-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Alimentární přenos klíšťové encefalitidy v České republice (1997–2008)
Alimentary Transmission of Tick-borne Encephalitis in the Czech Republic (1997–2008)
Objectives:
Analysis of cases of tick-borne encephalitis (TBE) with confirmed food-borne transmission in patients who were unaware of tick attachment prior to the onset of illness.Material and methods:
Data on laboratory confirmed cases of TBE reported in the Czech Republic (CR) in 1997-2008 were obtained from the EPIDAT system. Patient interview data were recorded in a standardised questionnaire form with multiple choices for locality (GIS) and route of transmission to be exported on a weekly basis in electronic form to a protected database of the Ministry of Health of CR. Statistical processing was conducted by ANOVA using Epi-Info CDC Atlanta and STATCALC software.Results:
TBE has been recognised in CR since 1948 when, for the first time ever in Europe, the TBE virus was isolated from patients and I. ricinus ticks in two Czech and Moravian areas simultaneously (and independently of one another). TBE cases in the Czech Republic have been reported since 1971. In 1997 – 2008 the incidence ranged between 422 cases (1998) to 1029 cases (2006) . Food-borne transmission of TBE was first reported in Czechoslovakia in 1954. At that time, nothing was known of the possibilities of arbovirus transmission by the food-borne route; this was discovered following the TBE epidemic of 1951 in the east Slovak town of Rožňava in which 660 persons were infected, and of these, 271 were hospitalised. The source of infection was contaminated goats’ milk which had been mixed into dairy milk at the local dairy and distributed without pasteurization. The risk of TBE transmission by unpasteurized goats’ milk is associated with the current trend of I. ricinus tick proliferation in foothills and mountainous areas. The shift of the line of their spread from 700 m above sea level to 1200 m means that to up to 8% of the area of the CR (6300 km2) have been newly colonized by ticks. In 1997 – 2008, 64 cases of TBE were recorded in patients who reported consumption of unpasteurized goats’ and dairy milk or unpasteurized sheep’s milk cheese. The majority of cases involved goats’ milk (36 patients, i.e. 56.3%) and sheep’s milk cheese (21 patients, i.e. 32.8%). Dairy milk-borne infection was responsible for 7 TBE cases (10.9%). Of the 64 patients with food-borne TBE, 33 were men (51.6%) and 31 women (48.4%). Thirty-three cases (51.6%) occurred in family outbreaks following purchase of cheese or milk from animal breeders. Twenty-two cases (34.4%) occurred in individual patients and for 9 cases (14.0%) the data are unavailable. The highest age-specific morbidity, i.e. 1.94/100 000, was observed amongst the 5 – 9 years age-group, while in the adult age-groups the rates ranged between 0.17/100 00 (75+ years) and 0.89/100 00 (35 – 44 years). The comparison of TBE cases in child and adult age groups revealed that children in the food-borne TBE group had a 2.5 fold risk of TBE infection over adults. None of the TBE patients was vaccinated against TBE.Conclusion:
In 1997 – 2008, a total of 7288 cases of TBE were reported. Sixty-four (0.9%) TBE cases were food-borne. In the majority of these cases, TBE virus was transmitted by unpasteurized goats’ milk and caused family outbreaks. The deciding factor in these outbreaks was an attempt to provide healthy diet to offspring.Key words:
Tick-borne encephalitis – food-borne infection – milk-borne – cheese-borne – goats’ milk.
