-
Medical journals
- Career
Down-regulation of TSGA10, AURKC, OIP5 and AKAP4 genes by Lactobacillus rhamnosus GG and Lactobacillus crispatus SJ-3C-US supernatants in HeLa cell line
Authors: Z. Nouri 1; N. Neyazi 1; MH. Modarressi 2; F. Karami 3; A. Abedin-Do 4; Z. Taherian-Esfahani 4; S. Ghafouri-Fard 4; E. Motevaseli 5
Authors‘ workplace: Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran 1; Department of Medical Genetics, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran 2; Department of Medical Genetics, Science and Research Branch, Islamic Azad University, Tehran, Iran 3; Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran 4; Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran 5
Published in: Klin Onkol 2018; 31(6): 429-433
Category: Original Articles
doi: https://doi.org/10.14735/amko2018429Overview
Background:
Cancer testis antigens (CTAs) are considered cancer biomarkers due to their highly specific expression pattern in human malignancies and near absence from normal somatic tissues. Their specific expression has made them potential targets for early diagnosis, assessment of patients’ prognosis and treatment of cancer in recent years. Lactobacilli are a group of probiotics with anti-cancer, immunomodulatory and other beneficial features. These bacteria have been shown to alter expression of several cancer-related genes.
Aim:
We investigated the effect of Lactobacillus rhamnosus GG supernatant (LRS) and Lactobacillus crispatus SJ-3C-US supernatant (LCS) on expression of four CTAs (TSGA10, AURKC, OIP5 and AKAP4) in HeLa cell line after synchronization using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay and quantitative real-time polymerase chain reaction.
Results:
LRS and LCS inhibited HeLa cell growth after 24 h as demonstrated by MTT assay. Expressions of all CTAs were down-regulated after treatment with both supernatants. Conclusion: This study showed the role of Lactobacilli in down-regulation of CTAs genes. Such expression change might be involved in the anticancer effects of these Lactobacilli. The underlying mechanisms of these observations are not clear but epigenetic modulatory mechanisms may participate in this process. Future studies are needed to assess functional roles of Lactobacilli in modulation of other cancer-related genes.
Key words:
probiotic – cancer testis antigen – biomarker – HeLa cell line
Background
Cancer testis antigens (CTAs) are broadly expressed in various cancer tissues and cancer cell lines, but not in normal tissues except for germ cells. This highly specific expression suggests CTAs play a role in carcinogenesis [1,2]. As they trigger immune response, they are considered targeted immunotherapy options for many cancers, including cervical and ovarian cancers [3,4]. Moreover, their cancer-specific pattern has made them diagnostic, prognostic and therapeutic biomarkers [5,6]. CTAs expression appears to be regulated through epigenetic mechanisms, such as deoxyribonucleic acid (DNA) methylation. Demethylation of promoter CpG islands in their coding genes has been associated with their expression in a range of solid tumors [7]. Lactobacilli are a group of probiotics whose sufficient administration has beneficial effects on host health [8]. They are also normal flora of vagina which protect genitourinary tract from microbial infections [9]. In addition to anti-tumoral effects, they modulate immunogenic responses, regulate cytokines production and alter expression of tumor biomarkers [10–12]. Lactobacillus rhamnosus GG (L. rhamnosus GG) and Lactobacillus crispatus SJ-3C-US (L. crispatus SJ-3C-US) are two most predominant species of vagina and cervix which adhere to cervicovaginal cells [13,14]. Our previous study demonstrated the effect of Lactobacilli on modulation of CTAs expression in breast cancer cell line [15]. In the current study, we aimed at assessment of their effect on expression of four CTAs (Testis specific 10 (TSGA10), Aurora kinase C (AURKC), Opa interacting protein 5 (OIP5) and A-Kinase anchoring protein 4 (AKAP4)) in HeLa cervical cancer cell line.
