, complications, further head growth, CI and cosmetic effect were evaluated for both groups. All craniosynostosis patients were subjected to clinical neurological and neurosurgical examination. CT and CT 3D scanning under general anesthesia were performed in all patients. The patients assessed also underwent clinical and laboratory genetic examination, syndromic analysis and estimation of genetic risk. The FGFR2 and FGFR3 genes were investigated for direct detection of the most common mutations, while sequence analysis of the TWIST1 gene coding region was done. Formal, signed, informed consent was given by the parents in all cases. Considering the usually significant peroperative blood loss in the light of subjects aged under one year, a comprehensive haematological pre-operative examination, with assessment of individual preparation for each particular patient, was carried out, in order to minimize the anticipated higher need of blood transfusion during and following the highly stressful and predictably prolonged remodelling surgery. To reduce the need for transfusions, patients should go to surgery with a maximum red blood count. All patients operated upon under the new protocol were subject to an initial laboratory examination – complete blood count with differential count, retikulocyte count and retikulocyte haemoglobin, blood smear, iron, transferrin and ferritin levels, total iron binding capacity, transferrin saturation, erythropoetin, cobalamin, folate, haptoglobin and bilirubin levels, gamma-glutamyl transpeptidase (GGT), glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT) and lactate dehydrogenase (LDH) serum levels. After that, all patients were started on Aktiferrin (ferrosi sulfas heptahydricus, serinum racemicum) at a dose of 0.16 ml/ kg per day (in the event of initially detected sideropenia at a dose of 0.32 ml/ kg per day), Pyridoxin (pyridoxini hydrochloridum) at a dose of 10 mg per day, Celaskon (acidum ascorbicum) at a dose of 50–200 mg per day, and acidum follicum at a dose of 5 mg per day for a period of three weeks. Repeated red blood counts usually revealed haemoglobin values at or slightly above the higher reference levels, if not, additional administration of Eprex (epoetinum alpha) at a dose of 450–900 IU/ kg twice a week was given for two weeks. This treatment was also continued after surgery. Consecutive surgery was performed by cranial vault remodeling in 14 patients, using resorbable plates and screws in one case. Fixation was expected to enhance calvarial rigidity and optimise final cosmetic effect. The surgery was documented by photographs and video recordings. Postoperatively, all patients wore protective helmets to prevent head injury. Routine postoperative outpatient checkups with evaluation of results were made in the third and sixth month postoperatively. Clinical and anthropometric checks with standard photographs were all performed. All photographic documentation was conducted with parental consent.
Results
Table 1 gives a summary of the data on all 44 patients operated upon for craniosynostosis in the years 2003–2009. Of this number, 30 (68.2%) underwent strip technique surgery, 14 (31.8%) remodelling surgery. Tables 2 and 3 give an overview of patients operated upon by the authors in the indicated period. Table 2 reviews the 14 patients operated upon under the new protocol and using remodelling surgery. The mean patient age was 6.8 ± 2.2 months, the mean blood transfusion quantity was 1.2 ± 0.4 transfusion units (TU) of 125 ml. Mean head circumference increment after 6 months was 4.0 ± 1.6 cm. Mean CI difference after 6 months in four dolichocephalic patients was 7.5 ± 0.5; in all craniosynostosis patients 6.7 ± 7.8. In one case, a positive TWIST1 c.310 T (p.Glu104X) mutation was detected. Surgery mean time was 168.6 ± 45.7 minutes. No peroperative or postoperative complications occured. Table 3 reviews the set of 14 patients operated upon by the authors using strip surgery without pre-operative preparation. The patients’ mean age was 8.9 ± 3.5 months, mean blood transfusion amount was 1.5 ± 0.8 TU. Eprex was adiministered in one patient postoperatively due to contraindication to blood transfusion. Mean head circumference increment after 6 months was 2.8 ± 1.7 cm. Mean CI difference after 6 months in 12 dolichocephalic patients was 2.4 ± 1.3; in all craniosynostosis patients 2.6 ± 1.8. No abnormal results were obtained in genetic tests. Mean surgery time was 161.1 ± 47.9 minutes. No peroperative or postoperative complications occured. For statistical evaluation, the paired t-test was performed to assess differences in age, head growth, CI, surgery time and transfusion amount across the two groups. In terms of