Autoři: B. Kříž 1,2; C. Beneš 1; M. Daniel 1
Působiště autorů: National Institute of Public Health, Praha 1; Charles University, 3rd Medical Faculty 2
Vyšlo v časopise: Epidemiol. Mikrobiol. Imunol. 58, 2009, č. 2, s. 98-103
Souhrn
Cíl práce:
Analýza případů onemocnění klíšťovou encefalitidou (KE), u kterých byl prokázán alimentární přenos infekce a kteří si nebyli vědomi přisátí klíštěte v časové souvislosti s onemocněním.Materiál a metodika:
Údaje o laboratorně potvrzených případech KE hlášených v České republice (ČR) v letech 1997-2008 získané prostřednictvím národního hlásícího systému EPIDAT. Data získaná pohovorem s pacienty byla zaznamenávána do standardizovaného dotazníku s nabídkami lokalit (GIS) a cest přenosu a týdně elektronicky exportována na chráněné úložiště dat Ministerstva zdravotnictví ČR. Statistické zpracování bylo provedeno v programu Epi-Info CDC v Atlantě testem ANOVA a programem STATCALC.Výsledky:
KE je v ČR známa od r. 1948, kdy byl virus KE (VKE) (poprvé v Evropě) izolován z pacientů a klíšťat I. ricinus, současně (a nezávisle na sobě) ve dvou oblastech v Čechách a na Moravě. Onemocnění KE v ČR jsou hlášena od r. 1971. V letech 1997 – 2008 se výskyt onemocnění pohyboval od 422 případů (r. 1998) do 1029 případů (r. 2006). Informace o přenosu KE alimentární cestou byla poprvé publikována v Československu v roce 1954. V té době nebylo o možnosti šíření arbovirových nákaz alimentární cestou dosud nic známo. Tento zcela prioritní objev byl založen na zkušenostech z epidemie KE, která vypukla na jaře 1951 ve východoslovenském městě Rožňava, při níž onemocnělo až 660 osob, z nichž 271 muselo být hospitalizováno. Zdrojem nákazy bylo infikované mléko koz, které bylo v místní mlékárně přimícháváno do kravského mléka a bez pasterizace dodáváno do distribuční sítě. S problematikou rizika přenosu VKE nepasterizovaným kozím mlékem je spojen i současný trend šíření klíšťat I. ricinus do podhorských a horských oblastí. Při zvýšení horní hranice jejich rozšíření ze 700 m na 1200 m n.m. bylo jimi nově kolonizováno území, kterému v uvedeném výškovém rozpětí odpovídá až 8 % celkové rozlohy státu (6 300 km2). V období let 1997-2008 bylo zjištěno 64 případů onemocnění KE, u kterých udávali pacienti pití tepelně neupraveného kozího či kravského mléka či požití tepelně neupraveného ovčího sýra. V naprosté většině případů se jednalo o kozí mléko 36 (56,3 %) a ovčí sýr 21 (32,8 %). Kravským mlékem se infikovalo 7 osob (10,9 %). V souboru nemocných bylo 33 (51,6 %) mužů a 31 (48,4 %) žen. Ve 33 případech (51,6 %) se jednalo o rodinné výskyty, kdy bylo postiženo několik členů rodiny, která zakoupila mléko či sýr u chovatelů zvířat. Ve 22 případech (34,4 %) se jednalo o jednotlivce, v 9 případech (14,0 %) není tato informace k dispozici. Nejvyšší věkově specifická nemocnost byla zjištěna ve skupině 5-9 letých 1,94/100 000. Ve věkových skupinách dospělých se pohybovala v rozmezí 0,17/100 00 (věk 75+) – 0,89/100 000 (věk 35-44). Ze srovnání věkových skupin dětí s dospělými ve skupině alimentárně infikovaných s analogickými skupinami celého souboru onemocnění KE vyplývá, že děti tohoto souboru byly 2,5 násobně více ohroženy než dospělí. Nikdo z nemocných nebyl proti KE očkován.Závěr:
V období let 1997-2008 bylo v ČR hlášeno celkem 7 288 případů onemocnění KE. U 64 nemocných došlo k přenosu infekce alimentárním způsobem (0,9 %). Ve většině těchto případů onemocnění došlo k přenosu viru KE prostřednictvím tepelně neupraveného kozího mléka a většinou se jednalo o rodinné výskyty, při kterých hrála rozhodující úlohu snaha rodičů zajistit svým dětem zdravou výživu.Klíčová slova:
klíšťová encefalitida – alimentární přenos – přenos lékem –přenos sýrem – kozí mléko.Introduction
Tick-borne encephalitis (TBE) is a serious acute neuroinfection caused by an RNA virus (genus Flavivirus, family Flaviviridae) (VTBE). In the Czech Republic this is represented by a European subtype of this virus, which is mainly transmitted by the common tick Ixodes ricinus. The infectious agent is part of the arbovirus group (viruses transmitted by arthropods). The characteristics of the disease are summarised [4]. TBE has been recognised in CR since 1948 when, for the first time ever in Europe, the TBE virus was isolated from patients and I. ricinus ticks in Berounsko [21, 5] and Vyskovsko [12,13] simultaneously (and independently of one another).
