-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRelationship between cold water swimming and increased cardiac markers: A pilot study
Vztah mezi zimním plaváním a zvýšením hladiny kardiálních markerů: pilotní studie
Cíl:
Zjistit možnou souvislost mezi zimním plaváním a koncentracemi běžně užívaných kardiálních markerů.Soubor a metody:
8 plavců (5 mužů), medián věku 44 (31-71) let, bylo vyšetřeno během soutěže v zimním plavání v listopadu 2014. Šest plavců (4 muži) plavalo 500 m dlouhou trať, 1 žena 750 m dlouhou trať a 1 muž plaval 1000 m dlouhou trať. Teplota vody byla 8,2°C. Koncentrace vysoce senzitivního troponinu T (hsTnT), vysoce senzitivního troponinu I (hsTnI) a NT-proBNP byly vyšetřeny den před závodem, ihned po závodě, 2 hodiny po závodě a 24 hodin po závodě. Průběhy hladin hsTnI, hsTnT a NT-proBNP byly testovány užitím ANOVA analýzy.Výsledky:
Doba plavání účastníků byla 9 min 36 s - 26 min 48 s v závislosti na zvolené trase a rychlosti závodníka. Medián body mass index (BMI) byl 24,8 (22,1-27,9) kg.m-2. Byl zjištěn statisticky významný vzestup hladiny hsTnI 2 hodiny po závodě (p=0,048, kvadratický trend). Nebyly zjištěny statisticky významné změny v koncentracích hsTnT a NT-proBNP (p=0,19, p=0,57 resp.).Závěr:
Zimní plavání může být asociováno s uvolněním kardiálních troponinů, nicméně není spojeno se zvýšením hladiny NT-proBNP. Další výzkum by měl odhalit možnou spojitost mezi uvolněním troponinů během zimního plavání a přítomností arytmií, případně zvýšeným rizikem kardiální příhody.Klíčová slova:
zimní plavání, tonutí, vysoce senzitivní troponiny, natriuretické peptidy, náhlá srdeční smrt.
Authors: P. Brož 1,3; D. Rajdl 1,3; J. Racek 1,3; V. Zeman 2; J. Novák 2; L. Trefil 1
Authors place of work: Institute of Clinical Biochemistry and Hematology, University Hospital, Pilsen, Czech Republic 1; Institute of Sports medicine, Faculty of medicine in Pilsen, Charles University, Czech Republic 2; Faculty of Medicine in Pilsen, Charles University in Prague, Prague, Czech Republic 3
Published in the journal: Klin. Biochem. Metab., 25, 2017, No. 1, p. 27-31
Summary
Aim:
Drowning causes about five hundred thousand deaths in the world every year. There is a higher risk of adverse medical events and death from cardiac causes in participants of cold water swimming (CWS).Material and methods:
Eight swimmers (five men, three women), with a median age of 40 years (range 31-71), were examined during winter swimming competition in November 2014. Six swimmers (four men, two women) swam 500 m long distance, one woman swam 750 m and one man swam 1000 m long distance. The swimmers’ body temperatures were measured immediately after the swim. Concentrations of high sensitivity troponin I (hsTnI), high sensitivity troponin T (hsTnT) and aminoterminal pro-BNP (NT-proBNP) were examined one day before, immediately after, two hours after and 24 hours after the competition. Trends of hsTnI, hsTnT and NT-proBNP were tested using the Analysis of Variance (ANOVA).Results:
Swimming times ranged from 9 min and 36 s to 26 min and 48 s depending on distance and velocity. The median body mass index (BMI) of swimmers was 24.8 kg.m-2 (range 22.1-27.9). There was a statistically significant increase of hsTnI two hours after CWS (p=0.048 for quadratic trend). Trends of hsTnT and NT-proBNP did not exhibit statistically significant differences (p=0.19 and p=0.57, respectively).Conclusion:
CWS can be connected with release of cardiac troponins. However, it is not connected with release of NT-proBNP. Future research should clarify whether release of cardiac troponins during CWS can be connected with presence of arrhythmias, higher cardiovascular risk or probability of incidental heart failure.Keywords:
cold water swimming, drowning, high sensitivity troponin, natriuretic peptides, cardiac death.Introduction
Drowning causes about five hundred thousand deaths in the world every year [1]. Many people participate in cold water swimming (CWS). With increasing numbers of CWS participants, there is a higher risk of adverse medical events and cardiac-related deaths. The presence of arrhythmias can be connected with hypothermia or cold shock response after immersion [2].