patient age, calculated t = 1.875; p = 0.083 indicated no significant difference. For head circumference, calculated t = 1.806; p = 0.094 also indicated no significant difference, but using the non-parametric Wilcoxon test for the assessment, t = 6.067; p = 0.052, the values almost reached statistical significance at the p <0.05 level. By evaluating the CI difference in scaphocephalic patients using ANOVA t-test summary data analysis, the means of the two groups were significantly different (mean group difference 5.08 ± 0.44, t (14) = 7.750; p < 0.001). By evaluating the CI difference in all craniosynostosis patients, t = 2.066; p = 0,059 almost reached significance at p <0.05 level. Minimal group mean difference –0.149; t = 0.520; p = 0.612 obtained by analyzing the transfusion amounts in both groups obviously indicated no significant difference. Surgery time was not significantly different between the groups ( t = 0.454; p = 0.657). The cosmetic postoperative effect was assessed largely visually by surgeons’ and parental assessment, and found to be better in patients in the remodelling surgery group, mainly due to early visible head shape improvement. Figures 1–3 show surgical remodeling technique and postoperative outcome in a patient with dolichocephaly operated upon at four months of age. Figures 4 and 5 show the pre-operative and postoperative state in a patient treated with remodeling technique for trigonocephaly.
Fig. 1. Preoperative state in patient with dolichocephaly in four months. 
Fig. 2. Remodeling technique surgery in dolichocephalic patient. 
Based on these results, we deduce that employment of cranial vault remodeling technique offers better and earlier visible cosmetic and therapeutic (psychomotor retardation prevention) effects compared to strip craniectomy. Anticipated higher transfusion needs [9] and higher complication rates in very young patients treated with more stressful and possibly not significantly prolonged remodelling technique surgery may be eliminated or even lowered by dose-tailored haematological preparation with maximized pre-operative red blood count levels. Photographic documentation conducted with parental consent allowed assessment of the cosmetic results of surgery.
Fig. 3. Postoperative remodeling technique outcome. 
Discussion
In recent decades there has been a growing tendency to avoid strip craniectomies because of their inadequacy in complex craniosynostoses. The timing for surgery has also changed, with treatment of younger children [10–14]. Despite the fact that some surgeons prefer endoscopic operations [15–18], minimally invasive techniques [19] or distraction devices [20–25], the remodeling technique still remains a gold standard, based on its greater effectiveness [26,27]. Suitable resorbable plates are available for the fixation of the calvarial fragments, and they guarantee maximum strength [28–31]. Demanding operations on very young children are also associated with higher surgical risk and especially with comparatively high blood loss, since overall blood volume and haemocoagulation systems are immature [32–34]. Transfusion requirements and risk can be lowered by maximizing red blood count before surgery. The risk of blood loss and its related need for blood transfusion can be to a great extent forestalled by comprehensive haematological examination and individual preparation with administration of vitamins, microelements and erythropoetine [35].
Fig. 4. Preoperative state in patient with trigonocephaly in five months. 
Fig. 5. Postoperative remodeling technique outcome after six months. 
Bearing these facts in mind, in 2007 the authors began a project for the improvement of surgical treatment for craniosynostosis patients and created an algorithm for early assessment, diagnostics and early remodelling operative treatment of craniosynostosis patients, using individualized pre-operative haematological preparation. Our results, in terms of visual assessment, head circumference increment and CI difference in dolichocephalic patients, clearly favored remodelation surgery when compared with data in the literature [14,36–38]. More demanding and not significantly prolonged remodelling surgery performed in very young children after individualized haematological preparation was associated with no need for additional transfusion in our study; on the contrary, transfusion consumption was slightly lower. Comparing these data with the literature [7,9], our results support the positive influence of pre-operative heamatological preparation on lowering transfusion need. Mortality and morbidity rates in our patient group were excellent and other parameters also compared well with data in the literature [14,36,38–43].
