-
Medical journals
- Career
Syntéza, charakterizace, studie molekulárního dokování nových alkylových derivátů 5-(2-brom-4-fluorfenyl)-4-ethyl - 4H-1,2,4-triazol-3-thiolu
Authors: Roman Shcherbyna; Valerü Kalchenko; Sergii Kulish; Volodymyr Salionov; Liubov Morozova; Natalia Nedorezaniuk; Olha Mazur
Published in: Čes. slov. Farm., 2023; 72, 190-200
Category: Original Articles
Overview
The main goal of this article is to present the results of the synthesis of new alkyl derivatives of 5-(2-bromo4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol and molecular docking studies against COX-1 and COX-2. Previous studies have established a wide range of biological activity of 1,2,4-triazole derivatives. Therefore, it was essential to determine how a new series of 1,2,4-triazole derivatives would provide potential anti-inflammatory activity. To reach the goal, raw alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiols (2a-2i) from 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole3-thiol (1e) were obtained. The structure of the synthesized compounds was confirmed by 1H-NMR elemental analyses. The individuality and purity of compounds were confirmed by the method of liquid chromatography-mass spectrometry. These compounds have a relatively simple synthesis scheme, which gives them an advantage in creating a potential drug, and the appearance of alkyl radicals in the molecule should positively affect pharmacokinetic indicators, stability, selectivity, and bioavailability. An in silico study was conducted for the synthesized compounds, namely molecular docking, in relation to the interaction with COX-1 and COX-2. Based on the selectivity indexes of binding modes observed for the selected compounds (2e, 2g) with active COX-1 centers, it was found that compounds can reliably exhibit their anti-inflammatory effect through the prostaglandin biosynthesis pathway, inhibiting COX-1 instead of COX-2. The effect of hydrophobic interactions of alkyl groups of 1,2,4-triazole derivatives on changes in affinity and selectivity to COX-1 or COX-2 has also been proven. Therefore, derivatives of 1,2,4 are promising candidates for improvement, further study, and future development of new, more powerful antiinflammatory drugs for therapeutic use.
Keywords:
1,2,4-triazole – synthesis – molecular docking – anti-inflammatory activity – in silico
Introduction
Inflammation is a complex biological response to harmful stimuli, such as pathogens, irritants, or tissue damage, and is a contributing factor to the development of a number of chronic diseases. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat inflammation and pain, but long-term use of such drugs has been associated with serious gastrointestinal side effects, such as ulcers, bleeding, and perforation, which limits their therapeutic efficacy1).
COX enzymes are responsible for the production of pro-inflammatory prostaglandins and are the main target of NSAIDs. In particular, selective COX-2 inhibitors have been developed to reduce gastrointestinal side effects associated with non-selective COX inhibitors2, 3). Unfortunately, many of these medications are also associated with an increased risk of cardiovascular events4). In addition, recent studies have shown that selective COX-2 inhibitors may contribute to certain cancers’ development5). Thus, there is an urgent need to develop new selective drugs with better safety profiles that can effectively inhibit both COX-1 and COX-2 without causing serious side effects6–8).
The scientific literature contains large amounts of data describing the synthesis of 9–12), biological13, 14), pharmacological15–20), and other types of activities of 21–25) derivatives of 1,2,4-triazole. On the basis of these heterocycles, powerful and harmless potential drug candidates are created and modern drugs are registered9, 13, 14, 21, 24). This is due to the fact that usually the compounds containing the 1,2,4-triazole nucleus in their structure are endowed with high biological activity13, 16, 19, 20) and low toxicity13, 14, 24). In this respect, 1,2,4-triazole derivatives have shown promising antiinflammatory activity due to their ability to inhibit cyclooxygenase (COX) enzymes in combination with low toxicity26–30). The binding of arachidonic acid, a natural substrate, to COX-1/2, is significantly affected by hydrophobic interactions of the aliphatic chain31). As a result, developing new analogues of 1,2,4-triazole with various aliphatic substitutions is crucial for evaluating their effect on COX-1/2 binding affinity.
1. Data from protocols used for molecular docking verification 
Materials and methods
The melting points data for synthesized compounds were obtained by the open capillarymethod with a MPA100 (OptiMelt, USA) device with a range of temperature measurements of 30–400 °C and 1 °C resolution. The elemental analysis of synthesized compounds was provided by an Elementar Vario L cube analyzer (Carbon, Hydrogen, Nitrogen, Sulfur (II)). The 1H NMR spectra were obtained by a Varian MR400 spectrometer with 400 MHz dimension and hexadeuterodimethyl sulfoxide (DMSO-d6) as the solvent with next analyzation by the ADVASP Analyzer program. The individuality and purity of the compounds were carried out using an Agilent 1260 Infinity HPLC System (Agilent Technologies, Germany) and an Agilent 6120 single quadrupole mass spectrometer with ionization in electrospray (ESI). All chemicals were obtained from UKRORGSYNTEZ Ltd. (Kyiv, Ukraine) with
documental approval of their purity and quality.
Molecular doсking
Ligand structures were drawn using Marvinsketch software and converted to SDF format using OpenBabel. The ligands were additionally subjected to energy minimization using Chimera. X-ray crystal structures of COX-1/2 cyclohexogenases (PDB ID: 1EGQ/1CX2) with co-crystallized ligands ibuprofen and SC-558 (bromocelecoxib), respectively, obtained from Protein Data Bank (PDB). Polar hydrogen atoms and the combined charges of Coleman atoms were then added to the resulting protein structures to prepare them for docking studies.
The DockingPie Vina32) plugin in PyMOL was used to perform the study of protein and ligand docking and their conversion to pdbqt format. To confirm the docking protocol, the data of which are shown in Table 1, it was validated.
