-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSelecting an Invertebrate Model Host for the Study of Fungal Pathogenesis
article has not abstract
Published in the journal: . PLoS Pathog 8(2): e32767. doi:10.1371/journal.ppat.1002451
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002451Summary
article has not abstract
Invertebrate Hosts Are a Valuable Tool in Studying Fungal Pathogens
The use of invertebrate hosts as infection models can greatly facilitate the study of pathogenesis (Table 1). Among invertebrate model hosts, the available options to select from include amoebae (Acanthamoeba castellanii and Dictyostellium discoideum) [1], [2], the nematode Caenorhabditis elegans [3], and insects (including Drosophila melanogaster, Galleria mellonella, and Bombyx mori) [4]–[8].
Tab. 1. Summary of findings generated by using the invertebrate infection models. 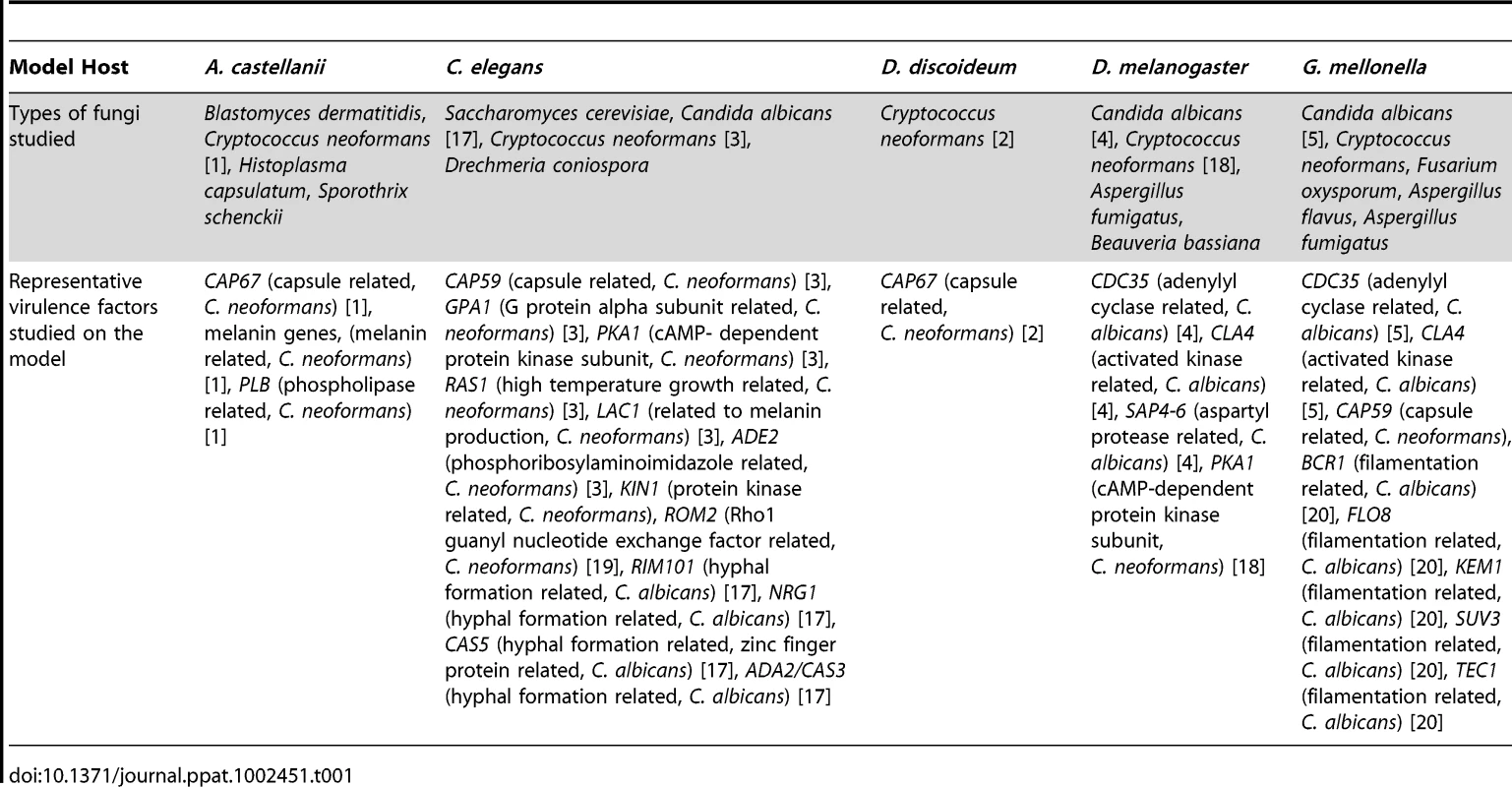
A critical step in addressing a question or hypothesis regarding host–pathogen interactions is to determine which infection model(s) best fit into the experimental criteria. For example, Cryptococcus neoformans and Candida albicans can infect amoebae, C. elegans, and several insect hosts. However, not all hosts are amenable to infection by every fungal pathogen, conditions for infections need to be optimized, and in some cases the host is not favorable for the study of the particular pathogenesis trait. For example, Pneumocystis murina cannot infect D. melanogaster or G. mellonella.
Available model hosts offer different advantages and disadvantages, and before choosing the right model host some basic questions should be posed: 1) are you interested in the host immune response to the infecting pathogen and what host-related tools, such as RNAi, sequenced genome, or mutants, are available and could be advantageous to such studies, 2) will the host be used for drug discovery, 3) will host tissue need to be removed and evaluated, 4) will host phagocytosis of the pathogen be studied, 5) is fungal hyphal formation of interest, and 6) what temperature conditions are best suited to address the research questions of interest or for studying a particular fungal gene (Figure 1).
Fig. 1. The basic characteristics of the more frequently used model hosts. 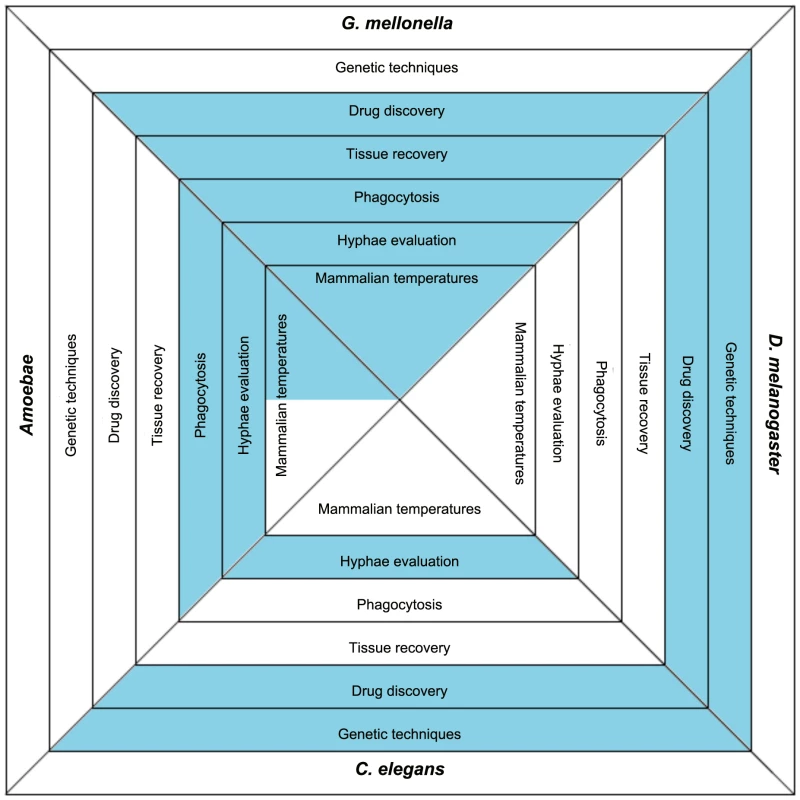
