-
Medical journals
- Career
Synthesis and anti-infective evaluation of 5-amino-N-phenylpyrazine-2-carboxamides
Authors: Jan Zitko; Fernando Franco; Pavla Paterová
Authors‘ workplace: Charles University in Prague ; Department of Pharmaceutical Chemistry and Drug Control, Faculty of Pharmacy
Published in: Čes. slov. Farm., 2015; 64, 19-24
Category: Original Articles
Overview
A series of eleven novel 5-amino-N-phenylpyrazine-2-carboxamides were synthesized and evaluated for in vitro anti-infective properties. Prepared compounds were characterized by IR, 1H NMR and 13C NMR spectra, elementary analysis and melting points. Lipophilicity parameters Log P and ClogP were calculated. None of the compounds was effective against any of tested mycobacterial strains (Mycobacterium tuberculosis H37Rv, M. kansasii, M. avium) up to concentration of 100 μg/mL. 5-amino-N-(2,5-dimethylphenyl) pyrazine-2-carboxamide (3) exerted moderate antibacterial activity against Staphylococcus aureus (MIC = 62.5 μM). No antifungal activity was detected. Several compounds exerted moderate antiviral activity against influenza A viruses at the level of tens of μM.
Key words:
pyrazinamide • antimycobacterial activity • antibacterial activity • antifungal activity • antiviral activityIntroduction
Even in the 21st century tuberculosis (TB) remains the 2nd leading cause of death among all infectious diseases. According to WHO worldwide estimates, there were 9 million new cases of TB in 2013 and 1.5 million of TB-associated deaths1). Although the incidence rates of TB (both in relative and absolute numbers) have been decreasing since the beginning of the new millennium, the rate of decline remains slow (2% per year worldwide). In 2013, 3.5% of new and 20.5% of previously treated TB cases were estimated to be multidrug-resistant1) (MDR, resistant to at least isoniazid and rifampicin, the two most powerful anti-TB drugs). The rising abundance of resistant tuberculosis is an imperative to develop new anti-TB drugs, and this imperative has recently led in development of several new compounds active against MDR-TB, namely bedaquiline (a diarylquinoline), delamanid and pretomanid (both nitroimidazoles), which have been introduced to clinical practice or are in late stages of clinical development2, 3).
The development of new antitubercular drugs is a long-term project on the Faculty of Pharmacy in Hradec Králové (Charles University, Czech Republic)4–6). Research of our laboratory is focused on design of pyrazinamide (PZA) derivatives as potential antituberculars. PZA is a first-line anti-TB drug and is used in all basic treatment regimens. Recently, PZA has been a subject of increased scientific attention and several studies have suggested new and specific mechanisms of action of this drug and its simple derivatives7–10). Practically, the sterilizing activity of PZA, which is given by its ability to kill non-replicating persisting sub-population of mycobacteria in the host’s organism, is the reason why PZA is a vital component of newly developed MDR-TB treatment regimens3, 11). In our previous publication we showed that 5‑alkylamino-N-phenylpyrazine-2-carboxamides possessed significant activity against M. tuberculosis H37Rv with MIC ranging from 0.78 to 3.13 ∝g/mL (approximately 3–12 μM)12). Based on the relatively insignificant differences in activity among derivatives with varied length of the alkyl chain (propylamino to octylamino derivatives), we hypothesized that all these derivatives could be metabolized via oxidative N‑dealkylation13, 14) to common metabolite of 5‑amino-N-phenylpyrazine-2-carboxamide. This compound was synthesized and tested to found it was completely inactive (MIC > 100 μg/mL), possibly due its low lipophilicity, which could have prevented effective diffusion into mycobacterial cell through its lipophilic envelope12). In the same study and under same conditions, 5‑amino-N-(2-chlorophenyl)pyrazine-2-carboxamide was inactive as well. To further support the finding that 5‑amino-N-phenylpyrazine-2-carboxamides lack in vitro activity against M. tbc H37Rv and other mycobacterial strains, we decided to synthesize and evaluate a small series of 5-amino-N-phenylpyrazine-2-carboxamides with various substituents in the phenyl part. Some substituents were chosen to increase lipophilicity, which could enhance the ability to penetrate the mycobacterial cell.
Experimental part
General
All chemicals (unless stated otherwise) were purchased from Sigma-Aldrich (Schnelldorf, Germany). The reaction process and the purity of final compounds were checked using Merck Silica 60 F254 TLC plates (Merck, Darmstadt, Germany). Microwave assisted reactions were performed in microwave reactor CEM Discover (focused field) connected to autosampler Explorer 24 (CEM Corporation, Matthews, NC, USA). Flash chromatography of the final compounds was run on automated chromatograph CombiFlash Rf (Teledyne Isco, Lincoln, NE, USA) using columns filled with Kieselgel 60, 0.040–0.063 mm (Merck, Darmstadt, Germany), detection wavelength 280 nm. NMR spectra were recorded on Varian VNMR S500 (Varian, Palo Alto, CA, USA) at 500 MHz for 1H and 125 MHz for 13C or at Varian Mercury VX-BB 300 at 300 MHz for 1H and 75 MHz for 13C. The spectra were recorded in DMSO-d6 or CDCl3 at ambient temperature. The chemical shifts as δ values in ppm are indirectly referenced to tetramethylsilane (TMS) via the solvent signal. IR spectra were recorded on Nicolet Impact 400 (Nicolet, Madison, WI, USA) using ATR-Ge method. Elemental analysis was performed on CE Instruments EA-1110 CHN analyser (CE Instruments, Wigan, United Kingdom). All values are given as percentages. Melting points were determined in open capillary on Stuart SMP30 melting point apparatus (Bibby Scientific Limited, Staffordshire, UK) and are uncorrected. Yields are given as percentages and refer to the amount of chromatographically pure product after all purification steps. Log P and ClogP was calculated using the program CS ChemBioDraw Ultra version 14.0 (CambridgeSoft, Cambridge, MA, USA).