In the USA between 2003 and 2011, 79% of deaths in triathlons occurred during the swim. The most probable cause seems to be cardiac death rather than hypothermia, because the immersion time was often too short to cause hypothermia [2,3]. During winter swimming, there are more frequent arrhythmias than in the summer, but many arrhythmias remain undetected [2]. Some authors see a possible explanation in a trigger called “autonomic conflict” caused by the combination of the cold shock and the diving response provoked by a rapid submersion in cold water (<15°C) [4]. Moreover, the probability of sudden cardiac death in CWS is more frequent in competitive situations rather than in non-competitive situations because of stress, anger, extended breath holding, or aspiration of water into the nasopharynx [5–7].
High sensitivity troponin (hsTn) examination is a modern assessment widely used in the diagnostic work up of patients where a myocardial lesion is suspected [8,9]. In comparison with conventional troponin assays, hsTn assays can detect minimal amounts of cardiac troponins (cTn) released by the myocardium even in healthy individuals [10,11]. High sensitivity troponin can be used as a predictor of cardiovascular disease, and increased levels are associated with increased risk of cardiovascular death or incidental heart failure [12,13]. Some authors published increased troponin or hsTn levels in healthy individuals after heavier physical activity [14,15]. To our knowledge, the influence of cold water swimming on hsTn levels hasn’t been published yet.
Natriuretic peptides, especially brain natriuretic peptide and aminoterminal pro-BNP (NT-proBNP), their effects on haemodynamics and electrolyte homeostasis and their role in the diagnostic algorithm in patients with dyspnea is well known [16,17]. Their role in asymptomatic persons and the association with prognosis and mortality have been studied. Natriuretic peptides can predict the risk of death, heart failure, stroke or transient ischemic attack [18,19]. Water immersion is connected with an increase in cardiac filling and central venous pressure and also with a decrease in heart rate. Elevation of natriuretic peptide levels after prolonged strenuous exercise [20,21] and after scuba diving can be present [22].
The goal of our pilot study was to examine changes in high sensitive troponin I (hsTnI), high sensitive troponin T (hsTnT) and NT-proBNP levels before and after winter swimming competitions.
Methods
Eight swimmers from 10 volunteers (seven men, three women) participating in winter swimming competitions in November 2014 were included in the study. Two swimmers (males) were excluded because of a previous history of cardiovascular disease. One of those excluded had a history of arterial hypertension treated for five years, and the other one had a history of stable angina with coronarography stenting two years ago. The median age of the swimmers inclu-ded was 40 years (range 31-71). All swimmers were well adapted to CWS and trained regularly 1-3 times per week. Five possible distances could have been chosen (100, 250, 500, 750 and 1000 metres). The swimming time for all swimmers was measured. The water temperature was 8.2°C. The body temperature of all swimmers was measured rectally immediately after the swim.
Blood samples were taken one day before (referred to as “before”), immediately after (“after”), two hours after (“2h after”) and 24 hours after (“24h after”) the swim. After collection, blood samples were immediately transported to the laboratory and centrifuged at 2000 g for 10 minutes. Samples were stored at -70°C prior to analysis. All samples were analysed batch-wise. Levels of hsTnI were analysed using the Abbott system (STAT High Sensitive Troponin-I, Abbott Architect i2000SR, Abbott), and hsTnT and NT-proBNP levels were analysed using the Cobas system (Cobas 8000 Analyzer - Cobas e602 module, Roche Diagnostics). The limit of quantification for hsTnI was 2 ng/l and for hsTnT was 5 ng/L. In all swimmers, routine screening (erythrocyte and leukocyte count, C-reactive protein, urea, creatinine and glucose levels) was performed using the sample “before”.
Trends of hsTnI, hsTnT and NT-proBNP levels were tested using the Analysis of Variance (ANOVA). Statistical significance was set at p<0.05. For statistical analysis, MedCalc software was used (MedCalc Software, version 12.0, MedCalc Software bvba, Ostend, Belgium).