The blue color indicates that this feature is found in the specific model host. Utilizing the features of the chart can aid in determining which host(s) are most amenable to a particular study. Host genetic tools aiding in understanding host–pathogen interaction include sequenced genomes, available mutant strains, or RNAi. Once infected, some hosts can be used to identify compounds with antifungal activity. Also, while infected, some hosts are large enough that individual portions or tissues from the hosts can be removed and further analyzed either for host responses or to evaluate tissue invasion by the pathogen. As part of the host response, some hosts have phagocytic cells that engulf the foreign fungi and can be studied to elucidate information about host–pathogen interactions. When some fungi are engulfed by phagocytes, or establish an infection within the hosts, they produce hyphae. Because of the transparency or ability to recover tissue from some of the hosts, fungal hyphae formation can be further evaluated. For all of the infecting pathogens, temperature conditions are a consideration. The various hosts have conditions that are ideal for meeting their own survival needs, and the fungi will react differently in terms of gene expression and growth rate based on the temperatures in which the hosts are maintained. Temperature features marked in grey on the chart indicate hosts that can survive at temperature ranges as high as 37°C. Other invertebrate model hosts including Bombyx mori, Culex quinquefasciatus, Blattella germanica, and even a plant model of Arabidopsis thaliana have been developed. They are not as widely used and not mentioned here in detail because of space limitations. Are Virulence Traits Equally Important in All Systems?
The pathogenicity of fungi in mammals has many similarities with the pathogenicity in non-vertebrate hosts. Throughout their evolution, fungi have been in continuous contact and interaction with other soil-dwelling organisms, and it has been suggested that many virulent factors have evolved in order to protect fungi from environmental predators. However, all virulence traits are not equally important for the pathogenesis in different systems. For example, an intact capsule is critical for C. neoformans pathogenesis in G. mellonella and amoebae, but this is not the case in the killing of C. elegans [1], [3]. Therefore, the right choice of a model host is crucial for successful research.