General synthetic procedure for compounds 1–11
Substituted 5-chloro-N-phenylpyrazine-2-carboxamide15) (1 mmol) was placed into special microwave tube (10 mL) with magnetic stirrer and approximately 3 mL of MeOH and 3 mL of 25% (v/v) aqueous ammonia was added. The tube was closed with septum and placed into microwave reactor. The reaction was set up for 160 °C, microwave power 100 W, PowerMAX Mode, 45 min. The reaction was performed in a closed tube (i.e. over-pressurized.). The generated pressure (dependent on the volume of solvents and the total volume of the reaction tube) was approx 7–8 bar. After reaction, the mixture was moved to round-bottom flask, adsorbed on silica gel/sea sand and subjected to flash-chromatography (silica gel, gradient elution EtOAc/hexane, detection wavelength 280 nm). Finally, compounds were recrystallized from ethanol/water. Analytical data were fully consistent with the proposed structures.
Analytical data of prepared compounds 1-11
5-amino-N-phenylpyrazine-2-carboxamide (1). White solid. Yield: 79%. mp 130.0–131.6 °C. 1H NMR (DMSO-d6, 300 MHz) δ 10.11 (1H, s, CONH), 8.61 (1H, d, J = 1.4 Hz, H3), 7.92 (1H, d, J = 1.4 Hz, H6), 7.84 (2H, d, J = 7.4 Hz, H2´, H6´), 7.31 (2H, t, J = 7.9 Hz, H3´, H5´), 7.23 (2H, bs, NH2), 7.06 (1H, t, J = 7.4 Hz, H4´). 13C NMR (DMSO-d6, 75 MHz) δ 162.5, 157.7, 143.5, 138.9, 132.7, 130.2, 128.8, 123.6, 120.2. IR (ATR-Ge, cm–1): 3384 (NH, NH2), 3352, 3152, 2359, 1670 (C = O, CONH), 1581, 1528, 1444, 1405, 1273, 1018, 753, 690. Anal. Calcd. For C11H10N4O (MW 214.23): C, 61.67; H, 4.71; N, 26.15. Found: C, 61.60; H, 4.52; N, 26.07.
5-amino-N-(3-hydroxyphenyl)pyrazine-2-carboxamide (2). White solid. Yield: 24%. mp 241.0–243.8 °C. 1H NMR (DMSO-d6, 500 MHz) δ 9.92 (1H, s, CONH), 9.38 (1H, s, OH), 8.59 (1H, s, H3), 7.90 (1H, s, H6), 7.45–7.40 (1H, m, H2´), 7.25–7.13 (3H, m, H4´, NH2), 7.07 (1H, t, J = 8.0 Hz, H5´), 6.52–6.42 (1H, m, H6´). 13C NMR (DMSO-d6, 125 MHz) δ 162.3, 157.7, 157.6, 143.3, 139.9, 132.7, 130.2, 129.4, 111.0, 110.7, 107.2. IR (ATR-Ge, cm–1): 3458 (NH, NH2), 3318, 3111 (OH), 2946, 1645 (C = O, CONH), 1583, 1531, 1450, 1404, 1285, 1225, 1160, 1019, 786, 690. Anal. Calcd. For C11H10N4O2 (MW 230.23): C, 57.39; H, 4.38; N, 24.34. Found: C, 57.11; H, 4.75; N, 24.19.
5-amino-N-(2,5-dimethylphenyl)pyrazine-2-carboxamide (3). White solid. Yield: 52%. mp 235.8–237.4 °C. 1H NMR (DMSO-d6, 300 MHz) δ 9.59 (1H, s, CONH), 8.58 (1H, s, H3), 7.89 (1H, s, H6), 7.65 (1H, s, H6´), 7.24 (2H, s, NH2), 7.10 (1H, d, J = 7.6 Hz, H4´), 6.88 (1H, d, J = 7.6 Hz, H3´), 2.26 (3H, s, CH3), 2.20 (3H, s, CH3). 13C NMR (DMSO-d6, 75 MHz) δ 162.0, 157.7, 143.1, 136.2, 135.4, 132.5, 130.4, 130.2, 126.9, 125.3, 123.6, 21.0, 17.3. IR (ATR-Ge, cm–1): 3366 (NH, NH2), 3150, 2925, 2855, 2359, 1653 (C = O, CONH), 1582, 1533, 1407, 1260, 1017, 792, 679. Anal. Calcd. For C13H14N4O (MW 242.28): C, 64.45; H, 5.82; N, 23.13. Found: C, 64.69; H, 5.92; N, 22.96.