TSGA10 has been primarily identified as CTAs by differential messenger ribonucleic acid (mRNA) display. Over-expression of TSGA10 was observed in many cancer cell lines compared to normal tissue. TSGA10 has been suggested as a target for immunotherapy in malignancies [16,17]. AURKC is a member of Aurora kinase family, which regulates different processes during cell division. AURKC is over-expressed in cervical and colorectal cancers and also in a wide range of cancer cell lines. Over-expression of AURKC can cause higher cell proliferation and migration through kinase activity [18]. OIP5 is another CTAs gene, which is over-ex-pressed in many types of cancer. OIP5 is a putative binding partner of lamina-associated polypeptide 2α (LAP2α) which participates in cell cycle regulation and chromatin organization. OIP5 knockdown has inhibited cell growth [19,20]. AKAP4 transcription has been only detected during spermatogenesis. Moreover, most proteins in fibrous sheath of sperm flagellum are encoded by this gene. In fact, it is a scaffold protein which is needed for effective sperm motility [21]. It has been recognized as a CTA in a variety of cancers, including breast, colorectal and cervical cancers [22–24].
Materials and Methods
Cell culture
Human cervical cancer (HeLa) cell line was purchased from National Cell Bank of Iran, Pasteur Institute of Iran. Cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, California, USA) and 1% penicillin/ streptomycin (Invitrogen, Carlsbad, California, USA). Cells were kept under condition of 37 °C in humidified atmosphere of 5% CO2 to allow adherence.
Cell synchronization
HeLa cells were cultured in RPMI medium containing 10% FBS, and 1% penicillin/ streptomycin for 24 h. Next, cells were counted and sub-cultured with equal numbers in four 25 ccm flasks and synchronized.
Bacterial supernatant preparation
L. rhamnosus and L. crispatus bacteria were inoculated in de Man, Rogosa and Sharpe (MRS) broth (Merck; pH 6.5) and incubated for 24 h in 37 °C. Overnight culture of these two Lactobacilli contained 108 CFU (colony-forming unit)/ mL. These cultures were centrifuged at 7,000 rpm for 7 min, and supernatant was isolated. Lactobacillus rhamnosus GG supernatant (LRS) and Lactobacillus crispatus SJ-3C-US supernatant (LCS) were filtered using 0.2 μm to remove any possible bacteria or debris. The pH of the LRS and LCS were reduced from 6.5 (MRS broth pH) to 4.3. A lactic acid control of MRS with similar pH of both Lactobacillus supernatants (LS) was used to clarify whether cytotoxic effects are related to acidic pH or compounds existing in the supernatant. The following conditions were tested – LCS, pH 4.3; LRS, pH 4.3; MRS, pH 6.5; MRS adjusted with lactate (MRL, pH 4.3).
MTT assay
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide] assay (Sigma,St. Louis, Missouri) was performed to assess the inhibitory effects of LRS and LCS on HeLa cells. In total, 104 cells were seeded in 96-well plate and after 24 h incubation cells were treated with different concentrations (5, 10, 15, 20, 25%) of LS in triplicates. Cells were incubated for 24 h under condition of 37 °C and 5% CO2. Medium was aspirated out, 0.5 mg/ mL MTT reagent was added and plates were kept in dark condition for 4 h. When MTT formazan crystals were produced, 100 μl of dimethyl sulfoxide was added and cell viability was measured by Elisa Plate Reader using the following formula:
Viability (percentage of control) = [(absorbance sample – absorbance blank) / (absorbance control – absorbance blank)] × 100
RNA extraction, cDNA synthesis and qRT-PCR
Total ribonucleic acid (RNA) was isolated from cultured cells (treated and un-treated cells) using TriPure Reagent kit (Roche Applied Science, Germany). RNA quality and quantity were assessed using Nanodrop 2000c spectrophotometer (Thermo Scientific). Complementary DNA (cDNA) was synthesized using PrimeScript RT reagent kit (Takara Bio, Ohtsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was used to analyze mRNA expression of target genes. Alteration in gene expression patterns of four CTAs (TSGA10, AURKC, OIP5 and AKAP4) was assessed by rotor gene 3,000 corbette detection system. PCR was done in final volume of 10μl containing 0.5μl cDNA, 0.5μl of forward and reverse primers (10 pmol), 5μl 2× master mix (Takara Bio, Ohtsu, Japan), and 3.5μl nuclease free water. Primer sequences were obtained from previous studies [1,12,15] and verified using online Primer 3 software and The National Center for Biotechnology Information (NCBI) and The Basic Local Alignment Search Tool (BLAST). The thermal cycling condition was initiated with DNA denaturation at 95 °C for 10 s, following 50 cycles of 2 step denaturation at 95 °C for 10 s and annealing/ extension at 65 °C for 30 s. For each condition, experiment was done in duplicate. Phosphoglucomutase 1 (PGM1) and hypoxanthine phosphoribosyltransferase 1 (HPRT1) genes were used as reference genes. Melting curve analysis was performed to confirm specificity of products. The nucleotide sequences of primers and amplicon sizes are shown in Tab. 1.