How to Study Temperature-Sensitive Virulence Traits?
Some, but not all, invertebrate hosts allow the study of pathogenesis at mammalian temperatures. For example, D. melanogaster and C. elegans are temperature restricted and cannot survive at high temperature testing conditions; C. elegans is better used at a temperature range from 15°C to 25°C, and D. melanogaster has an optimal temperature range from 18°C to 30°C. Although some model hosts have temperature ceilings that are lower than mammalian conditions, there are other more thermotolerant model hosts, such as some insects, including G. mellonella (which has a temperature range of 25°C to 37°C), some amoebae, or worms such as Panagrellus redivivus [9].
The higher thermotolerance presents conditions under which certain genes expressed at mammalian temperatures can be studied. However, mammalian temperatures are not always ideal for the study of a temperature-related trait. For example, multiple hosts were necessary to study ECA1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase type calcium pump [10]. An eca1 C. neoformans mutant exhibited reduced growth at 37°C, so association with virulence was difficult to ascertain with mammalian models or by using G. mellonella or amoeba at 37°C conditions. A role in virulence was found using G. mellonella at 30°C and C. elegans at 25°C. This approach is interesting because using an array of model hosts has the advantage that we can study fungal pathogenesis at temperatures ranging from mammalian conditions to those of natural fungal environments. Of note is that a variety of other traits have been found to play a role in virulence at lower temperatures (Table 1).
What System Is Better for Studying Phagocytosis?
If we want to reveal the phagocytosis process, unicellular organisms like amoeba or slime molds such as D. discoideum are amenable for such studies. For example, amoebae such as A. castellanii phagocytose fungi like C. neoformans, Saccharomyces cerevisiae, and C. albicans. These amoebae envelope the fungal cell into a vacuole [1]. Interestingly, during the interaction between A. castellanii or insect hemocytes and fungi, fungal structures such as capsule and phospholipase activity provide protection, as they would in mammalian macrophages [1].