5-amino-N-(3,5-dimethoxyphenyl)pyrazine-2-carboxamide (4). White solid. Yield: 50%. mp 227.1–230.0 °C. 1H NMR (DMSO-d6, 500 MHz) δ 10.01 (1H, s, CONH), 8.59 (1H, d, J = 1.4 Hz, H3), 7.90 (1H, d, J = 1.4 Hz, H6), 7.23 (2H, s, NH2), 7.17 (2H, d, J = 2.2 Hz, H2´, H6´), 6.22 (1H, t, J = 2.3 Hz, H4´), 3.72 (6H, s, CH3). 13C NMR (DMSO-d6, 125 MHz) δ 162.5, 160.6, 157.7, 143.4, 140.5, 132.6, 130.2, 98.4, 95.8, 55.3. IR (ATR-Ge, cm–1): 3439 (NH, NH2), 3373, 3117, 2976, 1674 (C = O, CONH), 1581, 1519, 1425, 1274, 1160, 1070, 1020, 827. Anal. Calcd. For C13H14N4O3 (MW 274.28): C, 56.93; H, 5.15; N, 20.43. Found: C, 57.05; H, 5.31; N, 20.47.
5-amino-N-(2-chloro-5-methylphenyl)pyrazine-2-carboxamide (5). White solid. Yield: 58%. mp 256.2–258.1 °C. 1H NMR (DMSO-d6, 500 MHz) δ 9.90 (1H, s, CONH), 8.61 (1H, s, H3), 8.21 (1H, s, H6), 7.89 (1H, s, H6´), 7.49 – 7.22 (3H, m, NH2, H3´), 6.95 (1H, d, J = 8.3 Hz, H4´), 2.30 (3H, s, CH3). 13C NMR (DMSO-d6, 125 MHz) δ 161.8, 158.0, 143.4, 137.6, 134.4, 131.5, 130.6, 129.0, 125.7, 122.1, 120.3, 21.0. IR (ATR-Ge, cm–1): 3377 (NH, NH2), 3332, 3149, 2926, 2358, 1671 (C = O, CONH), 1582, 1522, 1463, 1408, 1270, 1232, 1049, 1017, 793, 679. Anal. Calcd. For C12H11ClN4O (MW 262.7): C, 54.87; H, 4.22; N, 21.33. Found: C, 54.98; H, 4.17; N, 21.19.
5-amino-N-(2-chlorophenyl)pyrazine-2-carboxamide (6). White solid. Yield: 72%. mp 130.0–131.6 °C. 1H NMR (DMSO-d6, 500 MHz) δ 9.97 (s, 1H, CONH), 8.62 (d, J = 1.3 Hz, 1H, H3), 8.36 (dd, J = 8.3, 1.6 Hz, 1H, H6´), 7.90 (d, J = 1.4 Hz, 1H, H6), 7.53 (dd, J = 8.0, 1.5 Hz, 1H, H3´), 7.43–7.30 (3H, m, NH2, H5´), 7.17–7.11 (m, 1H, H4´). 13C NMR (DMSO-d6, 125 MHz) δ161.9, 158.0, 143.5, 134.8, 131.5, 130.6, 129.5, 128.1, 125.1, 123.4, 121.7. IR (ATR-Ge, cm–1): 3395, 3329, 3152, 2359, 1674 (C = O, CONH), 1585, 1517, 1441, 1406, 1269, 1018, 750, 684. IR (ATR-Ge, cm–1):. Anal. Calcd. for C11H9ClN4O (MW 248.67): C, 53.13; H, 3.65; N, 22.53. Found: C, 52.97; H, 3.59; N, 22.41.
5-amino-N-(3-chlorophenyl)pyrazine-2-carboxamide (7). White solid. Yield: 38%. mp 250.3–253.3 °C. 1H NMR (DMSO-d6, 300 MHz) δ 10.34 (1H, s, CONH), 8.61 (1H, d, J = 1.4 Hz, H3), 8.06 (1H, t, J = 2.1 Hz, H2´), 7.92 (1H, d, J = 1.4 Hz, H6), 7.84–7.74 (1H, m, H6´), 7.39–7.24 (3H, m, NH2, H5´), 7.16–7.05 (1H, m, H4´). 13C NMR (DMSO-d6, 75 MHz) δ 162.9, 157.7, 143.7, 140.5, 133.1, 132.3, 130.4, 130.2, 123.2, 119.7, 118.7. IR (ATR-Ge, cm–1): 3392 (NH, NH2), 3328, 3166, 2359, 1673 (C = O, CONH), 1585, 1540, 1481, 1403, 1274, 1018, 771, 683. Anal. Calcd. For C11H9ClN4O (MW 248.67): C, 53.13; H, 3.65; N, 22.53. Found: C, 53.37; H, 3.72; N, 22.48.