Previous studies show that the RMSD values, which represent the difference between the calculated and crystallographic conformations of the ligand complex, should not exceed 2.0 Å33, 34). Re-docking allowed us to obtain similarities in the overlap of crystallographic postures (orientation + conformation, blue) and calculated (yellow) postures, which illustrates the low RMSD value (Fig. 1).
PyMOL v. 2.5 and Discovery Studio Visualizer35) were used to create shapes of receptor-ligand complexes). The predicted inhibitory constant (pKi) was estimated using the following standardized equation36):
pKi=10[connectionenergyestimation1.336]

Synthesis and structural characterization
The initial compound 5-(2-bromo-4-fluorophenyl)-4--ethyl-4H-1,2,4-triazole-3-thiol (1e) was synthesized by known methods11, 16, 18, 19), 2-bromo-4-fluorobenzoic acid (1a, CAS#1006-41-3) was used as the starting substance, from which isopropyl 2-bromo-4-fluorobenzoate (1b) and the corresponding 2-bromo4-fluorobenzohydrazide (1c) were synthesized. Subsequently, 2-(2-bromo-4-fluorobenzoyl)-N-ethyl-hydrazine-1-carbothioamide (1d) was obtained by interacting the 1c compound in an alcoholic medium with ethyl isothiocyanate. 5-(2-Bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (1e) was obtained in two stages. In the first stage, the 1d compound was boiled in excess alkali, and in the second stage, the hydrochloric acid reaction mixture was neutralized with filtration of the resulting precipitate (Fig. 2)
Further synthesis was carried out by reacting the initial thiol 1e with the relevant quantity of halohenalkans (iodomethane, bromoethane, 1-bromopropane, 1-bromobutane, 1-bromopentane, 1-bromhexane, 1-bromoheptane, 1-bromooctane, 1-bromononane) in i-propanol medium and the presence of equivalent potassium hydroxide (Fig. 3).
The structure of the synthesized compounds (1e, 2a-i) was confirmed by: 1H-NMR (Nuclear Magnetic Resonance Spectroscopy), EA (Elemental analyses). Individuality and purity of compounds were confirmed by the method of LC/MS (liquid chromatography-mass spectrometry) (Figs. 4 and 5).
Analyzing the obtained nuclear magnetic resonance spectra, the structure of the synthesized compounds was confirmed. The first sign of the alkylation reaction of the initial thiol was the absence of an SH group signal indicating the formation of an alkyl derivative. Sets of signal protons of S-alkyl fragments were fixed in the corresponding magnetic field, and their parameters coincided with the literature data. For example, proton signals of the methyl group are expressed at 2.75 ppm as a singlet. Elongation of the alkyl chain provokes a shift in proton signals towards a stronger field (+ I – and + M-effects). Thus, the proton signals of the methyl fragment (2a-2i) gradually changed to 0.85 ppm. Proton signals of the methylene fragment were observed in a strong field in the form of triplets (3.12–3.23 ppm) or multiplets (1.21–1.43 ppm, 1.63–1.80 ppm). Signals in the form of doublets (7.27–7.81 ppm) are generated in the proton absorption region of the aromatic fragment.
1. Receptors in complexes with calculated and experimental ligand conformation: ibuprofen with COX-1(A), bromocelecoxib with COX-2 (B) 
2. Scheme of synthesis 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (1e) 
3. Scheme of synthesis of alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (2a-2i) 
4. LC/MS-spectrum of the 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(octylthio)-4H-1,2,4-triazole 2h 
5. L. LC/MS-spectrum 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(hexylthio)-4H-1,2,4-triazole 2f 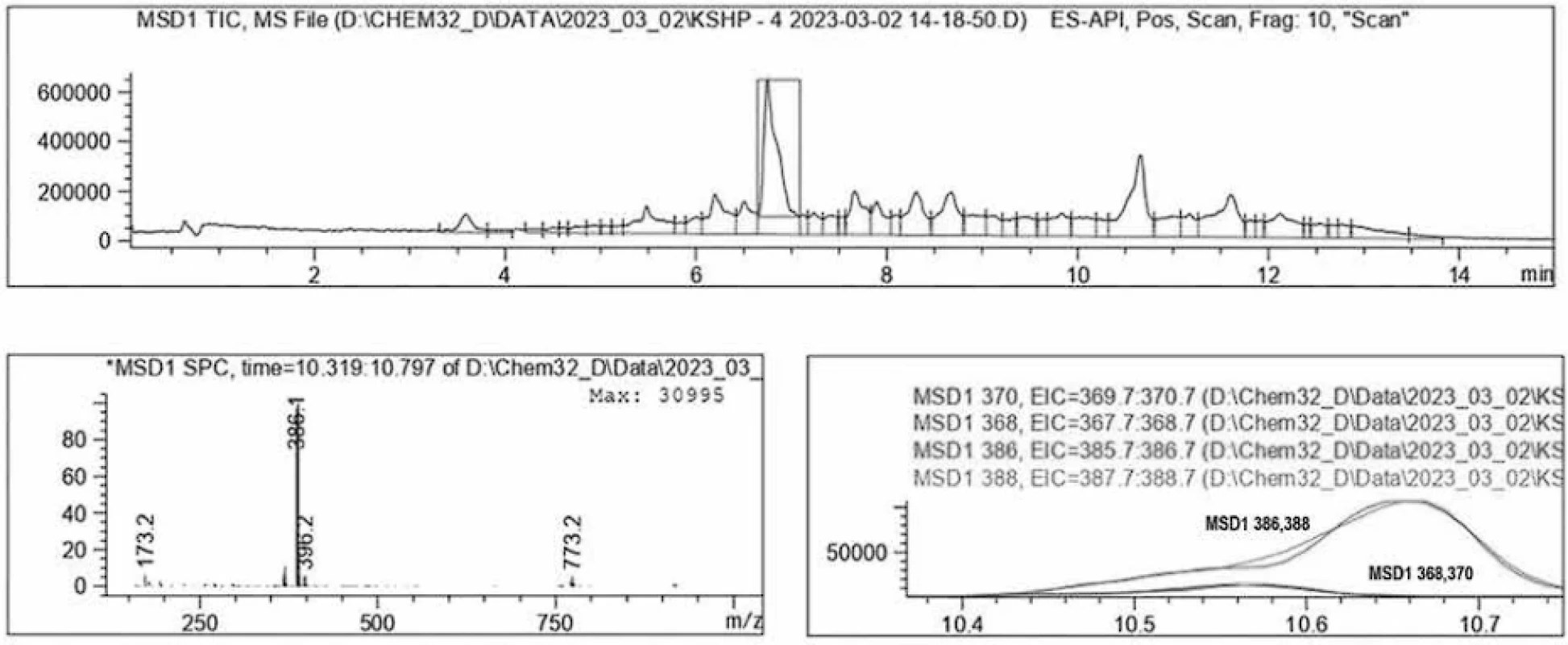
Synthesis of isopropyl 2-bromo-4-fluorobenzoate 1b. To 0.01 mol of the acid 1a in 50 ml i-propanol was added 0.001 mol acid sulfuric. The reaction mixture was heated during 6 h, cooled, and neutralized by a water solution of sodium carbonate to pH = 7. The obtained ether was retrieved from the solution by chloroform which was evaporated. The synthesized compound is a white crystalline substance. The ether was recrystallized from propane-2-ol for analysis.