Also, model hosts like G. mellonella and D. melanogaster utilize phagocytic cells as part of their host defense. For example, an indicator of the active response of G. mellonella to fungal infections is the number of hemocytes, the phagocytic cells for G. mellonella, present after pathogen infection. There is an inverse relationship between the virulence of the invading fungi and the number of hemocytes. Introduction of pathogenic strains does not garner an increase in hemocytes. However, infecting larvae with non-pathogenic strains of fungi causes a release of hemocytes and therefore an increase in the number of hemocytes in the hemolymph [11]. Interestingly, C. albicans evade and escape hemocytes utilizing hyphae, similar to the action taken against mammalian phagocytes. On the contrary, phagocytosis is not part of C. elegans response to infection. Also, although D. discoideum might be too small to phagocytose some of the larger fungi, this host has contributed to understanding phagocytic processes through the study of actin cytoskeleton, an integral part of the phagocytotic process [12].
Which System Is More Appropriate for Studying Antimicrobial Compounds?
The use of model hosts can facilitate the study of existing and discovery of new compounds. The model systems that have been used most frequently in the field of drug discovery are C. elegans and the insects D. melanogaster and G. mellonella. The nematode C. elegans has been the most amenable to the screening process in search of new antifungal compounds due to its small size and use in liquid assay format, making it ideal for implementing high-throughput assays utilizing automation [13]. During the application of this method, liquid infection assays are set up in 96 - or 384-well plate formats. Automated systems can supply the plates with the liquid media, nematodes, and specific quantities of compounds [14]. Thus, the testing of thousands of candidate compounds is accelerated. The process can identify not only antifungal compounds, but also those with immunomodulatory effects that bolster the immune response, effectively inhibiting the fungal infection.
Insects can be used for the study of smaller compound libraries. An insect model host system used for the discovery of new antifungal drugs is G. mellonella. The substance astemizole, which is an antihistamine drug, found to be active in combination with fluconazole, against C. neoformans. Even combination of a few (2–3) compounds can be studied in the survival of infected larvae [15]. In addition, the compound lovastatin was evaluated using D. melanogaster as a model for infection from zygomycetes. Lovastatin was active against the fungi Rhizopus homothallicus, Rhizopus oryzae, Mucor circinelloides, and Cunninghamella bertholletiae [16]. When D. melogaster is utilized as an infection model, a candidate antifungal compound is ingested by the host. However, the exact quantity of the consumed compound is unknown. In the case of G. mellonella, a standardized concentration of the compound is delivered via injection. Although more accurate in quantification, the process is time consuming.
Conclusion
There are several hosts used to model infections ranging from single cell protozoa to insects. For the best interrogation into host–pathogen interactions, researchers can select from a variety of invertebrate model hosts. However, no single model host can answer all scientific questions. The selection of the appropriate host should be based on the virulence trait or the host response under study and the financial, space, and time commitment required (for example, D. melanogaster requires incubators and a “fly room”, C. elegans requires incubators and microscopes however G. mellonella can be used in almost any laboratory). Importantly, scientists can also use the “multi host” approach and implement multiple complementary infection models as they try to understand the various mechanisms in the fungal arsenal to establish an infection, evade or cope with host defenses, and grow and reproduce within the confines of another organism.