5-amino-N-(3-cyanophenyl)pyrazine-2-carboxamide (8). White solid. Yield: 37%. mp 261.8–263.8 °C. 1H NMR (DMSO-d6, 500 MHz) δ 10.51 (1H, s, CONH), 8.62 (1H, s, H3), 8.33 (1H, s, H5), 8.16 (1H, d, J = 7.7 Hz), 7.92 (1H, s), 7.57–7.50 (2H, m), 7.29 (2H, s, NH2). 13C NMR (DMSO-d6, 125 MHz) δ 163.1, 157.8, 143.8, 139.9, 132.1, 130.3, 130.2, 127.0, 124.9, 123.0, 119.0, 111.5. IR (ATR-Ge, cm–1): 3287, 3129, 2926, 2854, 2358, 2232 (CN, nitrile), 1672 (C = O, CONH), 1585, 1541, 1434, 1408, 1287, 1254, 1018, 790, 684. Anal. Calcd. For C12H9N5O (MW 239.24): C, 60.25; H, 3.79; N, 29.27. Found: C, 60.37; H, 3.91; N, 29.45.
5-amino-N-(4-cyanophenyl)pyrazine-2-carboxamide (9). White solid. Yield: 34%. mp 284.2–290.7 °C. 1H NMR (DMSO-d6, 500 MHz) δ 10.57 (1H, s, CONH), 8.62 (1H, s, H3), 8.08 (2H, d, J = 8.5 Hz, H2´, H6´), 7.92 (1H, s, H6), 7.77 (2H, d, J = 8.5 Hz, H3´, H4´), 7.32 (2H, s, NH2). 13C NMR (DMSO-d6, 125 MHz) δ 163.2, 157.8, 144.0, 143.3, 133.2, 132.1, 130.3, 120.2, 119.3, 105.2. IR (ATR-Ge, cm–1): 3435 (NH, NH2), 3333, 3138, 2926, 2854, 2348, 2224 (CN, nitrile), 1671 (C = O, CONH), 1581, 1516, 1405, 1277, 1237, 1175, 1018, 833, 679. Anal. Calcd. For C12H9N5O (MW 239.24): C, 60.25; H, 3.79; N, 29.27. Found: C, 60.38; H, 3.80; N, 29.41.
5-amino-N-(3-(trifluoromethyl)phenyl)pyrazine-2-carboxamide (10). White solid. Yield: 42%. mp 223.5–227.0 °C. 1H NMR (DMSO-d6, 500 MHz) δ 10.50 (1H, s, CONH), 8.62 (1H, d, J = 1.4 Hz, H3), 8.38 (1H, bs, H2´), 8.11 (1H, d, J = 8.2 Hz, H2´), 7.93 (1H, d, J = 1.4 Hz, H4´), 7.54 (1H, t, J = 8.0 Hz, H5´), 7.39 (1H, d, J = 7.7 Hz, H6´), 7.27 (2H, s, NH2). 13C NMR (DMSO-d6, 125 MHz) δ 163.1, 157.8, 143.8, 139.8, 132.3, 130.3, 129.8, 129.5 (q, J = 31.5 Hz), 124.4 (q, J = 272.2 Hz), 123.8, 119.8 (q, J = 3.6 Hz), 116.4 (q, J = 4.0 Hz). IR (ATR-Ge, cm–1): 3385 (NH, NH2), 3352, 3155, 2932, 2855, 2358, 1673 (C = O, CONH), 1582, 1536, 1494, 1406, 1332, 1269, 1127, 1097, 1019, 796, 698. Anal. Calcd. For C12H9F3N4O (MW 282.23): C, 51.07; H, 3.21; N, 19.85. Found: C, 51.13; H, 3.30; N, 19.81.
5-amino-N-(4-(trifluoromethyl)phenyl)pyrazine-2-carboxamide (11). White solid. Yield: 45%. mp 268.0–270.3 °C. 1H NMR (DMSO-d6, 300 MHz) δ 10.49 (1H, s, CONH), 8.63 (1H, d, J = 1.4 Hz, H3), 8.09 (2H, d, J = 8.4 Hz, H3´, H5´), 7.93 (1H, d, J = 1.4 Hz, H5), 7.67 (2H, d, J = 8.6 Hz, H2´, H6´), 7.30 (2H, s, NH2). 13C NMR (DMSO-d6, 75 MHz) δ 163.1, 157.8, 143.9, 142.6, 132.3, 130.3, 126.0 (q, J = 3.9 Hz), 124.6 (q, J = 272.4 Hz), 123.5 (q, J = 31.7 Hz), 120.2. IR (ATR-Ge, cm–1): 3368 (NH, NH2), 3101, 2358, 1672 (C = O, CONH), 1579, 1526, 1407, 1320, 1125, 1065, 1018, 834. Anal. Calcd. For C12H9F3N4O (MW 282.23): C, 51.07; H, 3.21; N, 19.85. Found: C, 50.88; H, 3.17; N, 19.53.
Lipophilicity calculation
Log P (the logarithm of the partition coefficient for n-octanol/water) and ClogP (the logarithm of n-octanol/water partition coefficient P based on established chemical interactions) were calculated using the program CS ChemBioDraw Ultra ver. 14.0 (CambridgeSoft, Cambridge, MA, USA).