TBE is a zoonosis characterised by the phenomenon of natural foci. This means that the viral agent circulates in nature transmitted by I. ricinus amongst wildlife in an ecologically determined territory (e.g. natural focus of infection) and its transmission to domesticated animals and humans occurs after their entry into and activity within this focus, associated with attack by an infected tick [3]. A second way of transmission of VTBE infection can be milk from infected animals (particularly goats and sheep) which were infected by ticks during grazing. This alimentary route necessitates consumption of unpasteurized milk or milk products from these animals.
TBE cases in the Czech Republic have been reported since 1971. Prior to the late 1970s the incidence of TBE was characterized by intermittent periods of higher and lower morbidity ranging from 180 to 595 cases annualy; the 1980s were a period of low incidence and minimum variability. The early 1990s saw a sharp increase in the number of registered cases (particularly since 1993) which remains to this day with certain fluctuations every year. An exceptionally high incidence was recorded (745 cases) in 1995 and maximum (1029) in 2006.
Information concerning transmission of TBE by the alimentary route was first published in Czechoslovakia in 1954 [1, 22]. At that time, nothing was known of the possibilities of arbovirus spread via the alimentary system; this was discovered following the TBE epidemic of 1951 in the East Slovak town of Roznava in which 660 persons were taken ill, and of these, 271 were hospitalised. The source was infected goats’ milk which had been mixed into dairy milk at the local dairy and distributed without pasteurization. This has been repeatedly and experimentally verified. Recently, this problem has been highlighted by a report [18] of a local, small-scale, epidemic in the Vsetin district (1999) in which patients were infected following consumption of sheep’s cheese.
Method
Laboratory diagnostic involves determination of IgM antibodies in sera or fluids using ELISA, NIF (indirect immunofluorescence) or proof of seroconversion, significant elevation of IgG antibody levels or overall antibodies using ELISA, NIF or KFR. In patients recently vaccinated against yellow fever, Japanese encephalitis and persons returning from areas endemic with these viruses or dengue fever and West Nile virus it is necessary to confirm serology with viral neutralisation test.
Each case of disease with suspicion of TBE is reported by the attending physician to public health bodies which conduct epidemiological investigation. Interviews with patients by epidemiologists or infectious disease specialists yield anamneses including probable routes of transmission and localities where the infection was acquired. Data acquired from interviews with patients were recorded in a standardised questionnaire with locality multi-choice (GIS) and possible routes of transmission. These are exported on a weekly basis in electronic form to a protected Ministry of Health CR database. Statistical processing was conducted through Epi-Info software and, at CDC Atlanta, by STATCALC and ANOVA test.
Results
Reported cases of TBE in the 1997 – 2008 period were analysed. A total of 7288 cases were reported. In 64 cases (0.9%) patients had consumed unpasteurized goats’ or dairy milk or ate unpasteurized sheep’s milk cheese. The majority of cases involved goats’ milk 36 persons (56.3%) and sheep’s milk cheese 21 persons (32.8%). Dairy milk was responsible for 7 infected persons (10.9%).
The number of cases ranged from 0 to 21 annually.
In 33 cases (51.6%) the infections had a familial character in which several members of a family were infected following purchase of cheese or milk from animal breeders. In 22 cases 34.4%) the patients were individuals and in 9 cases (14.0%) the data are unavailable.
The set of patients comprised 33 men (51.6%) and 31 women (48.4%). Total TBE morbidity during the monitored period was 4416 men (60.6%) and 2872 women (39.4%). No statistically significant difference was found between men and women in routes of transmission (OR=0.69, 0.41 <OR<1.17).