1. The nucleotide sequences of primers. 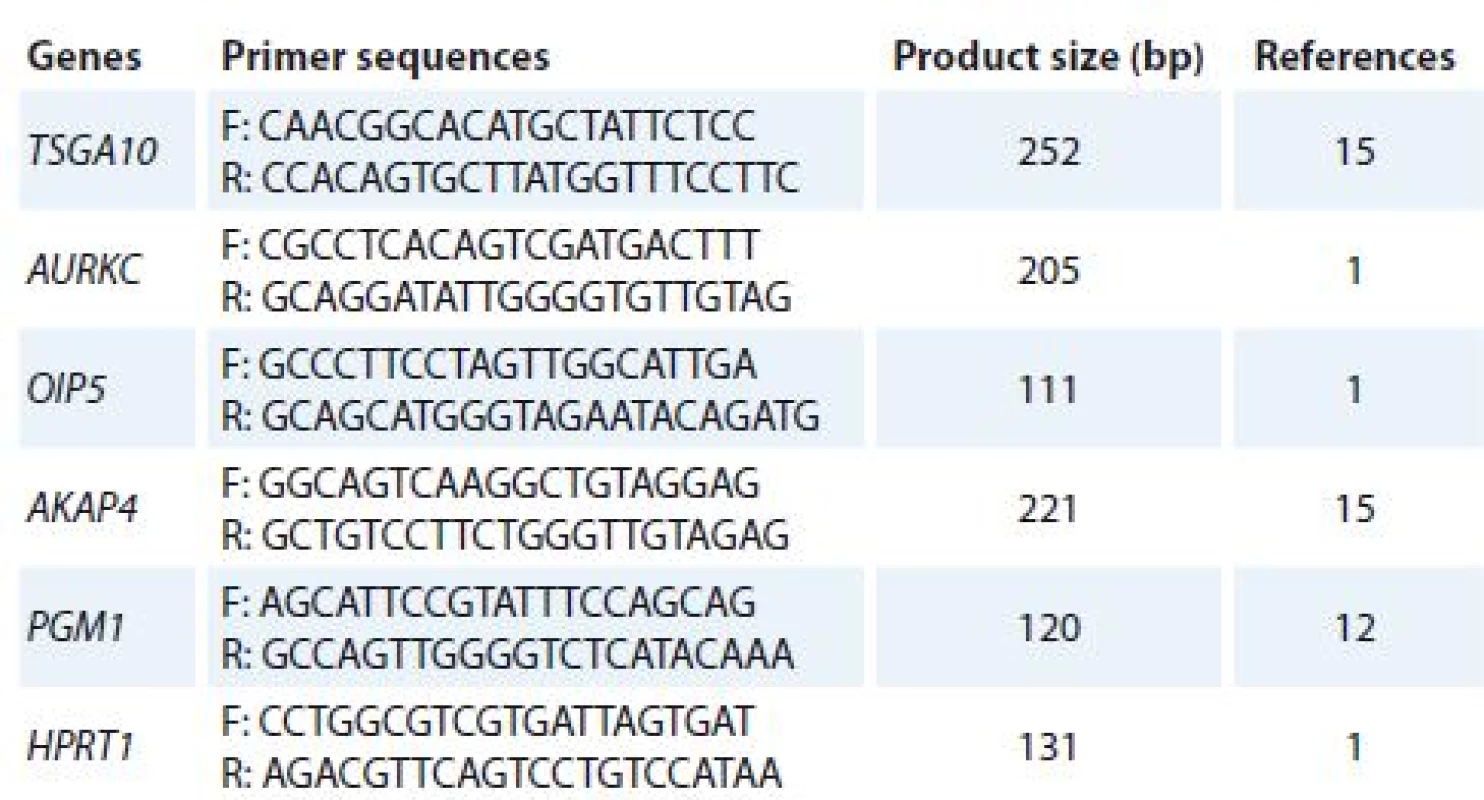
Statistical analysis
The half maximal inhibitory concentrations (IC50) of treatment strategies were compared using the Mann-Whitney test. Data were described as a mean ± SE of 3 distinct experiments. Expression of CTAs were compared between treated and control cells expression using relative expression software tool (REST).