Zdroje
1. SteenbergenJNShumanHACasadevallA 2001 Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 98 15245 15250
2. SteenbergenJNNosanchukJDMalliarisSDCasadevallA 2003 Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect Immun 71 4862 4872
3. MylonakisEAusubelFMPerfectJRHeitmanJCalderwoodSB 2002 Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 99 15675 15680
4. AlarcoAMMarcilAChenJSuterBThomasD 2004 Immune-deficient Drosophila melanogaster: a model for the innate immune response to human fungal pathogens. J Immunol 172 5622 5628
5. BrennanMThomasDYWhitewayMKavanaghK 2002 Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 34 153 157
6. LionakisMSKontoyiannisDP 2010 The growing promise of Toll-deficient Drosophila melanogaster as a model for studying Aspergillus pathogenesis and treatment. Virulence 1 488 499
7. FuchsBBO'BrienEKhouryJBMylonakisE 2010 Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1 475 482
8. HanaokaNTakanoYShibuyaKFugoHUeharaY 2008 Identification of the putative protein phosphatase gene PTC1 as a virulence-related gene using a silkworm model of Candida albicans infection. Eukaryot Cell 7 1640 1648
9. LawsTRSmithSASmithMPHardingSVAtkinsTP 2005 The nematode Panagrellus redivivus is susceptible to killing by human pathogens at 37 degrees C. FEMS Microbiol Lett 250 77 83
10. FanWIdnurmABregerJMylonakisEHeitmanJ 2007 Eca1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect Immun 75 3394 3405
11. BerginDBrennanMKavanaghK 2003 Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect 5 1389 1395
12. NoegelAASchleicherM 2000 The actin cytoskeleton of Dictyostelium: a story told by mutants. J Cell Sci 113 Pt 5 759 766
13. BregerJFuchsBBAperisGMoyTIAusubelFM 2007 Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog 3 e18
14. YanikMFRohdeCBPardo-MartinC 2011 Technologies for micromanipulating, imaging, and phenotyping small invertebrates and vertebrates. Annu Rev Biomed Eng 13 185 217
15. VuKGelliA 2010 Astemizole and an analogue promote fungicidal activity of fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii. Med Mycol 48 255 262
16. ChamilosGLewisREKontoyiannisDP 2006 Lovastatin has significant activity against zygomycetes and interacts synergistically with voriconazole. Antimicrob Agents Chemother 50 96 103
17. Pukkila-WorleyRPelegAYTampakakisEMylonakisE 2009 Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot Cell 8 1750 1758
18. ApidianakisYRahmeLGHeitmanJAusubelFMCalderwoodSB 2004 Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell 3 413 419
19. TangRJBregerJIdnurmAGerikKJLodgeJK 2005 Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect Immun 73 8219 8225
20. FuchsBBEbyJNobileCJEl KhouryJBMitchellAP 2010 Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect 12 488 496
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Mechanisms of Pathogenesis, Infective Dose and Virulence in Human Parasites
- Structural and Functional Insights into the Pilotin-Secretin Complex of the Type II Secretion System
- Characterising the Mucosal and Systemic Immune Responses to Experimental Human Hookworm Infection
- Population Genetic Analyses Reveal the African Origin and Strain Variation of var.
- How Do Microbial Pathogens Make s?
- Substance P Causes Seizures in Neurocysticercosis
- Phagosomal Rupture by Results in Toxicity and Host Cell Death
- Absence of HIV-1 Evolution in the Gut-Associated Lymphoid Tissue from Patients on Combination Antiviral Therapy Initiated during Primary Infection
- Five Questions about Viral Trafficking in Neurons
- Selecting an Invertebrate Model Host for the Study of Fungal Pathogenesis
- A Co-Opted DEAD-Box RNA Helicase Enhances Tombusvirus Plus-Strand Synthesis
- Biochemical Properties of Highly Neuroinvasive Prion Strains
- ClpP1 and ClpP2 Function Together in Protein Degradation and Are Required for Viability and During Infection
- Discrete Cyclic di-GMP-Dependent Control of Bacterial Predation versus Axenic Growth in
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Discrete Cyclic di-GMP-Dependent Control of Bacterial Predation versus Axenic Growth in
- Characterising the Mucosal and Systemic Immune Responses to Experimental Human Hookworm Infection
- How Do Microbial Pathogens Make s?
- Substance P Causes Seizures in Neurocysticercosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