In vitro whole cell antimycobacterial activity
Broth microdilution panel method – modified Microplate Alamar Blue Assay. Tested strains of M. tuberculosis H37Rv CNCTC My 331/88, M. kansasii Hauduroy CNCTC My 235/80, and M. avium ssp. avium Chester CNCTC My 80/72 were obtained from Czech National Collection of Type Cultures (CNCTC), National Institute of Public Health, Prague, Czech Republic. Middlebrook 7H9 broth enriched with OADC supplement (both from Sigma Aldrich) of declared pH = 6.6 was used for cultivation. Tested compounds were dissolved and diluted in DMSO, mixed with broth (25 μL of DMSO solution in 4.475 mL of broth and placed (100 μL) into microplate wells. Mycobacterial inocula were suspended in isotonic saline solution and the density was adjusted to 0.5–1.0 McFarland. These suspensions were diluted by 10–1 and used to inoculate the testing wells, adding 100 μL of suspension to 100 μL of the DMSO/broth solution of tested compound. Final concentrations of tested compounds in wells were 100–50–25–12.5–6.25–3.13–1.56 μg/mL. Isoniazid (INH) was used as positive control (inhibition of growth). Negative control consisted of broth plus DMSO. 30 μL of Alamar Blue working solution (1 : 1 mixture of 0.1% resazurin sodium salt (aq. sol.) and 10% Tween 80) was added usually after 5 days of incubation. Results were then determined after 24 h of incubation and interpreted according to Franzblau et al.16) The minimum inhibitory concentration (MIC, μg/mL) was determined as the lowest concentration which prevented the blue to pink colour change.
In vitro antibacterial activity
Microdilution broth method12). Reference strains from Czech Collection of Microorganisms (Brno, Czech Republic): Staphylococcus aureus CCM 4516/08, Escherichia coli CCM 4517, Pseudomonas aeruginosa CCM 1961. Clinical isolates from Department of Clinical Microbiology, University Hospital and Faculty of Medicine in Hradec Králové, Charles University in Prague, Czech Republic: Staphylococcus aureus H 5996/08-methicilin resistant (MRSA), Staphylococcus epidermidis H 6966/08, Enterococcus sp. J 14365/08, Klebsiella pneumoniae D 11750/08, Klebsiella pneumoniae J 14368/08-ESBL positive. Highest concentrations achieved for individual compounds: 500 μM for compound 1, 250 μM for 11, and 125 μM for the rest of the tested compounds (3, 5, 6, 7, 10).
In vitro antifungal activity
Microdilution broth method12). Tested species: Candida albicans ATCC 44859, C. tropicalis 156, C. krusei E28, C. glabrata 20/I, Trichosporon asahii 1188, Aspergillus fumigatus 231, Lichtheimia corymbifera 272 and Trichophyton mentagrophytes 445. Highest concentrations achieved for individual compounds: 500 μM for compound 1; and 125 μM for the rest of the tested compounds (3, 5, 6, 7, 10, 11).
Antiviral evaluation
Antiviral activity in cell culture was assessed by cytopathic effect (CPE) reduction assays with a broad panel of viruses17–19). The following viruses were examined on human embryonic lung fibroblast cells: herpes simplex virus type 1 (HSV-1); a thymidine kinase-deficient (TK–) HSV‑1 KOS strain resistant to aciclovir; herpes simplex virus type 2 (HSV-2); vaccinia virus; human adenovirus type 2; and vesicular stomatitis virus (VSV). The viruses examined on human cervix carcinoma HeLa cells were: VSV; Coxsackie B4 virus; and respiratory syncytial virus (RSV). African Green Monkey Vero cells were used to determine the antiviral effect on para-influenza-3 virus; reovirus-1; Sindbis virus; Coxsackie B4 virus and Punta Toro virus. Human influenza A/H1N1, A/H3N2 and B viruses were assessed on Madin-Darby canine kidney (MDCK) cells. Finally, activity against human immunodeficiency virus (HIV) type 1 and type 2 was studied in human MT-4 lymphoblast cells. To perform the tests, the virus was added to semiconfluent cell cultures in 96-well plates and, simultaneously, serial dilutions of the test compounds were added. The plates were incubated until clear CPE was reached (typically 3–6 days). Microscopic scoring was then performed to determine the antiviral activity and expressed as 50% effective concentration (EC50). In the case of HIV-1, HIV-2 and influenza virus, virus-induced CPE was determined by the colorimetric formazan-based MTS cell viability assay.
Results and discussion
Summary of prepared compounds
5-amino-N-phenylpyrazine-2-carboxamides 1–11 were prepared from original samples of 5‑chloro-N-phenylpyrazine-2-carboxamides left from our previous study 15). The nucleophilic substitution of chlorine for amino group was achieved by treating the corresponding starting compound with aqueous solution of ammonia with MeOH as co-solvent (Fig. 1). The reaction was performed in a closed tube under microwave irradiation. The closed system allowed us to work above the atmospheric boiling point of the solvents, under elevated pressure, and, importantly, prevented the volatile ammonia to escape from the reaction. Under these conditions, the products were obtained in reasonable yields (ranging from 24 to 79% of alter all purification steps), while the same reaction attempted in boiling MeOH or EtOH in open atmosphere did not yield satisfactory results even after several hours. See Table 1 for the summary of prepared compounds.