The highest rates were recorded in the following age groups: 5 – 9 years (10 cases), 25 – 34 years (12 cases), 35 – 44 (12 cases). The highest age-specific morbidity occurred amongst the 5 – 9 years age-group 1.94/100 000; in the adult age-groups this ranged 0.17/100 00 (75+ years) – 0.89/100 00 (35 – 44 years).
Tab. 1. Number of cases of tick-borne encephalitis with alimentary transmission, CR, 1997-2008 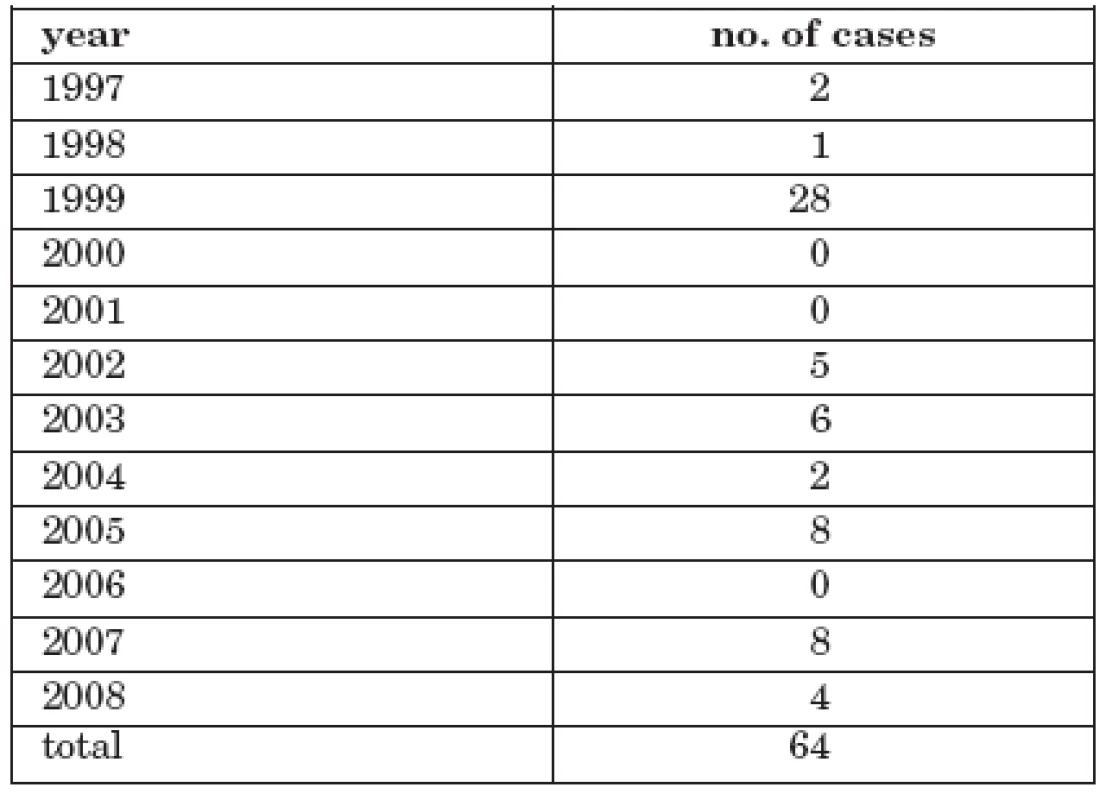
Tab. 2. Cases of tick-borne encephalitis with alimentary transmission in the family 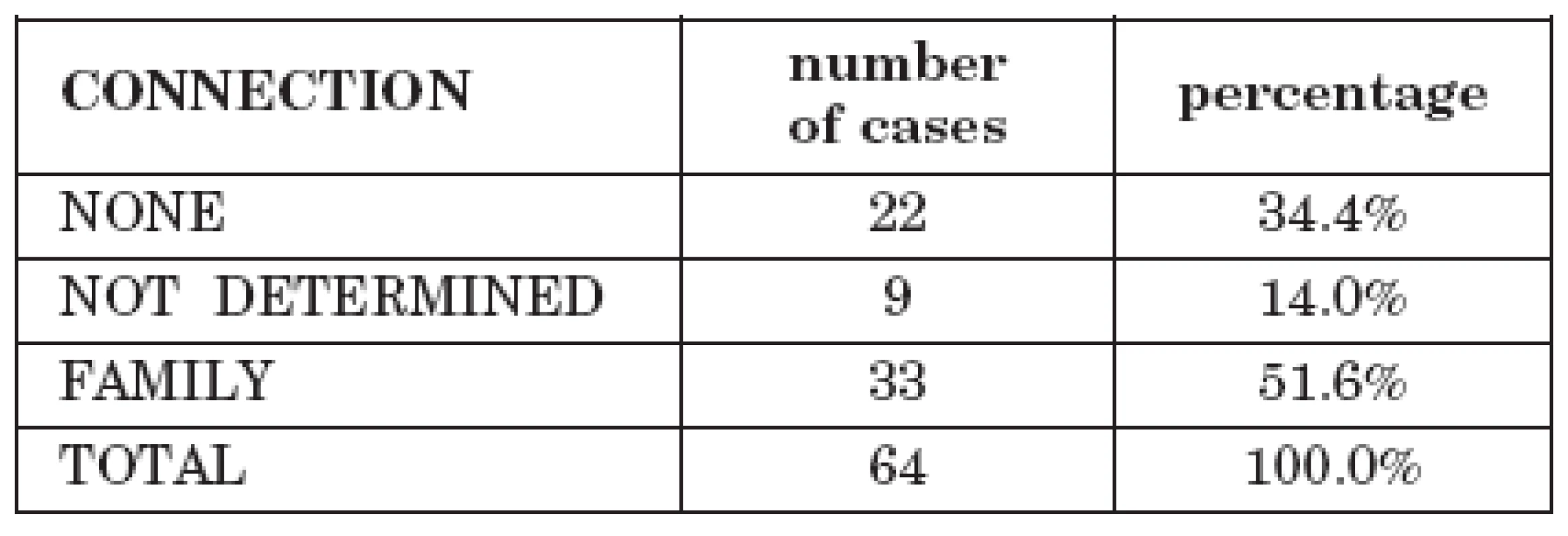
Tab. 3. No. of cases and morbidity of tick-borne encephalitis with alimentary transmission in CR by age group, 1997 - 2008 morbidity 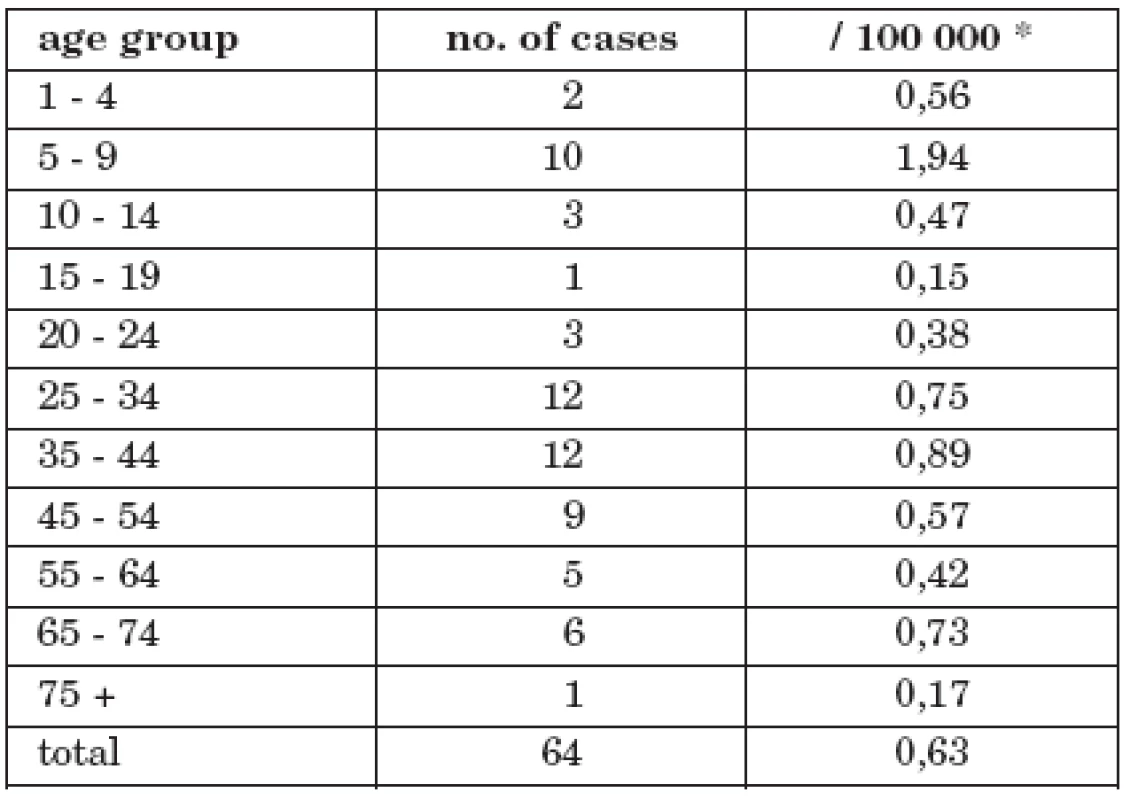
* number of population in the middle of the time interval Comparison of child and adult age-groups (alimentary infections) with analogous groups in the whole TBE set reveals that children had a 2.5 fold risk of infection over adults OR=2.48 (1.32<OR<4.58)
Fig. 1. No. of cases and morbidity of tick-borne encephalitis with alimentary transmission in CR by age group, 1997 – 2008 
Fig. 2. presents an overview of districts with occurrence of TBE alimentary transmission. These comprise 23 districts mainly in the vicinity of Vysocina and pastures in the foothills where use of arable land is limited. In these areas a total of 3123 