Both diagnostic and postoperative head shape evaluation is classically based on 3D CT and craniometric evaluation, largely employing CI [44]. In our opinion, simple head circumference is still useful in evaluating postoperative head growth with respect to normal brain development and prevention of psychomotor retardation [45]. CI computed from two-dimensional craniometric data is unsatisfactory for evaluation of cosmetic outcome, particularly in non-dolichocephalic patients, although it is routinely recorded worldwide. Usually, sets of photographs taken in the course of patient follow-up may document cosmetic outcome. This method was used for our patients. In our opinion, photographic documentation is currently highly valuable to the assessment of pre-operative and postoperative status and the cosmetic results of surgery. Despite this, all these methods fail in objective, comparable, fast and easy head shape assessment and follow-up. Therefore new optical 3D morphometric methods have been developed and are coming into use to obtain non-invasively stereoscopic and metrical information, avoiding radiation and any need for general anaesthesia [46]. These methods also open up the possibility of virtual pre-operatively-tailored planning of the stages of cranial vault remodeling [47–53], with possible robotic surgery implementation in the future. Despite all these perspectives, more or less subjective, individual visual outcome assessment and parent satisfaction are likely to remain significant tools in the assessment of outcome.
Conclusion
The established algorithm for diagnosis and therapy in craniosynostosis is capable of diagnostic specification, improvement of operative results and reduction of perioperative risk, especially blood-transfusion-related. The cranial vault remodeling surgical technique, together with with compehensive, tailored pre-operative care is a safe and efficient procedure in the treatment of craniosynostosis even in very young children.
doc. MUDr. Eva Brichtová, Ph.D.
Pediatric Surgery, Orthopaedics and Traumatology Clinic
Brno Faculty Hospital
Cernopolni 9
613 00 Brno
e-mail: brichtovae@seznam.czAccepted for review: 27. 7. 2010
Accepted for press: 1. 10. 2010
Zdroje
1. Cohen MM. Craniosynostosis: Diagnosis, Evaluation and Management. New York: Raven Press 1986 : 1–20.
2. San P, Persing A. Craniosynostosis. In: Albright L, Pollack I, Andelson D (eds). Principles and Practise of Pediatric Neurosurgery. New York: Thieme; 1999 : 219–242.
3. Meyer P, Renier D, Arnaud E, Jarreau MM, Charron B, Buy E et al. Blood loss during repair of craniosynostosis. Br J Anaesth 1993; 71(6): 854–857.
4. Faberowski LW, Black S, Mickle JP. Blood loss and transfusion practice in the perioperative management of craniosynostosis repair. J Neurosurg Anesthesiol 1999; 11(3): 167–172.
5. Kearney RA, Rosales JK, Howes WJ. Craniosynostosis: an assessment of blood loss and transfusion practices. Can J Anaesth 1989; 36(4): 473–477.
6. Scholtes JL, Thauvoy C, Moulin D, Gribomont BF. Craniofaciosynostosis: anesthetic and perioperative management. Report of 71 operations. Acta Anaesthesiol Belg 1985; 36(3): 176–185.
7. Tuncbilek G, Vargel I, Erdem A, Mavili ME, Benli K, Erk Y. Blood loss and transfusion rates during repair of craniofacial deformities. J Craniofac Surg 2005; 16(1): 59–62.
8. White N, Marcus R, Dover S, Solanki G, Nishikawa H, Millar C et al. Predictors of blood loss in fronto-orbital advancement and remodeling. J Craniofac Surg 2009; 20(2): 378–381.
9. Ririe DG, David LR, Glazier SS, Smith TE, Argenta LC. Surgical advancement influences perioperative care: a comparison of two surgical techniques for sagittal craniosynostosis repair. Anesth Analg 2003; 97(3): 699–703.