Fig. 1. Synthesis and structure of 5-amino-N-phenylpyrazine-2-carboxamides 1–11 
1. Summary of prepared 5-amino-N-phenylpyrazine-2-car- boxamides 1–11 and their calculated lipophilicity parameters 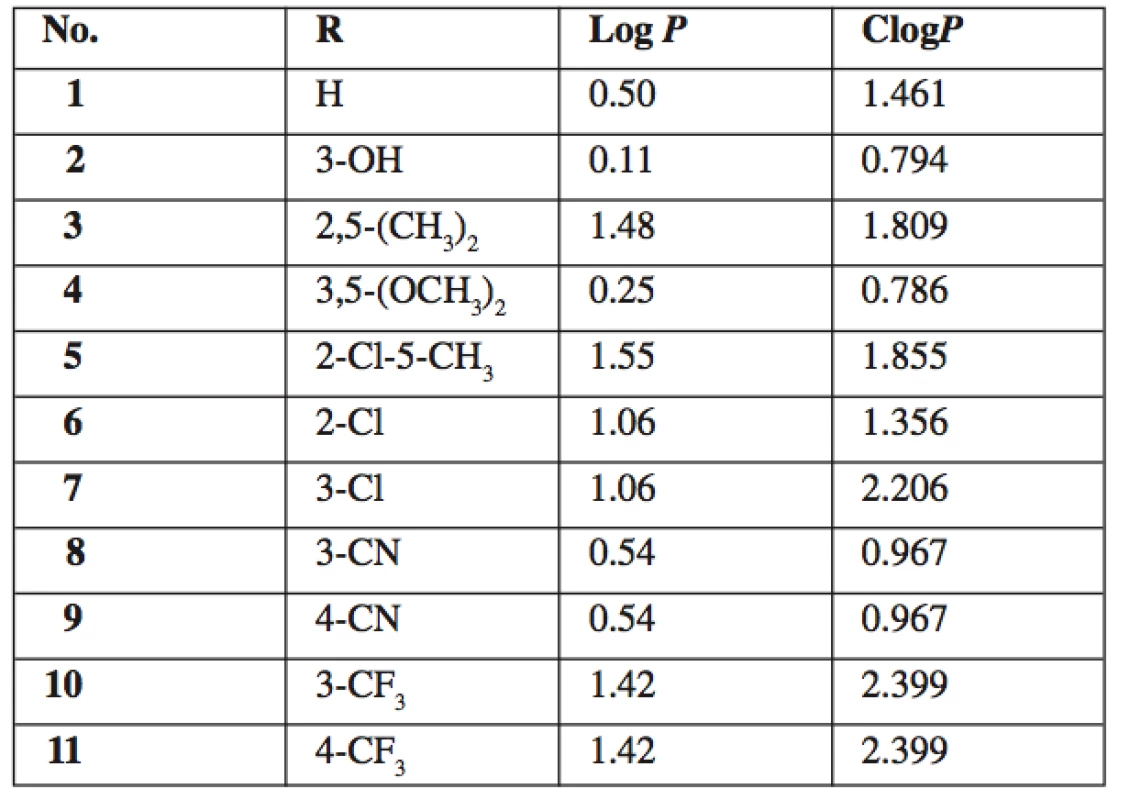
In vitro antimycobacterial activity
All final compounds 1–11 were tested for in vitro whole cell antimycobacterial activity against M. tuberculosis H37Rv, M. kansasii and M. avium. No detectable activity was observed up to the concentration of 100 μg/mL (MIC ≥ 100 μg/mL). This inactivity is in concordance with the results of previously performed evaluation of compounds 1 and 6 12). The MIC values for standard isoniazid were 0.2–0.39 μg/mL for M. tbc, 1.56–6.25μg/mL for M. kansasii and 12.5 μg/mL for M. avium. On the contrary, most of the corresponding parent 5-chloro-N-phanylpyrazine-2-carboxamides, from which final compounds 1–11 were prepared, exerted good antimycobacterial activity with MIC = 0.78–6.25μg/mL against M. tbc H37Rv15, 20). Therefore we can conclude that substitution of chorine in 5-chloro-N-phanylpyrazine-2-carboxamides for amino group completely abolishes antimycobacterial activity. This fact can be partially explained by a significant decrease of lipophilicity (the difference in calculated log P between parent 5-chloro derivatives and 5-amino derivatives 1–11 is 0.99).
In vitro antibacterial and antifungal activity
As a complementary test, compounds 1, 3, 5–7, 10, and 11 were tested for in vitro inhibitory activity against selected bacterial and fungal strains of clinical importance. Compound 3 exhibited low activity against Staphylococcus aureus with MIC = 62.5 μM as read after 24 hours of incubation. Compound 11 exerted modest activity against Pseudomonas aeruginosa with MIC = 250 μM (after 24 h). The rest of compounds were completely inactive against all of the tested bacterial strains. No antifungal activity was detected for any of the tested compounds. See Experimental section for the list of tested strains and for tested concentrations achieved for individual compounds.
Antiviral activity
As a complementary test, compounds 1, 3–6, 10, and 11 were tested for potential activity against diverse DNA and RNA viruses. The virus panel (see Experimental for complete list of tested viruses) included pathogens of medical importance such as herpesviruses, HIV and influenza virus. Most compounds did not produce any visible antiviral activity, however compounds 5, 6 and 7 exerted moderate activity against several influenza A viruses (see Table 2). Interestingly, simultaneously tested influenza virus type B was completely resistant. The basis for the antiviral effect of 5–7 remains to be identified.