The study was approved by the local ethics committee and all participants signed written informed consent to participate in the study.
Results
Six swimmers (four men and two women) swam 500 m long distance, one woman swam 750 m long distance and one man swam 1000 m long distance. Swimming times ranged from 9 min and 36 s to 26 min and 48 s depending on distance and velocity. The median (min-max) body mass index (BMI) of swimmers was 24.8 (22.1-27.9) kg.m-2. The median body temperature after the swim was 36.2°C (range 33.4-36.9°C). Trends in hsTnI levels exhibited statistically significant differences (p=0.048 for quadratic trend). In four cases (62.5%), hsTnI values (2h after compared to the sample before) had a tendency to increase and then to decrease back to the imaginary baseline in three of samples (in one case sample 24h after was not obtained). In three cases (37.5%), hsTnI levels did not exhibit a tendency to change (or differences were too small or values were below the limit of detection). In one case (12.5%), there was a further tendency to increase (24h after compared to the sample 2h after). Individual trends of hsTnI levels are shown in Fig. 1. Concentrations of hsTnT showed similar trends but did not reach statistical significance (p=0.19).
Fig. 1 Individual levels of hsTnI measured before, immediately after, two hours after and 24 hours after the swim. Body temperature measured rectally immediately after the swim is noted in plots. The number of swimmers in study included in upper right corner of each graph, individual parameters stated in Table 1. F-female, M-male. Swim distances: 500 m, 750 m, 1000 m. 
Tab. 1. Individual parameters of each swimmer. 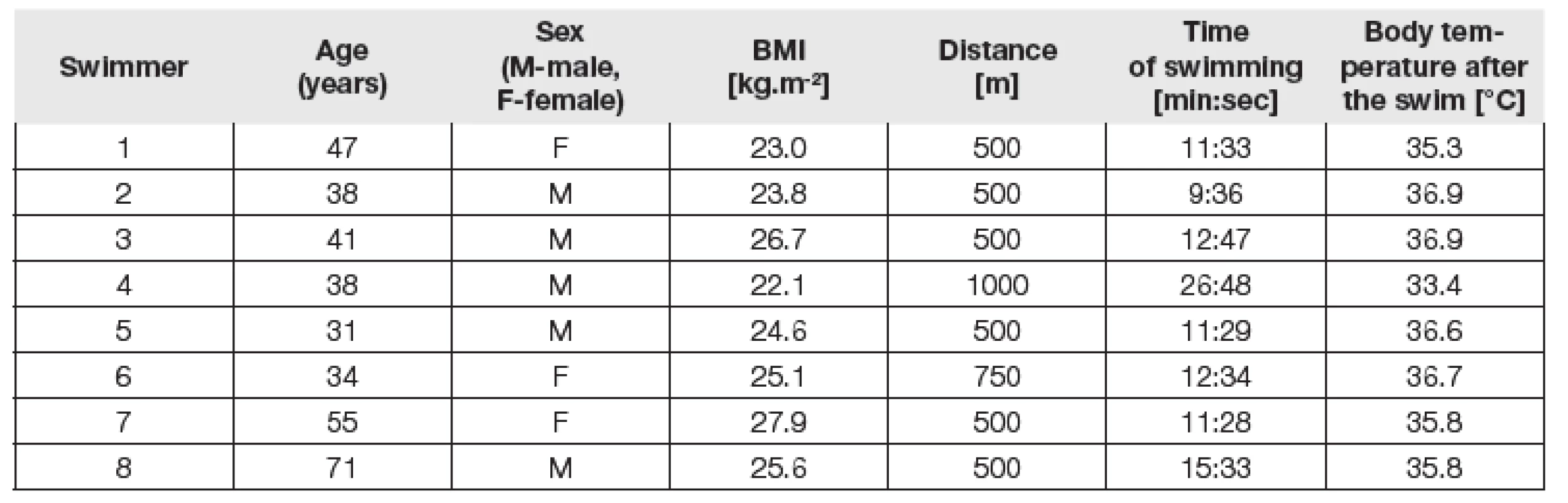
We did not observe statistically significant diffe-rences in concentrations of NT-proBNP (p=0.57). However, one case showed significantly higher levels of NT-proBNP in all samples (before, after, 2h after and 24h after) compared to the median of the other swimmers (before: 217 vs. 35.5 ng/L, after: 240 vs. 43.0 ng/L, 2h after: 255 vs. 44.0 ng/L, 24h after: 200 ng/L vs. 64.0 ng/L). The trends of measured parameters of all swimmers are summarised in Fig. 2.