Results
The effects of LRS and LCS on HeLa cells
The results of cell growth inhibition based on MTT assay have shown that LRS and LCS have IC50 of 16% and 12% (v/ v), respectively. Cytotoxic effects of both LSs on HeLa cells were meaningfully greater than MRS and MRL. The results of MTT assay are shown in Graph 1.
1. Cytotoxic effects of different concentrations of LCS, LRS, MRL and MRS with on HeLa cell line as measured by MTT assay. The mean values of 3 separate experiments are shown in each point.
LCS – Lactobacillus crispatus SJ-3C-US supernatant, LRS – Lactobacillus rhamnosus GG supernatant, MRS – Man, Rogosa and Sharpe, HeLa – human cervical cancer cell line, MRL – MRS adjusted with lactate, MTT – [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], v/v – volume/volume![Cytotoxic effects of different concentrations of LCS, LRS, MRL and MRS with
on HeLa cell line as measured by MTT assay. The mean values of 3 separate experiments
are shown in each point.<br>
LCS – <i>Lactobacillus crispatus</i> SJ-3C-US supernatant, LRS – <i>Lactobacillus rhamnosus</i> GG supernatant,
MRS – Man, Rogosa and Sharpe, HeLa – human cervical cancer cell line, MRL
– MRS adjusted with lactate, MTT – [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide], v/v – volume/volume](https://pl-master.mdcdn.cz/media/image_pdf/0b497e7fdeed3c5a901aef8a94bfa725.jpeg?version=1544536505)
Down-regulation of CTAs by LCS and LRS
Expressions of CTAs were evaluated in HeLa cells after synchronization and treatment with supernatant of Lactobacilli. Supernatant of these two Lactobacilli significantly down-regulated transcripts of all CTA genes in HeLa cell line (Graph 2).
2. Expression levels of all CTAs were signifi cantly decreased following treatment of HeLa cells with LCS and LRS. Asterisks show signifi cance.
CTAs – cancer testis antigens, HeLa – human cervical cancer cell line, LCS – Lactobacillus crispatus SJ-3C-US supernatant, LRS – Lactobacillus rhamnosus GG supernatant, MRS – Man, Rogosa and Sharpe, MRL – MRS adjusted with lactate
Discussion
CTA genes characteristics such as cancer-restricted expression profile, immunogenicity, association with tumor progression and induction of their expression by hypomethylation and/ or histone acetylation have suggested their potential as cancer biomarker and therapeutic targets [25]. On the other hand, probiotics possess many anti-tumoral effects, such as anti-proliferative properties, mutagens eliminating effects, moderating side effects of chemotherapy, promoting survival and delaying tumor onset [26]. They also inhibit tumor growth by stimulation of host anti-tumor immune responses [27].
Immunotherapeutic response of L. rhamnosus GG (live or lyophilized) has been observed in female C57BL/ 6 mice implanted with MB49 bladder cancer cells. Such effects have been mainly exerted through recruitment of neutrophils and macrophages in the tumor site [28]. The effects of Lactobacilli depend on preparation method and the administered dose. For example, L. rhamnosus GG (lived and heat-killed) can reduce production of TNFa - -induced interleukin-8 (IL-8) through the NFĸB/ IĸB pathway. While high dose (10^10 CFU/ L) of live L. rhamnosus GG increases IL-8 production, heat-killed Lactobacilli does not. Moreover, doses between 108 and 106 CFU/ L of both preparations diminished TNFα - induced IL-8 production [29].
L. rhamnosus has anti-proliferative effects on ME-180 cell line (a human cervical epithelial-like adenocarcinoma cell line) via moderating cell cycle progression. Treatment with this Lactobacilli resulted in accumulation of host cells in G1 phase through enhancement of expression and nuclear accumulation of p21 [30]. Anti-proliferative effect of L. crispatus SJ-3C-US has been reported in MDA-MB-231 cell line, which has been accompanied by down-regulation of ODF4, PIWIL2, RHOXF2, and TSGA10 [15]. In our previous study, we showed that L. crispatus and L. rhamnosus exist in cervix of healthy women and have cytotoxic effects on cervical cancer cells [9]. In this study, we reported the effect of these strains on expression of four CTAs gene. TSGA10 expression has been previously detected in testes and malignant tissues. This gene encodes a protein of fibrous sheath that is a major constituent of sperm tail in mouse mature spermatozoa [17,31]. We previously reported down-regulation of TSGA10 by Lactobacillus acidophilus and L. crispatus culture supernatants in MDA-MB-231 cells [15]. We hypothesize that its down-regulation by Lactobacilli might affect tumor cell mobility in HeLa or MDA-MB-231 cells. Future studies are needed to assess such effects in cancer cells.