2. In vitro antiviral activity against influenza A viruses. Activity expressed as EC50, that is compound concentration (μM) that reduces virus-induced cytopathic effect (CPE) to MDCK cells as measured microscopically (CPE score) and by formazan- based colorimetric assay (MTS) 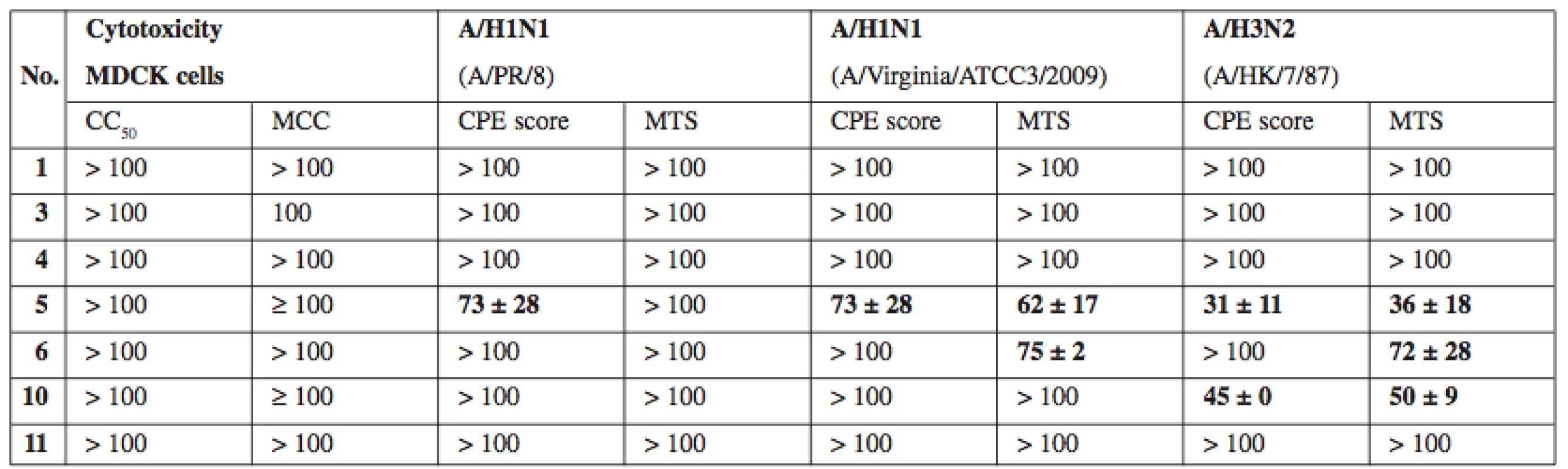
CC50 – concentration reducing the cell viability by 50% as measured by formazan-based colorimetric assay, MCC – minimum compounds concentration to cause microscopically detectable alteration of normal cell morphology, MDCK – Madin-Darby canine kidney cells. Values expressed as average ± SD (n = 2 or 3) Conclusion
The present study confirms our previously published preliminary conclusion that 5-amino-N-phenylpyrazine-2-carboxamides possess no in vitro antimycobacterial activity. However, these compounds were non-toxic in non-cancer mammalian cell line (MDCK – Madin-Darby canine kidney cells) and can potentially found other uses as biologically active compounds. For example, we have documented a moderate activity against influenza A viruses for three of the prepared compounds.
Acknowledgement
This work was financially supported by IGA NT 13346 and SVV 260 183. Authors wish to thank Ida Dufková for performing in vitro antifungal and antibacterial screening and Assoc. Professor Lieve Naesens (Rega Institute for Medical Research, Laboratory of Virology and Chemotherapy, Leuven, Belgium) for coordination of antiviral testing.
Conflicts of interest: none.
Received 27 February 2015 / Accepted 24 March 2015
PharmDr. Jan Zitko, Ph.D. • F. Franco
Department of Pharmaceutical Chemistry and Drug Control, Faculty of Pharmacy
Charles University in Prague
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: jan.zitko@faf.cuni.cz
P. Paterová
Department of Clinical Microbiology, University Hospital, Hradec Králové, Czech Republic
Sources
1. WHO. Global Tuberculosis Report 2014. WHO/HTM/TB/2014.08. Available online: http://apps.who.int/ iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1 (accessed on 26th February 2015).
2. Butler M. S., Blaskovich M. A., Cooper M. A. Antibiotics in the clinical pipeline in 2013. J. Antibiot. (Tokyo) 2013; 66, 571–591.
3. Zumla A. I., Gillespie S. H., Hoelscher M., Philips P. P. J., Cole S. T., Abubakar I., McHugh T. D., Schito M., Maeurer M., Nunn A. J. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect. Dis. 2014; 14, 327–340.
4. Waisser K., Novotna E., Kunes J., Solarikova J. Phenyl salicylates - a new group of potential antituberculotics. Čes. slov. Farm. 2012; 61, 282–284.
5. Opletalova V., Dolezel J. Thiosemicarbazones and their antimycobacterial effects. Čes. slov. Farm. 2013; 62, 78–83.
6. Dolezal M., Vobickova J., Servusova B., Paterova P. Aminopyrazinoic acid esters as potential antimycobacterial drugs. Čes. slov. Farm. 2013; 62, 84–88.