Fig. 2 Trends of hsTnI, hsTnT and NT-proBNP before, after, two hours after and 24 hours after the swim. 
We found no correlation (p = ns) between body temperature and differences in hsTnI, hsTnT and NT-proBNP levels (difference between sample before and 2h after). However, the male swimmer with a swim distance of 1000 m had the greatest difference in cTn levels (sample before in comparison with 2h after), and in this case we measured the lowest temperature immediately after the swim (33.4°C).
Discussion
Studies focused on cTn levels after physical exercise describe a release of cTn after prolonged or extreme exercise, e.g. iron man, marathon or ultra-marathon competitions [14]. The mechanism of cTn release in healthy individuals is not fully understood [15]. In contrast with the studies mentioned above, exposure to cold water in our study was for a relatively short period of time. The recent work by Shattock and Tipton (2012) suggests that in CWS, simultaneous activation of the sympathetic and parasympathetic nervous systems and their influence on the heart can provoke fatal arrhythmias [4]. When muscle temperature decreases below 27 °C, reduced contractile force due to reduced enzyme activity is expected, as well as decreased rates of diffusion and decreased repolarisation [27]. A consequence of cooled muscles is a left shift of the oxygen-haemoglobin dissociation curve and an earlier rise in lactate concentration [27–29]. Compared to the normal environment, higher oxygen consumption during CWS contributes to higher energy expenditure [2]. Peripheral vasoconstriction and hydrostatic pressure can cause “cold diuresis”, and the circulation volume can be reduced by 24%. Muscular function can be decreased in this situation [30]. After CWS, body temperature can further decrease because of temperature gradients the body establishes while in the water [2].
This is the first report of an increase in cardiac troponin after moderate physical exercise (swimming) in cold water. In the case of one male swimmer with a swim distance of 1000 m, we observed the greatest difference in cTn and the lowest temperature after the swim (33.4°C). It is uncertain whether the release of cardiac troponin was caused by exercise or the cold water effect or both. The small number of swimmers in this study can explain why there was no correlation between body temperature after the swim and differences in cTn levels. We admit that monitoring of body temperature before and after the swim would be more suitable for assessing the relationship between cTn values and hypothermia. Finally, the trends in hsTnT were similar to those in hsTnI, however they were not statistically significant. One possible explanation for this observation is the differences in the analytical characteristics of each troponin kit, as the hsTnI assay is considered to be “more sensitive” than the hsTnT assay [23].
Our results did not show any statistically significant changes in NT-proBNP levels, in contrast with studies focused on changes after prolonged strenuous exercise or scuba diving [20–22]. A possible explanation for this is the short period of time spent in the water, the shallow depth of immersion or the fact that the exercise was not significantly strenuous. One case with significantly higher levels of NT-proBNP in all samples was the oldest participant of our study (71 years compared to the median age of 44 years). There was no history of cardiac disease in this swimmer. Moreover, this was the only case where hsTnI and hsTnT concentrations were higher in sample 24h after compared to sample 2h after. Thus, it is possible that cardiovascular disease was present in this case or that this could be a “physio-logical” pattern of markers in the elderly.
There were some limitations of this pilot study. First, the number of participants was small. All swimmers did not swim the same distance, and the time spent in cold water differed as well. The subjects were examined during the competition, and competitions are usually more stressful than non-competitive situations. To elucidate hsTn and NT-proBNP release during CWS, a comparison of each swimmer in cold water and water with a temperature of approximately 25 °C would be beneficial. Monitoring the temperature before and after the swim can clarify the influence of hypothermia. A detailed questionnaire and medical examination before swimming and ECG monitoring before and after swimming would be beneficial to assess whether arrhythmias as a consequence of CWS-induced hypothermia could be present in connection with the release of cTn or natriuretic peptides. Finally, future studies should clarify whether there is a higher incidence of arrhythmias or risk of cardiac events in the group of swimmers with elevated cTn.