AURKC is a regulatory serine/ threonine kinase, which is involved in mitotic cell division, cytokinesis and meiosis. Abnormal cell division has been seen as a result of AURKC over-expression in vitro [32]. Down-regulation of AURKC can enhance the chemotherapeutic effects of some drugs [33]. Thus, observed down-regulation of AURKC by Lactobacilli might have clinical implications. Future studies are needed to assess whether AURKC suppression by Lactobacilli influences mitotic cell division in cancer cells.
OIP5 accumulation occurs at telo-phase-G1 centromere and is necessary for formation and structure of centromeres/ kinetochores. Knockdown of OIP5 expression in gastric and colorectal cell line increased cell apoptosis [18]. OIP5 also modulates growth and metastasis of hepatocellular malignant cells through AKT/ mTORC1 and β-catenin signaling pathways. MiR-15b-5p inhibits these pathways in hepatocellular carcinoma by targeting OIP5 [34]. The observed down-regulation of OIP5 following treatment with Lactobacilli might affect cell growth and apoptosis which should be assessed in future studies.
AKAP4 gene and protein expressions have been detected in 86% of cervical cancer cell lines where its silencing has led to inhibition of cell proliferation, migration, invasion and colony-forming capacity [24]. Its expression in different malignant tumors potentiated it as a target for cancer immunotherapy [35–37]. The present study demonstrated the negative effects of LRS and LCS on transcriptional activity of AKAP4 in HeLa cells. Future investigations are needed to elaborate the detailed mechanism of probiotics action on invasive and proliferative characteristics of cervical cancer.
Herein, it was demonstrated that LRS and LCS treatment led to down-regulation of four CTAs in HeLa cells. The underlying mechanisms of these observations are not clear but epigenetic modulatory mechanisms may participate in this process.
This study was financially supported by Research Deputy of Tehran University of Medical Sciences. The authors are thankful of Genetic and Biotechnology lab staffs for their cooperation in this research.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 19. 8. 2018
Accepted: 30. 9. 2018
Soudeh Ghafouri-Fard, MD, PhD
Department of Medical Genetics
Shahid Beheshti University of Medical Sciences
Bldg No. 2 SBUMS
Arabi Ave, Daneshjoo Blvd, Velenjak
Tehran, Iran
e-mail: s.ghafourifard@sbmu.ac.ir
Elahe Motevaseli, MD, PhD
Department of Molecular Medicine
School of Advanced Technologies in Medicine
Tehran University of Medical Sciences
Keshavarz Blv. Vali-e Asr Ave.
Tehran, Iran
e-mail: e_motevaseli@tums.ac.ir
Sources
1. Yazarloo F, Shirkoohi R, Mobasheri MB et al. Expression analysis of four testis-specific genes AURKC, OIP5, PIWIL2 and TAF7L in acute myeloid leukemia: a gender-dependent expression pattern. Med Oncol 2013; 30(1): 368. doi: 10.1007/ s12032-012-0368-8.
2. Por E, Byun HJ, Lee EJ et al. The cancer/ testis antigen CAGE with oncogenic potential stimulates cell proliferation by up-regulating cyclins D1 and E in an AP-1 - and E2F-dependent manner. J Biol Chem 2010; 285(19): 14475–14485. doi: 10.1074/ jbc.M109.084400.
3. Ghafouri-Fard S, Modarressi MH. Cancer-testis antigens: potential targets for cancer immunotherapy. Arch Iran Med 2009; 12(4): 395–404.
4. Cheng YH, Wong EW, Cheng CY. Cancer/ testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis 2011; 1(3): 209–220. doi: 10.4161/ spmg.1.3.17990.
5. Sammut SJ, Feichtinger J, Stuart N et al. A novel cohort of cancer-testis biomarker genes revealed through meta-analysis of clinical data sets. Oncoscience 2014; 1(5): 349–359.
6. Kulkarni P, Uversky VN. Cancer/ testis antigens: “smart” biomarkers for diagnosis and prognosis of prostate and other cancers. Int J Mol Sci 2017; 18(4): e740. doi: 10.3390/ ijms18040740.