Isopropyl 2-bromo-4-fluorobenzoate 1b: White residue; yield 87 %; m. p. 258–260 °C.; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.86 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.58c(d, 1H, Н-3, 2-Br-4-F-C6H3), 7.23 (d, 1H, Н-5, 2-Br-4-F-C6H3), 5.22–5.13 (m, 1H, O-CH-(CH3)2), 1.31–1.26 (m, 6H, O-CH-(CH3)2). ESI-MS: m/z = 262 [M+H]+, Anal. Calcd. For C10H10BrFO2: C, 46.00; H, 3.86. Found: C, 45.55; H, 3.95.
Synthesis of 2-bromo-4-fluorobenzohydrazide 1c. To 0.01 mol of the ether 1b in 50 ml i-propanol was added 1,2 ml hydrazine hydrate solution (60%). The reaction mixture was heated for 2h, cooled, and evaporated. The synthesized compound is a white crystalline substance. The hydrazide was recrystallized from propane-2-ol for analysis.
2-Bromo-4-fluorobenzohydrazide 1c. White residue; yield 81 %; m. p. 272–274 °C.; 1H NMR (400 Mz, DMSOd6) δ ppm: 9.77 (t, 1H, NH-NH2), 7.80 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.60 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.29 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.61 (d, 2H, NH-NH2). ESI-MS: m/z = 234 [M+H]+, Anal. Calcd. For C7H6BrFN2O: C, 36.08; H, 2.60; N, 12.02. Found: C, 35.55; H, 2.95; N, 12.19.
Synthesis of 2-(2-bromo-4-fluorobenzoyl)-N-ethylhydrazine-1-carbothioamide 1d. To 0.01 mol of the hydrazide 1c in 50 ml i-propanol was added 0.01 mol ethyl isothiocyanate. The reaction mixture was heated to full solving of reagents and left to cool at room temperature to form the residue, which was filtered. The synthesized compound is a white crystalline substance. The carbothioamide was recrystallized from propane-2-ol for analysis.
2-(2-bromo-4-fluorobenzoyl)-N-ethylhydrazine-1-carbothioamide 1d. White residue; yield 78 %; m. p. 242 – 244 °C.; 1H NMR (400 Mz, DMSOd6) δ ppm: 10.44 (d, 1H, NH-NH), 9.40 (d, 1H, NH-NH), 7.79 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.63 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.33–7.21 (m, 2H, Н-5, 2-Br-4-F-C6H3, S = C-NH-CH2-CH3), 3.65–3.55 (m, 2H, S = C-NH-CH2-CH3), 1.17 (t, 3H, S = C-NH-CH2-CH3). ESIMS: m/z = 321 [M+H]+, Anal. Calcd. For C10H11BrFN3OS: C, 37.51; H, 3.46; N, 13.12; S, 10.01. Found: C, 37.01; H, 3.40; N, 13.31; S, 9.81.
Synthesis of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol 1e. To 0.01 mol of carbothioamide 1d in 50 ml distilled water was added 0.02 mol sodium hydroxide. The reaction mixture was heated for 4h, cooled, and neutralized by hydrochloric acid to pH = 7. The forming residue was filtered and dried on air. The synthesized compound is a white crystalline substance that was recrystallized from propane-2-ol for analysis.
5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole3-thiol 1e. White residue; yield 76 %; m. p. 180–182 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.81 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.65 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.24 (d, 1H, Н-5,2-Br-4-F-C6H3), 6.64 (s, 1H, SH), 4.32 (t, 2H, N-CH2-CH3), 1.46 (t, 3H, N-CH2-CH3). ESI-MS: m/z = 303 [M+H]+, Anal. Calcd. For C10H9BrFN3S: C, 39.75; H, 3.00; N, 13.91; S, 10.61. Found: C, 40.21; H, 3.09; N, 13.80; S, 10.55.