10. Di Rocco F, Arnaud E, Meyer P, Sainte-Rose C, Renier D. Focus session on the changing “epidemiology” of craniosynostosis (comparing two quinquennia: 1985–1989 and 2003–2007) and its impact on the daily clinical practice: a review from Necker Enfants Malades. Childs Nerv Syst 2009; 25(7): 807–811.
11. Di Rocco F, Arnaud E, Renier D. Evolution in the frequency of nonsyndromic craniosynostosis. J Neurosurg Pediatr 2009; 4(1): 21–25.
12. Ozgur BM, Aryan HE, Ibrahim D, Soliman MA, Meltzer HS, Cohen SR et al. Emotional and psychological impact of delayed craniosynostosis repair. Childs Nerv Syst 2006; 22(12): 1619–1623.
13. Persing JA. Immediate correction of sagittal synostosis. J Neurosurg 2007; 107 (Suppl 5): 426.
14. Maugans TA, McComb JG, Levy ML. Surgical management of sagittal synostosis: a comparative analysis of strip craniectomy and calvarial vault remodeling. Pediatr Neurosurg 1997; 27(3): 137–148.
15. Jimenez D, Barone CM. Endoscopic craniectomy for early surgical correction of sagittal craniosynostosis. J Neurosurg 1998; 88(1): 77–81.
16. Hinojosa J, Esparza J, Muñoz MJ. Endoscopic-assisted osteotomies for the treatment of craniosynostosis. Childs Nerv Syst 2007; 23(12): 1421–1430.
17. Murad JA, Clayman M, Seagle MB, White S, Perkins LA, Pincus DW. Endoscopic-assisted repair of craniosynostosis. Neurosurg Focus 2005; 19(6): E6.
18. Keshavarzi S, Hayden MG, Ben-Haim S, Meltzer HS, Cohen SR, Levy ML. Variations of endoscopic and open repair of metopic craniosynostosis. J Craniofac Surg 2009; 20(5): 1439–1444.
19. Di Rocco C, Caldarelli M, Massimi L, Romani R, Tamburrini G. A minimally invasive technique for the surgical correction of sagittal synostosis: preliminary experience. Childs Nerv Syst 2004; 20 : 653–685.
20. Pelo S, Gasparini G, Di Petrillo A, Tamburrini G, Di Rocco C. Distraction osteogenesis in the surgical treatment of craniostenosis: a comparison of internal and external craniofacial distractor devices. Childs Nerv Syst 2007; 23(12): 1447–1453.
21. Kim SW, Shim KW, Plesnila N, Kim YO, Choi JU, Kim DS. Distraction vs remodeling surgery for craniosynostosis. Childs Nerv Syst 2007; 23(2): 201–206.
22. Nonaka Y, Oi S, Miyawaki T, Shinoda A, Kurihara K. Indication for and surgical outcomes of the distraction method in various types of craniosynostosis. Childs Nerv Syst 2004; 20(10): 702–709.
23. Imai K, Komune H, Toda C, Nomachi T, Enoki E, Sakamoto H. Cranial remodeling to treat craniosynostosis by gradual distraction using a new device. J Neurosurg 2002; 96(4): 654–659.
24. Akai T, Iizuka H, Kawakami S. Treatment of craniosynostosis by distraction osteogenesis. Pediatr Neurosurg 2006; 42(5): 288–292.
25. Arai H, Nakanishi H, Miyajima M, Komuro Y, Yanai A. Cranial remodeling using gradual distraction method for craniosynostosis. Childs Nerv Syst 2004 : 20 : 653–685.
26. Heller JB, Heller MM, Knoll B, Gabbay JS, Duncan C et al. Intracranial volume and cephalic index outcomes for total calvarial reconstruction among nonsyndromic sagittal synostosis patients. Plast Reconstr Surg 2008; 121(1): 187–195.
27. Mori K, Sakamoto T, Nakai K. Expanding cranioplasty for craniosynostosis and allied disorders. Childs Nerv Syst 1992; 8(7): 399–405.