7. Linnekamp JF, Butter R, Spijker R et al. Clinical and biological effects of demethylating agents on solid tumours – a systematic review. Cancer Treat Rev 2017; 54 : 10–23. doi: 10.1016/ j.ctrv.2017.01.004.
8. Begum PS, Madhavi G, Rajagopal S et el. Probiotics as functional foods: potential effects on human health and its impact on neurological diseases. Int J Nutr Pharmacol Neurol Dis 2017; 7(2): 23–33.
9. Motevaseli E, Shirzad M, Raoofian R et al. Differences in vaginal Lactobacilli composition of Iranian healthy and bacterial vaginosis infected women: a comparative analysis of their cytotoxic effects with commercial vaginal probiotics. Iranian Red Crescent Med J 2013; 15(3): 199–206. doi: 10.5812/ ircmj.3533.
10. Abedin-Do A, Taherian-Esfahani Z, Ghafouri-Fard S et al. Immunomodulatory effects of Lactobacillus strains: emphasis on their effects on cancer cells. Immunotherapy 2015; 7(12): 1307–1329. doi: 10.2217/imt.15.92.
11. Eslami S, Hadjati J, Motevaseli E et al. Lactobacillus crispatus strain SJ-3C-US induces human dendritic cells (DCs) maturation and confers an anti-inflammatory phenotype to DCs. APMIS 2016; 124(8): 697–710. doi: 10.1111/ apm.12556.
12. Nouri Z, Karami F, Neyazi N et al. Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J 2016; 18(2): 127–134.
13. Motevaseli E, Shirzad M, Akrami SM et al. Normal and tumour cervical cells respond differently to vaginal Lactobacilli, independent of pH and lactate. J Med Microbiol 2013; 62(Pt7): 1065–1072. doi: 10.1099/ jmm.0.057521-0.
14. Kwok L, Stapleton AE, Stamm WE et al. Adherence of Lactobacillus crispatus to vaginal epithelial cells from women with or without a history of recurrent urinary tract infection. J Urol 2006; 176(5): 2050–2054. doi: 10.1016/ j.juro.2006.07.014.
15. Azam R, Ghafouri-Fard S, Tabrizi M et al. Lactobacillus acidophilus and Lactobacillus crispatus culture supernatants downregulate expression of cancer-testis genes in the MDA-MB-231 cell line. Asian Pac J Cancer Prev 2014; 15(10): 4255–4259.
16. Modarressi MH, Cameron J et al. Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene 2001; 262(1–2): 249–255.
17. Tanaka R, Ono T, Sato S et al. Over-expression of the testis-specific gene TSGA10 in cancers and its immunogenicity. Microbiol Immunol 2004; 48(4): 339–345.
18. Tsou JH, Chang KC, Chang-Liao PY et al. Aberrantly expressed AURKC enhances the transformation and tumourigenicity of epithelial cells. J Pathol 2011; 225(2): 243–254. doi: 10.1002/ path.2934.
19. Chun HK, Chung KS, Kim HC et al. OIP5 is a highly expressed potential therapeutic target for colorectal and gastric cancers. BMB Rep 2010; 43(5): 349–354.
20. Koinuma J, Akiyama H, Fujita M et al. Characterization of an Opa interacting protein 5 involved in lung and esophageal carcinogenesis. Cancer Sci 2012; 103(3): 577–586. doi: 10.1111/ j.1349-7006.2011.02167.x.
21. Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod 2011; 17(8): 524–538. doi: 10.1093/ molehr/ gar034.
22. Saini S, Jagadish N, Gupta A et al. A novel cancer testis antigen, A-kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS One 2013; 8(2): e57095. doi: 10.1371/ journal.pone.0057095.
23. Jagadish N, Parashar D, Gupta N et al. A-kinase anchor protein 4 (AKAP4) a promising therapeutic target of colorectal cancer. J Exp Clin Cancer Res 2015; 34 : 142. doi: 10.1186/ s13046-015-0258-y.
24. Saini S, Agarwal S, Sinha A et al. Gene silencing of A-kinase anchor protein 4 inhibits cervical cancer growth in vitro and in vivo. Cancer Gene Ther 2013 Jul; 20(7): 413–420. doi: 10.1038/ cgt.2013.32.
25. Scanlan MJ, Gure AO, Jungbluth AA et al. Cancer/ testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002 Oct; 188 : 22–32.