General synthesis of the alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (2a-2i). To 0.01 mol of the thiol 1e solution in 50 ml i-propanol was added 0.01 mol of potassium hydroxide which was previously dissolved in a minimal amount of distilled water. The reaction mixture was heated until the thiol dissolved. After the 0.01 mol of appropriate halohenalkan (iodomethane, bromoethane, 1-bromopropane, 1-bromobutane, 1-bromopenta-ne, 1-bromhexane, 1-bromoheptane, 1-bromooctane, 1-bromononane) was added and continued heating to boiling on water to pH = 7. The obtained solutions were filtered, and the filtrates were evaporated. The synthesized compounds are white crystalline substances (2a, 2b), yellow (2c, 2d, 2e, 2g, 2h), and orange (2f, 2i) color. The compounds 2a-2i were recrystallized from propane-2-ol for analysis.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(methylthio)--4H-1,2,4-triazole 2a: White residue; yield 81 %; m. p. 156–158 °C.; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.86 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.58 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.23 (d, 1H, Н-5, 2-Br-4-F-C6H3), 5.22-5.13 (m, 1H, O-CH-(CH3)2), 1.31–1.26 (m, 6H, O-CH-(CH3)2); ESI-MS: m/z = 317 [M+H]+; Anal. Calcd. For C11H11BrFN3S: C, 41.79; H, 3.51; N, 13.29; S, 10.14. Found: C, 41.31; H, 3.58; N, 13.17; S, 10.01.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(ethylthio)-4H-1,2,4-triazole 2b: White residue; yield 72 %; m. p. 120 – 118 °C.; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.82 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.67 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.25 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.34 (t, 2H, N-CH2-CH3), 3.15 (t, 2H, S-CH2-CH3), 1.41 (t, 3H, N-CH2-CH3), 1.36 (t, 2H, S-CH2-CH3), ESI-MS: m/z = 331 [M+H]+; Anal. Calcd. For C12H13BrFN3S: C, 43.65; H, 3.97; N, 12.73; S, 9.71. Found: C, 43.99; H, 3.94; N, 12.65; S, 9.79.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(propylthio)-4H-1,2,4-triazole 2c: Yellow residue; yield 68 %; m. p. 109–107 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.81 (d,1H, Н-6, 2-Br-4-F-C6H3), 7.68 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.27 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.30 (t, 2H, N-CH2-CH3), 3.10 (t, 2H, S-CH2-CH2-CH3), 1.81–1.71 (m, 2H, S-CH2-CH2-CH3), 1.42 (t, 3H, N-CH2-CH3), 1.05 (t, 3H, S-(CH2)2-CH3); ESI-MS: m/z = 344 [M+H]+; Anal. Calcd. For C13H15BrFN3S: C, 45.36; H, 4.39; N, 12.21; S, 9.31. Found: C, 45.89; H, 4.36; N, 12.29; S, 9.26.
- 3-(2-bromo-4-fluorophenyl)-5-(butylthio)-4-ethyl-4H-1,2,4-triazole 2d: Yellow residue; yield 63 %; m. p. 113 – 115 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.79 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.70 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.26 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.34 (t, 2H, N-CH2-CH3), 3.15 (t,2H, S-CH2-(CH2)2-CH3), 1.71–1.62 (m, 2H, S-CH2-CH2-CH2-CH3), 1.47–1.33 (m, 5H, S-(CH2)2-CH2-CH3, N-CH2-CH3), 0.89 (t, 3H, S-(CH2)3-CH3); ESI-MS: m/z = 358 [M+H]+; Anal. Calcd. For C14H17BrFN3S: C, 46.93; H, 4.78; N, 11.73; S, 8.90. Found: C, 46.23; H, 4.76; N, 11.65; S, 8.81.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(pentylthio)-4H-1,2,4-triazole 2e: Yellow residue; yield 64 %; m. p. 103–105 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.80 (d,1H, Н-6, 2-Br-4-F-C6H3), 7.68 (d, 1H, Н-3, 2-Br-4-F-C6H3),p 7.27 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.35 (t, 2H, N-CH2-CH3), 3.12 (t, 2H, S-CH2-(CH2)3-CH3), 1.78–1.65 (m, 2H, S-CH2-CH2-(CH2)2-CH3), 1.46–1.31 (m, 7H, S-(CH2)2-(CH2)2-CH3,N-CH2-CH3), 0.95–0.83 (t, 3H, S-(CH2)4-CH3); ESI-MS:m/z = 372 [M+H]+; Anal. Calcd. For C15H19BrFN3: C,48.39; H, 5.14; N, 11.29; S, 8.61. Found: C, 48.65; H, 5.17; N, 11.37; S, 8.55.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(hexylthio)-4H-1,2,4-triazole 2f: Orange residue; yield 67 %; m. p. 118 120 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.82 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.65 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.25 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.34 (t, 2H, N-CH2-CH3), 3.11 (t, 2H, S-CH2-(CH2)4-CH3), 1.72–1.64 (m, 2H, S-CH2-CH2-(CH2)3-CH3), 1.48 (t, 3H, N-CH2-CH3), 1.39-1.25 (m, 6H,S-(CH2)2-(CH2)3-CH3,) 0.91–0.82 (t, 3H, S-(CH2)5-CH3); ESI--MS: m/z = 386 [M+H]+; Anal. Calcd. For C16H21BrFN3S: C, 49.74; H, 5.48; N, 10.88; S, 8.30. Found: C, 49.41; H, 5.46; N, 10.81; S, 8.36.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(heptylthio)- 4H-1,2,4-triazole 2g: Yellow residue; yield 71 %; m. p. 93–95 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.79 (d, 1H, Н-6, 2-Br-4-F-C6H3), 7.68 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.27 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.31 (t, 2H, N-CH2-CH3), 3.12 (t, 2H, S-CH2-(CH2)5-CH3), 1.73–1.65 (m, 2H, S-CH2-CH2-(CH2)4-CH3), 1.45–1.39 (m, 5H, N-CH2-CH3, S-(CH2)2-CH2-(CH2)3-CH3), 1.30–1.23 (m, 6H, S-(CH2)3-(CH2)3-CH3) 0.95–0.81 (t, 3H, S-(CH2)6-CH3); ESI-MS: m/z = 401 [M+H]+; Anal. Calcd. For C17H23BrFN3S: C, 51.00; H, 5.79; N, 10.50; S, 8.01. Found: C, 51.25; H, 5.75; N, 10.54; S, 8.05.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(octylthio)-4H-1,2,4-triazole 2h: Yellow residue; yield 74 %; m. p. 87–89 °C;1H NMR (400 Mz, DMSOd6) δ ppm: 7.81 (d, 1H, Н-6,2-Br-4-F-C6H3), 7.66 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.28 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.34 (t, 2H, N-CH2-CH3), 3.10 (t, 2H, S-CH2-(CH2)6-CH3), 1.71–1.64 (m, 2H, S-CH2-CH2 - (CH2)5-CH3), 1.46 (t, 3H, N-CH2-CH3) 1.37–1.31 (m, 4H,S-(CH2)2-C(H2)2-(CH2)3-CH3), 1.29–1.23 (m, 6H, S-(CH2)4-(CH2)3-CH3) 0.95–0.81 (t, 3H, S-(CH2)7-CH3); ESI-MS: m/z = 414 [M+H]+; Anal. Calcd. For C18H25BrFN3S: C, 52.17; H,6.08; N, 10.14; S, 7.74. Found: C, 52.44; H, 6.04; N, 10.20;S, 7.76.