28. Ahmad N, Lyles J, Panchal J. Outcomes and complications based on experience with resorbable plates in pediatric craniosynostosis patients. J Craniofac Surg 2008; 19(3): 855–860.
29. Kang JK. Cranial remodelling techniques in the treatment of craniosynostosis during the first year of life: evaluation of loose, rigid, and limited fixation. Jpn J Neurosurg 2000; 9(1): 4.
30. Arai H, Sato K, Okuda O, Miyajima H, Hishii M, Nakanishi H et al. Early experience with poly L-lactic acid bioabsorbable fixation system for paediatric craniosynostosis surgery. Report of 3 cases. Acta Neurochirurgica 2000; 142(2): 187–192.
31. Sikorski CW, Iteld L, McKinnon M, Yamini B, Frim DM. Correction of sagittal craniosynostosis using a novel parietal bone fixation technique: results over a 10-year period. Pediatr Neurosurg 2007; 43(1): 19–24.
32. Haas T, Fries D, Velik-Salchner C, Oswald E, Innerhofer P. Fibrinogen in craniosynostosis surgery. Anesth Analg 2008; 106(3): 725–731.
33. Carver E. Blood loss during repair of craniosynostosis. Br J Anaesth 2004; 93(5): 747.
34. Steinbok P, Heran N, Hicdonmez T, Cochrane DAP. Minimizing blood transfusions in surgery for craniosynostosis. Childs Nerv Syst 2004; 20(7): 653–685.
35. Przybylo HJ, Przybylo JH. The use of recombinant erythropoietin in the reduction of transfusion rates in craniosynostosis repair in infants and children. Plast Reconstr Surg 2003; 111(7): 2485–2486.
36. Panchal J, Marsh JL, Park TS, Kaufman B, Pilgram T, Huang SH. Sagittal craniosynostosis outcome assessment for two methods and timings of intervention. Plast Reconstr Surg 1999; 103(6): 1574–1584.
37. Marsh JL, Jenny A, Galic M, Picker S, Vannier MW. Surgical management of sagittal synostosis. A quantitative evaluation of two techniques. Neurosurg Clin N Am 1991; 2(3): 629–640.
38. Boop FA, Shewmake K, Chadduck WM. Synostectomy versus complex cranioplasty for the treatment of sagittal synostosis. Childs Nerv Syst 1996; 12(7): 371–375.
39. Ferreira MP, Collares MV, Ferreira NP, Kraemer JL, Pereira FA, Pereira FG. Early surgical treatment of nonsyndromic craniosynostosis. Surg Neurol 2006; 65 (Suppl 1): 22–26.
40. Esparza J, Hinojosa J. Complications in the surgical treatment of craniosynostosis and craniofacial syndromes: apropos of 306 transcranial procedures. Childs Nerv Syst 2008; 24(12): 1421–1430.
41. Mackenzie KA, Davis C, Yang A, MacFarlane MR. Evolution of surgery for sagittal synostosis: the role of new technologies. J Craniofac Surg 2009; 20(1): 129–133.
42. Bizzi J, Bedin A. Surgery for craniosynostosis: experience in 150 cases and strategies to avoid complications. Childs Nerv Syst 2010; 26 : 545–592.
43. Sloan GM, Wells KC, Raffel C, McComb JG. Surgical treatment of craniosynostosis: outcome analysis of 250 consecutive patients. Pediatrics 1997; 100(1): E2.
44. Van Lindert E, Ettema A, Borstlap W. Validation of cephalic index measurements in scaphocephaly. Childs Nerv Syst 2010; 26 : 545–592.
45. Lindley AA, Benson JE, Grimes C, Cole TM, Herman AA. The relationship in neonates between clinically measured head circumference and brain volume estimated from head CT-scans. Early Hum Dev 1999; 56(1): 17–29.
46. Messing-Jünger M, Röhrig A, Persits S, Martini. Morphometric assessment of pre - and postoperative craniofacial shape in craniosynostosis patients. Childs Nerv Syst 2010; 26 : 545–592.