26. Patel S, Goyal A. Evolving roles of probiotics in cancer prophylaxis and therapy. Probiotics Antimicrob Proteins 2013; 5(1): 59–67. doi: 10.1007/ s12602-012-9124-9.
27. Hu J, Wang C, Ye L et al. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J Biosci 2015; 40(2): 269–279.
28. Seow SW, Cai S, Rahmat JN et al. Lactobacillus rhamnosus GG induces tumor regression in mice bearing orthotopic bladder tumors. Cancer Sci 2010; 101(3): 751–758. doi: 10.1111/ j.1349-7006.2009.01426.x.
29. Zhang L, Li N, Caicedo R et al. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-a–induced interleukin-8 production in caco-2 cells. J Nutr 2005; 135(7): 1752–1756. doi: 10.1093/ jn/ 135.7.1752.
30. Vielfort K, Weyler L, Söderholm N et al. Lactobacillus decelerates cervical epithelial cell cycle progression. PLoS One 2013; 8(5): e63592. doi: 10.1371/ journal.pone.0063592.
31. Dianatpour M, Mehdipour P, Nayernia K et al. Expression of testis specific genes TSGA10, TEX101 and ODF3 in breast cancer. Iran Red Crescent Med J 2012; 14(11): 722–726. doi: 10.5812/ ircmj.3611.
32. Dieterich K, Soto Rifo R, Faure AK et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet 2007; 39(5): 661–665. doi: 10.1038/ ng2027.
33. Tang A, Gao K, Chu L et al. Aurora kinases: novel therapy targets in cancers. Oncotarget 2017; 8(14): 23937–23954. doi: 10.18632/ oncotarget.14893.
34. Li H, Zhang J, Lee MJ et al. OIP5, a target of miR-15b-5p, regulates hepatocellular carcinoma growth and metastasis through the AKT/ mTORC1 and b-catenin signaling pathways. Oncotarget 2017; 8(11): 18129–18144. doi: 10.18632/ oncotarget.15185.
35. Kumar V, Jagadish N, Suri A. Role of A-Kinase anchor protein (AKAP4) in growth and survival of ovarian cancer cells. Oncotarget 2017; 8(32): 53124–53136. doi: 10.18632/ oncotarget.18163.
36. Han J, Gao W, Su D et al. Silencing of A-Kinase anchor protein 4 (AKAP4) inhibits proliferation and progression of thyroid cancer. Oncol Res 2017; 25(6): 873–878. doi: 10.3727/ 096504016X14783701102564.
37. Mesic A, Rogar M, Hudler P et al. Association of the AURKA and AURKC gene polymorphisms with an increased risk of gastric cancer. IUBMB Life 2016; 68(8): 634–644. doi: 10.1002/ iub.1521.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2018 Issue 6-
All articles in this issue
- Consequences of Hypoacidity Induced by Proton Pump Inhibitors – a Practical Approach
- Urinary Tract and Gynecologic Malignancies
- Effect and Toxicity of Radiation Therapy in Selected Palliative Indications
- Undifferentiated Carcinoma of the Pancreas – a Case Report
- Non-Small Cell Lung Cancer with Estrogen Receptors and ALK Positivity
- Long Non-Coding RNA Signature in Cervical Cancer
- Infiltration of Prostate Cancer by CD204+ and CD3+ Cells Correlates with ERG Expression and TMPRSS2-ERG Gene Fusion
- Down-regulation of TSGA10, AURKC, OIP5 and AKAP4 genes by Lactobacillus rhamnosus GG and Lactobacillus crispatus SJ-3C-US supernatants in HeLa cell line
- Use of the Metal Deletion Technique for Radiotherapy Planning in Patients with Cardiac Implantable Devices
- Diagnostic Challenges and Extraordinary Treatment Response in Rare Malignant PEComa Tumor of the Kidney
- Animal-Type Melanoma – a Mini-Review Concerning One of the Rarest Variants of Human Melanoma
- Influence of Gastrointestinal Flora in the Treatment of Cancer with Immune Checkpoint Inhibitors
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Undifferentiated Carcinoma of the Pancreas – a Case Report
- Effect and Toxicity of Radiation Therapy in Selected Palliative Indications
- Consequences of Hypoacidity Induced by Proton Pump Inhibitors – a Practical Approach
- Diagnostic Challenges and Extraordinary Treatment Response in Rare Malignant PEComa Tumor of the Kidney
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