- 3-(2-bromo-4-fluorophenyl)-4-ethyl-5-(nonylthio)-4H-1,2,4-triazole 2i: Orange residue; yield 65 %; m. p. 78–80 °C; 1H NMR (400 Mz, DMSOd6) δ ppm: 7.80 (d,1H, Н-6, 2-Br-4-F-C6H3), 7.69 (d, 1H, Н-3, 2-Br-4-F-C6H3), 7.26 (d, 1H, Н-5, 2-Br-4-F-C6H3), 4.31 (t, 2H, N-CH2-CH3),3.12 (t, 2H, S-CH2-(CH2)7-CH3), 1.69–1.63 (m, 2H, S-CH2-CH2-(CH2)6-CH3), 1.44 (t, 3H, N-CH2-CH3) 1.36–1.30 (m,2H, S-(CH2)2-CH2-(CH2)5-CH3), 1.26–1.16 (m, 10H, S-(CH2)3-(CH2)5-CH3), 0.93–0.81 (t, 3H, S-(CH2)8-CH3); ESI-MS:m/z = 429 [M+H]+; Anal. Calcd. For C19H27BrFN3S: C,53.27; H, 6.35; N, 9.81; S, 7.40. Found: C, 53.49; H, 6.31;N, 9.79; S, 7.48.
Results and discussion
Uptake, distribution, metabolism, and elimination ADME properties (uptake, distribution, metabolism, and elimination) were predicted using Lipinsky’s rule of five36) in the ADMETlab and SWISSADME Web Services (Table 2). According to Lipinski’s rule of five, a molecule violates this rule if it has more than 5 hydrogen bond donors, a molecular weight of more than 500, a log p value greater than 5, and the sum of N and O atoms greater than 10, resulting in poor absorption or penetration of the drug. A lipophilicity logP value of less than 5.0 implies a good distribution coefficient. The water solubility value of logS suggests that molecules in the range of –5.0 to 0.5 have druglike properties. Higher logD and logP values, as well as fewer hydrogen bonds, predict greater bioavailability of drugs. The topological polar surface (TPSA) area should not exceed 140 Å2, and exceeding this indicator is associated with low penetration through the bloodbrain barrier and poor membrane permeability. In addition, the hydrogen bond donor and acceptor sum must be less than or equal to 12.
2. Drug similarity and bioavailability profile of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (1e) and alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4–triazole-3-thiol (2a–2i) 
3. Pharmacokinetic profile of uptake and metabolism of the studied 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (1e) and alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (2a–2i) 
Compounds 1e, 2a, 2b, 2c, 2d, 2e, and 2f have demonstrated properties that indicate their potential use as therapeutic agents. Their molecular weight was less than 500 Daltons, and they showed fewer than five hydrogen bond donors and fewer than ten acceptors. The Consensus LogP values also corresponded to Lipinsky’s rule. The polar surface area of all the compounds studied ranged from 56.01 Å2 and 69.51 Å2, respectively, which indicates acceptable membrane permeability37).
When analyzing the uptake and distribution parameters, it was found that 1e-2e ligands have a positive rate of passing the blood-brain barrier (Table 3). None of the ligands are a substrate of P-glycoprotein, but all of them can act as inhibitors of at least one of the main cytochromes of P450.
Molecular docking
Molecular docking studies allow us to understand the efficiency of ligand binding to target cyclooxygenases and evaluate their selectivity. The results of the study, including binding affinity, inhibition constants, and selectivity indices, are presented in Table 4. Comparing the affinity values of the studied compounds, we can state a certain pattern: with an increase in the hydrocarbon chain, the selectivity of COX-2 with respect to COX-1 decreases. The optimal hydrocarbon radical was the С5–С7 range for COX-1 cyclooxygenase. Docking showed that a number of compounds (1e-2i) have a potential to bind to COX-1/2, and their values range from –6.672 to –7.843 kcal/mol. Compounds 2e and 2g showed binding energies of –7.843 and –7.796 kcal/mol and predicted inhibition constants of 0.135 and 0.146 µm, respectively, indicating a higher affinity for the enzyme compared to ibuprofen. In addition, the pKi ratio values showed that compounds 2e and 2g had a higher potential selectivity to COX-1 than ibuprofen. On the other hand, none of the compounds studied showed a COX-2 binding energy greater than that of the native SC-588 ligand. Substance 2e had a pKi = 5.019 coefficient, which indicates that 2e is 5 times higher than the potential selectivity for COX-1 inhibition compared to COX-2. There was also a noticeable increase in the pKi ratio for 2g, which indicates a relatively higher COX-1 selectivity index. Undoubtedly, the celecoxib derivative was the most selective for COX-2, confirming practical use and consistent with previous studies38).