cases of TBE were reported. Therefore, 64 cases of alimentary transmission represent 2% of all reported cases in these districts. In Vsetin district, the site of an epidemic caused by consumption of unpasteurized sheep’s milk this figure rose to 48%.
Fig. 2. Districts with reported alimentary infection by tickborne encephalitis in 1997 - 2008 
Discussion
Over the past two decades public opinion has changed as regards healthy diet. So-called biofoods, ostensibly manufactured with natural raw materials and minimum chemical additives, are becoming increasingly popular. A part of the attempt to return to a natural lifestyle involves consumption of unpasteurized milk and milk products despite the fact that they are scarcer and more expensive. This approach is mostly practised by young persons, particularly young parents, who feed their children with these products in an attempt to promote good health. Adults have greater options for independent selection of foods than children. The age structure of the presented set confirms this hypothesis.
The majority of documented cases of alimentary TBE infection were caused by consumption of unpasteurized goats’ milk which has become particularly fashionable. The danger of milk from grazing goats stems from the fact that these animals are attacked by ticks to a greater extent than sheep or cows. The reason for this is that goats prefer to graze on buds and young bushes and therefore occupy places with increased incidence of ticks. Moreover, they are sent to graze in early spring when the grass cover does not afford adequate nourishment.
The results acquired in the Czech Republic are comparable to those from abroad.
Unpasteurized goats’ milk and products thereof has been a vehicle of VTBE in the following cases:
In eastern part of CR 9 cases of TBE were caused by consumption of unpasteurized goat milk [2]. In West-Bohemia region family outbreak of three cases was associated with eating of yogurt made from unpasteurized milk [19] and alimentary transmission of TBE caused by consumption of unpasteurized milk was detected in 4.8% of reported TBE cases during 1960 – 2005 [20].
In Slovakia, 13 cases alimentary transmitted cases were reported [26]. 33 cases of TBE after consumption of unpasteurized goats’ and sheep’s milk [14] and a family epidemic of 7 persons. VTBE was isolated from ticks in the places where the goats grazed [11], two family epidemics with 14 persons taken ill [25].
Seropositivity in humans that correlated with consumption of goats’ milk was reported in Lithuani [9],
In Estonia, 27 cases were reported in 2005. Virus neutralisation antibodies were detected at a farm that supplied milk. A total of 16 cases were reported during the previous five year period [10].