Conclusions
Cold water swimming can be connected with the release of cardiac troponins; however, it is not connected with the release of NT-proBNP. The highest increase in cTn was observed in the participant with the lowest body temperature after the swim and who swam the longest distance.
Declaration of conflicting interests
The authors declare that there are no competing interests.
The study was supported by the Ministry of Health, Czech Republic – Conceptual Development of Research Organisation (FNPl – 00669806).
Do redakce došlo 25. 10. 2016
Adresa pro korespondenci
MUDr. Pavel Brož
ÚKBH FN Plzeň
Alej Svobody 80
304 60 Plzeň
e-mail: brozp@fnplzen.cz
Zdroje
1. Szpilman, D., Bierens, J. J. L. M., Handley, A. J. et al. Drowning. N Engl. J Med., 2012, 31, 366(22), p. 2102–10.
2. Tipton, M., Bradford, C. Moving in extreme environments: open water swimming in cold and warm water. Extreme Physiol. Med., 2014, 11, 3, p. 12.
3. Golden, F., Tipton, M. Essentials of Sea Survival. Champaign, IL: Human Kinetics c2002;2002.
4. Shattock, M. J., Tipton, M.J. “Autonomic conflict”: a different way to die during cold water immersion? J Physiol., 2012, 15, 590(Pt 14), p. 3219–30.
5. Taggart, P., Boyett, M. R., Logantha, S. J. R. J. et al. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol., 2011, 19. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3196868/ (accessed 18 Jan 2016).
6. Tipton, M. J. Sudden cardiac death during open water swimming. Br. J Sports Med., 2014, Aug, p. 1134–5.
7. Rainville, P., Bechara, A., Naqvi, N. et al. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int. J Psychophysiol., 2006, 61(1), p. 5–18.
8. Friedecký, B., Jabor, A., Kratochvíla, J., Rajdl, D., Kettner, J., Franeková, J. et al. Doporučení ČSKB: Používání kardiálních troponinů při podezření na akutní koronární syndrom. Klin. Biochem. Metab., 2015, 23(2), p. 71–7.
9. Li, W. J., Chen, X. M., Nie, X. Y. et al. The early dia-gnostic and prognostic utility of high-sensitive troponin assays in acute myocardial infarction: a meta-analysis. Intern. Med. J., 2015, 45(7), p. 748-56.
10. Hammerer-Lercher, A., Ploner, T., Neururer, S. et al. High-sensitivity cardiac troponin T compared with standard troponin T testing on emergency department admission: how much does it add in everyday clinical practice? J Am. Heart. Assoc. Cardiovasc. Cerebrovasc. Dis., 2013, 21, 2(3). http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3698787/.
11. Jarolim, P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin. Chem. Lab. Med., 2014, 25, 53(5), p. 635-52. http://www.degruyter.com/view/j/cclm.2015.53.issue-5/cclm-2014-0565/cclm-2014-0565.xml.
12. deFilippi, C. R., de Lemos, J. A., Christenson, R. H. et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA., 2010, 8, 304(22), p. 2494–502.
13. Thygesen, K., Alpert, J. S., Jaffe, A. S. et al. Third universal definition of myocardial infarction. J Am. Coll. Cardiol., 2012, 16, 60(16), p. 1581–98.
14. Vilela, E. M., Bastos, J. C. C., Rodrigues, R. P. et al. High-sensitivity troponin after running--a systematic review. Neth. J Med., 2014, 72(1), p. 5–9.
15. Shave, R., Baggish, A., George, K. et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am. Coll. Cardiol., 2010, 56(3), p. 169–76.
16. Gaggin, H. K., Januzzi, J. L. Natriuretic peptides in heart failure and acute coronary syndrome. Clin. Lab. Med., 2014, 34(1), p. 43–58.
17. Volpe, M., Rubattu, S., Burnett, J. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur. Heart. J., 2014, 14, 35(7), p. 419–25.
18. Daniels, L. B. Natriuretic peptides and assessment of cardiovascular disease risk in asymptomatic persons. Curr. Cardiovasc. Risk Rep., 2010, 4(2), p. 120–7.