4. Results of the study of the attachment of synthesized compounds to COX-1 (pdb: 1EQG) and COX-2 (pdb: 1CX2) compared to re–attached native ligands (ibuprofen and SC-588/ bromocelecoxib) 
6. Graphical representation of the binding position and interaction of 2e with COX-1 cyclooxygenase 
Accordingly, compounds 2e and 2g were selected for further study as potential scaffolds with high COX-1 form a pair of hydrogen bonds with AGR120 (SER35339), compound 2e achieves a higher affinity value of –7.843 kcal/mol by forming alkyl hydrophobic bonds between the amino acids ILE523, ALA527, VAL349, TYR355, LEU531, VAL116, LEU359 and the aliphatic substituent and π-alkyl contacts between residues leu352, Le523, ala527, val349 and the aromatic system of 1,2,4-triazole. There was also a stabilizing π-π t-like interaction between the π-electron cloud of the aromatic ring of 4-fluorophenyl and the aromatic ring of indole TPR387 (Fig. 6).
7. 2D and 3D interactions of the studied 2g ligand with COX-1 
For the 2g compound, a similar binding profile was recorded as for 2e, with a binding energy of –7.796 kcal/mol. The difference was the presence of alkyl hydrophobic contacts between ILE345, MET113 residues, and the heptyl radical. In total, there were 18 hydrophobic interactions of the alkyl-π, π-π, and alkyl types (4.01–6.09 Å) with ILE523, ALA527, VAL349, TYR355, LEU531, VAL116, LEU359, LEU352, ILE345, MET113, and TPR387 (Fig. 7).
Conclusion
As a result of the study, 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole-3-thiol (1e) and alkyl derivatives of 5-(2-bromo-4-fluorophenyl)-4-ethyl-4H-1,2,4-triazole3-thiol (2a-2i) were synthesized. The structure of the synthesized compounds was confirmed by using 1H-NMR, elemental analyses, and by the method of LC/MS. For the synthesized compounds, in silico studies were performed, namely molecular docking in relation to the interaction with COX-1 and COX-2. The compounds (2e, 2g) show a higher affinity or preference for binding to COX-1 over COX-2, which indicates their potential to selectively target COX-1 and inhibit its activity, thereby reducing the production of inflammatory prostaglandins. However, it is important to acknowledge the limitations of our study. First, we focused on the in-silico molecular docking approach and some aspects of metabolism and pharmacokinetics, which provide valuable information on ligand binding and the predicted bioactivity of compounds. However, future studies should include in vitro and in vivo experiments to confirm the inhibitory activity and evaluate the pharmacological properties of these compounds.
The effect of hydrophobic interactions of alkyl groups of 1,2,4-triazole derivatives on changes in affinity and selectivity to COX-1 or COX-2 has also been proven. Furthermore, the structure-activity relationship analysis revealed that increasing the hydrocarbon chain length led to decreased selectivity for COX-2 inhibition. This observation suggests that further exploration of different substitutions and modifications in the 1,2,4-triazole scaffold is necessary to enhance selectivity while maintaining potent anti-inflammatory activity. Certain chemical simulations, along with pharmacophore search, are also needed to improve the pharmacokinetic properties and increase the probability of binding to the ARG120 residue, which is considered critical for the selectivity of the COX-1 enzyme40). To overcome the limitations, comprehensive in vitro studies using human cell-based assays or tissue samples are used to evaluate the efficacy and safety profiles of compounds 2e and 2g. Additionally, performing structure-activity relationship studies focusing on optimizing selectivity for COX-1 inhibition without compromising potency against COX-2 would provide valuable information on the potential of 1,2,4-triazole derivatives as anti-inflammatory agents. Thus, the derivatives of 1,2,4-triazole are promising candidates for improvement, further study, and future development of new, more powerful antiinflammatory drugs for therapeutic use.
Conflict of interests: none.
Assoc. Prof. Roman Shcherbyna, Dr. Pharm. Sci. (*) • V. Kalchenko Department of natural sciences for foreign students and toxicological chemistry
Maiakovskyi avenue 26, 69035 Zaporizhzhia, Ukraine
e-mail: rscherbyna@gmail.comV. Salionov
Department of biological chemistry, Zaporizhzhya, UkraineL. Morozova
Department technologies, processing of livestock products and feeding, Vinnytsia National Agrarian University, UkraineN. Nedorezaniuk • O. Mazur
Department of Pharmaceutical Chemistry, National Pirogov Memorial Medical University, Vinnitsia, Ukraine
Sources
- Laine L. Gastrointestinal effects of NSAIDs and coxibs. J. Pain Symptom Manage. 2003; 25(2), 32–40.
- Hawkey C., Skelly M. Gastrointestinal safety of selective COX-2 inhibitors. Curr. Pharm. Des. 2002; 8(12), 1077–1089.
- Van der Vijver R. J., van Laarhoven C. J. H. M., Lomme R. M. L. M., Hendriks T. Carprofen for perioperative analgesia causes early anastomotic leakage in the rat ileum. BMC Vet. Res. 2012; 8(1), 247.
- Mukherjee D. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001; 286(8), 954.
- Vinogradova Y., Coupland C., Hippisley-Cox J. Exposure to cyclooxygenase-2 inhibitors and risk of cancer: Nested case–control studies. Br. J. Cancer 2011; 105(3), 452–459.
- Chen Z., Wang Z-C., Yan X-Q., Wang P-F., Lu X-Y., Chen L-W., Zhu H-L., Zhang H-W. Design, synthesis, biological evaluation and molecular modeling of dihydropyrazole sulfonamide derivatives as potential COX-1/COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2015; 25(9), 1947–1951.