7. Sayahi H., Zimhony O., Jacobs W. R., Shekhtman A., Welch J. T. Pyrazinamide, but not pyrazinoic acid, is a competitive inhibitor of NADPH binding to Mycobacterium tuberculosis fatty acid synthase I. Bioorg Med. Chem. Lett 2011; 21, 4804–4807.
8. Sayahi H., Pugliese K. M., Zimhony O., Jacobs W. R., Shekhtman A., Welch J. T. Analogs of the Antituberculous Agent Pyrazinamide Are Competitive Inhibitors of NADPH Binding to M. tuberculosis Fatty Acid Synthase I. Chem. Biodivers 2012; 9, 2582–2596.
9. Shi W. L., Zhang X. L., Jiang X., Yuan H. M., Lee J. S., Barry C. E., Wang H. H., Zhang W. H., Zhang Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 2011; 333, 1630–1632.
10. Yang J., Liu Y., Bi J., Cai Q., Liao X., Li W., Guo C., Zhang Q., Lin T., Zhao Y., Wang H., Liu J., Zhang X., Lin D. Structural basis for targeting the ribosomal protein S1 of Mycobacterium tuberculosis by pyrazinamide. Mol. Microbiol. 2015; 95, 791–803.
11. Zumla A., Nahid P., Cole S. T. Advances in the development of new tuberculosis drugs and treatment regimens. Nature Reviews Drug Discovery 2013; 12, 388–404.
12. Zitko J., Servusova B., Janoutova A., Paterova P., Mandikova J., Garaj V., Vejsova M., Marek J., Dolezal M. Synthesis and antimycobacterial evaluation of 5-alkylamino-N-phenylpyrazine--2-carboxamides. Bioorg Med. Chem. 2015; 23, 174–183.
13. Coutts R. T., Su P., Baker G. B. Involvement of CYP2D6, CYP3A4, and other cytochrome-P-450 isozymes in N-dealkylation reactions. J. Pharmacol. Toxicol. Methods 1994; 31, 177–186.
14. Coutts R. T., Foster B. C. Metabolism of amphetamines by mycobacterium-smegmatis. Can. J. Microbiol. 1980; 26, 343–349.
15. Zitko J., Servusová B., Paterová P., Mandíková J., Kubíček V., Kučera R., Hrabcová V., Kuneš J., Soukup O., Doležal M. Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides. Molecules 2013; 18, 14807–14825.
16. Franzblau S. G., Witzig R. S., McLaughlin J. C., Torres P., Madico G., Hernandez A., Degnan M. T., Cook M. B., Quenzer V. K., Ferguson R. M., Gilman R. H. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998; 36, 362–366.
17. Naesens L., Stephens C. E., Andrei G., Loregian A., De Bolle L., Snoeck R., Sowell J. W., De Clercq E. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antiviral Res. 2006; 72, 60–67.
18. Naesens L., Vanderlinden E., Roth E., Jeko J., Andrei G., Snoeck R., Pannecouque C., Illyes E., Batta G., Herczegh P., Sztaricskai F. Anti-influenza virus activity and structure-activity relationship of aglycoristocetin derivatives with cyclobutenedione carrying hydrophobic chains. Antiviral Res. 2009; 82, 89–94.
19. Vanderlinden E., Goktas F., Cesur Z., Froeyen M., Reed M. L., Russell C. J., Cesur N., Naesens L. Novel Inhibitors of Influenza Virus Fusion: Structure-Activity Relationship and Interaction with the Viral Hemagglutinin. J. Virol. 2010; 84, 4277–4288.
20. Servusova B., Vobickova J., Paterova P., Kubicek V., Kunes J., Dolezal M., Zitko J. Synthesis and antimycobacterial evaluation of N-substituted 5-chloropyrazine-2-carboxamides. Bioorg Med. Chem. Lett 2013; 23, 3589–3591.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2015 Issue 1-2-
All articles in this issue
- Effectiveness of phytotherapy in supportive treatment of type 2 diabetes mellitus Billberry (Vaccinium myrtillus)
- Bioavailability and factors influencing its rate
- The effect of the size of a conical hopper aperture on the parameters of the flow equation of sorbitol and its size fractions
- Synthesis and anti-infective evaluation of 5-amino-N-phenylpyrazine-2-carboxamides
- Alzheimer’s disease: cost cuts call for novel drugs development and national strategy
- Use of selected OTC drugs: comparing Greece and the Czech Republic
- 5. postgradual and 3. postdoctoral conference Faculty of Pharmacy UK
- Jewish pharmacists in pharmacies of interwar Czechoslovakia and their lives during World War II
- Computational approach to search for novel antituberculostics
- Alkaloids from hydrastidis canadensis and their cholinesterase and prolyl oligopeptidase inhibitory
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Bioavailability and factors influencing its rate
- Effectiveness of phytotherapy in supportive treatment of type 2 diabetes mellitus Billberry (Vaccinium myrtillus)
- Use of selected OTC drugs: comparing Greece and the Czech Republic
- The effect of the size of a conical hopper aperture on the parameters of the flow equation of sorbitol and its size fractions
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