19. Daniels, L. B., Laughlin, G. A., Clopton, P. et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am. Coll. Cardiol., 2008, 5, 52(6), p. 450–9.
20. Ohba, H., Takada, H., Musha, H. et al. Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am. Heart. J., 2001, 141(5), p. 751–8.
21. Scharhag, J., Herrmann, M., Urhausen, A. et al. Independent elevations of N-terminal pro–brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am. Heart. J., 2005, 150(6), p. 1128–34.
22. Passino, C., Franzino, E., Giannoni, A. et al. B-type natriuretic peptide secretion following scuba diving. Biomark. Med., 2011, 5(2), p. 205–9.
23. Apple, F. S., Collinson, P. O. Biomarkers for the ITF on CA of C. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin. Chem., 2012, 1, 58(1), p. 54–61.
24. Sherwood, M. W., Newby, L. K. High-sensitivity troponin assays: evidence, indications, and reasonable use. J Am. Heart. Assoc., 2014, 28, 3(1), e000403.
25. Jaffe, A. S. The 10 commandments of troponin, with special reference to high sensitivity assays. Heart Br. Card. Soc., 2011, 97(11), p. 940–6.
26. Tipton, M., Eglin, C., Gennser, M. et al. Immersion deaths and deterioration in swimming performance in cold water. The Lancet, 1999, 21, 354(9179), p. 626–9.
27. Holmér, I., Bergh, U. Metabolic and thermal response to swimming in water at varying temperatures. J Appl. Physiol., 1974, 1, 37(5), p. 702–5.
28. Jacobs, I., Romet, T. T., Kerrigan-Brown, D. Muscle glycogen depletion during exercise at 9 degrees C and 21 degrees C. Eur J Appl. Physiol., 1985, 54(1), p. 35–9.
29. Martineau, L., Jacobs, I. Muscle glycogen utilization during shivering thermogenesis in humans. J Appl. Physiol., 1988, 1, 65(5), p. 2046–50.
30. Ayme, K., Gavarry, O., Rossi, P. et al. Effect of head-out water immersion on vascular function in healthy subjects. Appl. Physiol. Nutr. Metab., 2014, 39(4), p. 425–31.
Štítky
Biochemie Nukleární medicína Nutriční terapeut
Článek Editorial
Článek vyšel v časopiseKlinická biochemie a metabolismus
Nejčtenější tento týden
2017 Číslo 1- GLP-1RA a PCOS: Je to „jenom“ o hmotnosti?
- Moderní přístupy zvyšující efektivitu antibiotické léčby v nemocniční praxi
- Farmakologická léčba obezity u pacientek se syndromem polycystických ovarií – systematický přehled a klinická doporučení
- Zpracované masné výrobky a červené maso jako riziko rozvoje kolorektálního karcinomu u žen? Důkazy z prospektivní analýzy
- Efektivita léčby a možné indikace liraglutidu v gynekologii
-
Všechny články tohoto čísla
- Comparison of the serum levels of heavy/light chain pairs of immunoglobulin (Hevylite™) and analysis of parameters of bone metabolism in patients with multiple myeloma.
- Mild Hyperhomocysteinemias From Deficiency of MTHFR (C677T and C1298A) in Adults and Adolescents Attending Metabolic Unit: Is There Any Necessity for Their Differentiation and Treatment?
- Relationship between cold water swimming and increased cardiac markers: A pilot study
- Editorial
- Intoxication with ethylene glycol and falsely increased plasma lactate concentration
- Opinion of Czech expert societies to consensus of European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine to determination of blood lipids and to interpretation of its values
- Nutrition and metabolism of the bone
- Doporučení k vyšetřování mozkomíšního moku
- Doporučení k vyšetřování mozkomíšního moku
- Klinická biochemie a metabolismus
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mild Hyperhomocysteinemias From Deficiency of MTHFR (C677T and C1298A) in Adults and Adolescents Attending Metabolic Unit: Is There Any Necessity for Their Differentiation and Treatment?
- Doporučení k vyšetřování mozkomíšního moku
- Intoxication with ethylene glycol and falsely increased plasma lactate concentration
- Nutrition and metabolism of the bone
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