- Alegaon S.G., Hirpara M.B., Alagawadi K.R., Hullatti K.K., Kashniyal K. Synthesis of novel pyrazole–thiadiazole hybrid as potential potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2014; 24(22), 5324–5329.
- Gouda A., Ali H., Almalki W., Azim M., Abourehab M., Abdelazeem A. Design, synthesis, and biological evaluation of some novel pyrrolizine derivatives as Cox inhibitors with anti-inflammatory/analgesic activities and low ulcerogenic liability. Molecules 2016; 21(2), 201–221.
- Gotsulya A., Brytanova T. Synthesis, properties and biological potential some condensed derivatives 1,2,4-triazole. J. Fac. Pharm. Ankara Univ. 2022; 46(2), 308–321.
- Fedotov S., Gotsulya A., Zaika Y., Brytanova T. Design, synthesis and molecular docking of some derivatives of 9-methylpyrazolo[1,5-d][1,2,4]triazolo[3,4-f][1,2,4]triazine-3-thiol. J. Fac. Pharm. Ankara Univ. 2023; 47(2), 336–348.
- Ihnatova T., Kaplaushenko A., Frolova Y., Pryhlo E. Synthesis and antioxidant properties of some new 5-phenethyl-3-thio-1,2,4-triazoles. Pharmacia 2021; 68(1), 129-133.
- Varynskyi B. A., Scherback M. A., Kaplaushenko A. G., Yurchenko I. A. The study of thione-thiol tautomerism of 4-amino-5-(4-nitrophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione by HPLC-MS method. J. Chem. Pharm. Res. 2014; 6(5), 1342–1350.
- Sameliuk Y., Kaplaushenko A., Diakova F., Ostretsova L., Nedorezaniuk N., Gutyj B. Prospects For the Search For New Biologically Active Compounds Among the Derivatives of the Heterocyclic System of 1,2,4-Triazole. Hac. Univ. J. Fac. Pharm. 2022; 42(3), 175–186.
- Shcherbyna R., Parchenko V., Martynyshyn V., Hunchak V. Evaluation of acute and subacute toxicity of oil liniment based on 4-((5-(decylthio)-4-methyl-4H-1,2,-4-triazol-3-yl)methyl)morpholine. Ank. Univ. Ecz. Fak. Derg. 2018; 42(1), 43–52.
- Frolova Y., Kaplaushenko A., Yurii S., Romanina D., Morozova L. Investigation of the antimicrobial and antifungal activities of some 1,2,4-triazole derivatives. Ces. Slov. Farm. 2022; 71(4), 151–160.
- Safonov A. A. Derivatives of 3-(alkylthio)-5-(thiophen2-ylmethyl)-4H-1,2,4-triazol-4-amines as anti-fatigue substances. Indones. J. Pharm. 2018; 29(3), 167–172.
- Zvenihorodska T., Hotsulia A., Kravchenko S., Fedotov S., Kyrychko B. Synthesis and antimicrobial action of 1,2,4-triazole derivatives containing theophylline and 1,3,4-thiadiazole fragments in their structure. Afr. J. Biomed. Res. 2021; 24(1), 159–163.
- Karpun Y., Polishchuk N. Synthesis and antimicrobial activity of S-substituted derivatives of 1,2,4-triazol-3-thiol. SciR Pharm. Sci. 2021; 31(3), 64–69.
- Shcherbyna R., Panasenko O., Polonets O., Nedorezaniuk N., Duchenko M. Synthesis, antimicrobial and antifungal activity of ylidenhydrazides of 2-((4-R-5-R1-4H-1,2,4-triazol-3-yl)thio)acetaldehydes. Ank. Univ. Ecz. Fak. Derg. 2021; 45(3), 504–514.
- Shcherbyna R., Pruhlo Y., Duchenko M., Kulagina M., Kudria V., Vashchuk V. Evaluation of antioxidant activity of 1, 2, 4-triazole derivatives with morpholine moiety. Hac. Univ. J. Fac. Pharm. 2022; 42(2), 73–82.
- Safonov A., Demianenko D., Vashchyk Y., Larianovska Y., Lytkin D., Shcherbyna R., Ocheretniuk A., Romanova S. Histological study of a corrective influence of sodium 2-((4-amino-5-(thiophen-2-ylmethyl)-4H-1,2,-4-triazol-3-yl)thio)acetate on the state of rats liver under conditions of acute immobilization stress. Ank. Univ. Ecz. Fak. Derg. 2022; 46(2), 330–341.
- Karpun Y., Fedotov S., Khilkovets A., Karpenko Y., Parchenko V., Klochkova Y., Bila Y., Lukina I., Nahorna N., Nahornyi V. An in silico investigation of 1,2,4-triazole derivatives as potential antioxidant agents using molecular docking, MD simulations, MM-PBSA free energy calculations and ADME predictions. Pharmacia 2023; 70(1), 139–153.
- Shcherbyna R., Vashchyk Y. The research of 1,2,4-triazole derivatives hepatoprotective activity under tetracycline and infectious hepatitis. Ank. Univ. Ecz. Fak. Derg. 2019; 43(2), 135–146.
- Shcherbyna R. An investigation of the pharmacokinetics and potential metabolites of potassium 2-((4-amino5-(morfolinometyl)-4H-1,2,4-triazol-3-yl)thio) acetate on rats. Ank. Univ. Ecz. Fak. Derg. 2020; 44(2), 233–241.
- Vashchyk Y., Shcherbyna R., Parchenko V., Bushueva I., Gutyj B., Fotina H., Fotina T., Stronskyi Y. Histological study of a corrective influence of a compound potassium 2-((4-amino-5-(morpholinomethyl)-4H-1,2,4-triazol-3-yl)thio)acetate (PKR-173) on the state of chicken’s liver under infection by Pseudomonas aeruginosa. Ank. Univ. Ecz. Fak. Derg. 2020; 44(1), 1–17.