47. Clijmans T, Gelaude F, Mommaerts M, Suetens P, Sloten JV. Computer Supported Pre-Operative Planning of Craniosynostosis Surtgery: a Mimics-Integrated Approach. In: Abstracts of the Annual Medical Innovations Conference. Barcelona 2006 : 1–12.
48. Clijmans T, Gelaude F, Suetens P, Mommaerts M, Sloten JV. Computer supported pre-operative planning of craniofacial surgery: from patient to template. In: Abstracts of the 3rd European Medical and Biological Engineering Conference; Prague 2005 : 2023.
49. Clijmans T, Mommaerts M, Gelaude F, Suetens P, Sloten JV. Skull reconstruction planning transfer to the operation room by thin metallic templates: Clinical results. J Craniomaxillofac Surg 2008; 36(2): 66–74.
50. Clijmans T, Mommaerts M, Suetens P, Slotena SV. Computer supported pre-operative simulation of neonatus cranial bone bending in craniosynostosis surgery planning. Int J CARS 2006; 1 (Suppl 1): 251–263.
51. Teschner M, Girod S, Girod B. Optimization Approaches for Soft–Tissue Prediction in Craniofacial Surgery Simulation. In: Abstracts of the Medical Image Computing and Computer-Assisted Intervention – MICCAI’99 Berlin. Berlin/Heidelberg Springer; 1999.
52. Levi D, Rampa F, Barbieri C, Pricca P, Franzini A, Pezzotta S. True 3D reconstruction for planning of surgery on malformed skulls. Childs Nerv Syst 2002; 18(12): 705–706.
53. Fruhwald J, Schicho KA, Figl M, Benesch T, Watzinger F, Kainberger F. Accuracy of craniofacial measurements: computed tomography and three-dimensional computed tomography compared with stereolithographic models. J Craniofac Surg 2008; 19(1): 22–26.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2011 Číslo 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Restless Legs Syndrome
- Using a Combination of Magnetic Resonance Techniques for Tumour Diagnosis
- Hyperkinetic Disorder/Attention Deficit Hyperactivity Disorder in Children with Epilepsy
- Invasive Fungal Sinusitis
- Remodelling Surgery in Craniosynostosis
- SOUTĚŽ ČNS
- Deep Brain Stimulation in Patients Suffering from Movement Disorders – Stereotactic Procedure and Intraoperative Findings
- Treatment of Peroneal Nerve Injury by Operation
- Radiation-Induced Meningiomas
- Bilateral Syphylitic Chorioretinitis in a 33-year-old Pervitin User
- Pantothenate Kinase-Associated Neurodegeneration – a Case Report
- Progressive Axonal Sensory and Motor Multifocal Polyneuropathy in a Patient with Chronic Hepatitis C
- Virus hepatitidy C a nervový systém: Komentář k práci Ambler et al Progredující axonální senzitivně-motorická multifokální polyneuropatie u pacientky s chronickou hepatitidou C
-
The Greenberg Rapid Review. A Companion to the 7th Edition
L. I. Kranzler. - A Case of Creutzfeldt-Jakob Disease Showing Decreased Cerebral Blood Flow on Tc-99m ECD SPECT at an Early Stage
- Sudden Dyspnoea as a First Symptom Leading to a Diagnosis of Amyotrophic Lateral Sclerosis – a Case Report
- Webové okénko
-
Analýza dat v neurologii
XXVI. Hodnocení diagnostických testů – věrohodnostní poměr a diagnostický poměr šancí - Průběh a závěry mezioborového setkání „Winter GLIO TRACK Meeting“ 2011
- Vzpomínky na neurology, s nimiž jsme rádi spolupracovali
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Restless Legs Syndrome
- Treatment of Peroneal Nerve Injury by Operation
- Sudden Dyspnoea as a First Symptom Leading to a Diagnosis of Amyotrophic Lateral Sclerosis – a Case Report
- Invasive Fungal Sinusitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