- Tariq S., Kamboj P., Alam O., Amir M. 1,2,4-Triazole-based benzothiazole/benzoxazole derivatives: Design, synthesis, p38α MAP kinase inhibition, anti-inflammatory activity and molecular docking studies. Bioorg. Chem. 2018; 81, 281–290.
- Hamoud M. M. S., Osman N.A., Rezq S., Abd El-wahab H. A. A., Hassan A. E. A., Abdel-Fattah H. A., Romero D. G., Ghanim A. M. Design and synthesis of novel 1,3,4-oxadiazole and 1,2,4-triazole derivatives as cyclooxygenase-2 inhibitors with anti-inflammatory and antioxidant activity in LPS-stimulated raw264.7 macrophages. Bioorg. Chem. 2022; 124, 105–808.
- Paprocka R., Kołodziej P., Wiese-Szadkowska M., Helmin-Basa A., Bogucka-Kocka A. Evaluation of anthelmintic and anti-inflammatory activity of 1,2,4-triazole derivatives. Molecules 2022; 27(14), 44–88.
- Mohassab A. M., Hassan H. A., Abdelhamid D., Abdel-Aziz M., Dalby K. N., Kaoud T. S. Novel quinoline incorporating 1,2,4-triazole/oxime hybrids: Synthesis, molecular docking, anti-inflammatory, Cox Inhibition, ulceroginicity and histopathological investigations. Bioorg. Chem. 2017; 75, 242–259.
- Li S-M., Tsai S-E., Chiang C-Y., Chung C-Y., Chuang T-J., Tseng C-C., Jiang W-P., Huang G-J., Lin C-Y., Yang Y-C., Fuh M-T., Wong F.-F. New methyl 5-(halomethyl)-1-aryl-1H-1,2,4-triazole-3-carboxylates as selective COX-2 inhibitors and anti-inflammatory agents: Design, synthesis, biological evaluation, and Docking Study. Bioorg. Chem. 2020; 104,104–333.
- Malkowski M. G., Ginell S. L., Smith W. L., Garavito R. M. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science 2000; 289(5486), 1933–1937.
- Rosignoli S., Paiardini A. DockingPie: a consensus docking plugin for PyMOL. Bioinformatics 2022; 38(17), 4233–4234.
- Hevener K. E., Zhao W., Ball D. M., Babaoglu K., Qi J., White S. W., Lee R. E. Validation of Molecular Docking Programs for Virtual Screening against Dihydropteroate Synthase. J. Chem. Inf. Model 2009; 49(2), 444–460.
- Costa J. S., Costa K. S., Cruz J. V., Ramos R. S., Silva L. B., Brasil D. S. B., Silva C. H. T .P., Santos C. B. R., Macedo W. J. C. Virtual Screening and Statistical Analysis in the Design of New Caffeine Analogues Molecules with Potential Epithelial Anticancer Activity. Int. J. Mol. Sci. 2018; 24(5), 576–594.
- Dassault Système. 3DS Discovery Studio Visualizer. https//discover.3ds.com/discovery-studio-visualizer-down load. (01.03.2023).
- Alamri M. A., Tahir ul Qamar M., Mirza M. U., Bhadane R., Alqahtani S. M., Muneer I., Froeyen M., Salo-Ahen O. M. H. Pharmacoinformatics and molecular dynamics simulation studies reveal potential covalent and FDA-approved inhibitors of SARS-COV-2 main protease 3CLpro. J. Biomol. Struct. Dyn. 2020; 39(13), 4936–4948.
- Veber D. F., Johnson S. R., Cheng H. Y., Smith B. R., Ward K. W., Kopple K. D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002; 45(12), 2615–2623.
- Szabó G., Fischer J., Kis-Varga Á., Gyires K. New celecoxib derivatives as anti-inflammatory agents. J. Med. Chem. 2008; 51(1), 142–147.
- Selinsky B. S., Gupta K., Sharkey C. T., Loll P. J. Structural Analysis of NSAID Binding by Prostaglandin H2 Synthase: Time-Dependent and Time-Independent Inhibitors Elicit Identical Enzyme Conformations. Biochemistry 2001; 40(17), 5172–5180.
- Sejdiu B. I., Tieleman D. P. COX-1 – lipid interactions: arachidonic acid, cholesterol, and phospholipid binding to the membrane binding domain of COX-1. bioRxiv. 2020; 1–29.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2023 Issue 4-
All articles in this issue
- Celkové intravenózne anestetiká – farmakodynamika, farmakokinetika a chirálne vlastnosti
- Diazotační titrace v lékopisné kontrole jakosti léčiv a návrhy pro jejich revizi v Evropském lékopisu*
- Vliv digoxinu, valproátu sodného a celekoxibu na mozkovou cyklooxygenázovou dráhu a neuron-specifickou enolázu při pentylenetetrazolem podnícené záchvaty u myší
- Studium protizánětlivých vlastností hustého extraktu Tribulus terrestris L.
- Syntéza, charakterizace, studie molekulárního dokování nových alkylových derivátů 5-(2-brom-4-fluorfenyl)-4-ethyl- 4H-1,2,4-triazol-3-thiolu
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Celkové intravenózne anestetiká – farmakodynamika, farmakokinetika a chirálne vlastnosti
- Studium protizánětlivých vlastností hustého extraktu Tribulus terrestris L.
- Vliv digoxinu, valproátu sodného a celekoxibu na mozkovou cyklooxygenázovou dráhu a neuron-specifickou enolázu při pentylenetetrazolem podnícené záchvaty u myší
- Diazotační titrace v lékopisné kontrole jakosti léčiv a návrhy pro jejich revizi v Evropském lékopisu*
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