Of 63 consumers of unpasteurized goats’ milk in Poland a total of 48 were infected by TBE. Antibodies against VTBE were detected in one of 19 goats [17]. Fifteen persons from 4 families who consumed milk from a single supplier were taken ill [8].
The risk of TBE transmission by unpasteurized goats’ milk is associated with the current trend of I. ricinus tick proliferation in foothills and mountainous areas [15, 16]. Their spread from 700 m above sea level to 1200 m constitutes a newly colonised area up to 8% of the area of the CR (6300 m2). This area contains minimal agricultural elements and mainly comprises areas suitable for ticks. Likewise, these areas contain a high concentration of recreational sites and recreational activities which largely coincide with periods of increased tick incidence. Moreover, these areas tend to be used as pastures for sheep or goats, often as part of ecological preservation of the countryside. The production of milk is not, as such, economically viable but the soft cheeses manufactured from this milk are tempting for the tourist trade and have become marketable items. This conclusion is supported by the case of four persons taken ill in the Austrian Alps (Voralberg) after consumption of goats’ cheese from animals grazing at 1500 m above sea level [7]. The source of infection was subsequently identified as one of the milked goats.
Unpasteurized dairy milk was the vehicle of VTBE in the following cases:
5 persons following consumption of milk and products infected by TBE (3 mortalities). Antibodies against VTBE detected in cows from the incriminated herd in Chabarovsko [23].
Unpasteurized sheep’s milk (or products thereof) has been the vehicle of VTBE in the following cases:
The first documented epidemic in Slovakia caused by consumption of sheep’s milk cheese was published in 1975 [6].
In 1999, a local outbreak occurred in the Vsetin district, CR, with 21 cases following consumption of sheep’s milk cheese from unpasteurized milk [18]. Examination of 41 sheep from the herd revealed virus neutralising antibodies against TBE in 13 cases [24].
It is probable that the greater amount of data on goats’ milk as the vehicle of infection is due to the fact that such milk is, as opposed to dairy milk, almost exclusively obtainable from the manufacturers in unpasteurized form, and that the implicit rearing methods present greater risk of infection. In the case of sheep as sources of infection, transmission is mainly effected through unpasteurized cottage cheese.
Conclusion
The alimentary route of TBE transmission does not play a decisive role in the spread of TBE in endemic areas (0.9%). Districts where alimentary transmission has occurred represent 2% of the overall TBE morbidity. These cases are the result of the fallacious view of persons who believe that consumption of unpasteurized milk or such milk products is positive for their health or that of their children. The results of the present study show that in the majority of 64 cases of TBE transmission occurred via goats’ milk and was largely familial in nature. Children had a 2.5 fold risk of infection over adults.
Do redakce došlo 21.1.2009
Doc. MUDr. Bohumir Kriz, CSc.
National Institute of Public Health
Srobarova 48
100 42 PRAHA 10
Czech Republic
e-mail: bohukriz@szu.cz
Zdroje
1. Blaskovic, D. (Ed.) The epidemic of encephalitis in Roznava natural focus of infection. 1954 .Bratislava, Slovac Academy of Sciences 1954, 1 - 314).
2. Czimova, M., Raszka, J., Januska, F., Heinz, P. et al. Familiar Incidence of Tick-borne Encephalitis with Alimentary Transmission. Epidemiol Mikrobiol Imunol 1981, 30, 6, 334-339.
3. Danielova, V. Natural foci of tick-borne encephalitis and prerequisites for their existence. Int J Med Microbiol 2002, 291, Suppl. 33, 183-186.
4. Danielova, V., Daniel, M., Kriz, B. Tick-borne encephalitis in Europe. In: Ebert R. A., Progress in Encephalitis Research, Nova Science Publisher, New York, 2006, 59 – 103.
5. Gallia, F., Rampas, J., Hollender, L. Laboratory infection by TBE virus. CasLekCes.1949, 88, 224 – 229.
6. Gresikova, M. Sekeyova, M. Stupalova, S. Necas, S. Sheep milk-borne epidemic of tick-borne encephalitis in Slovakia. Intervirology. 1975, 5, 1-2, 57-61.
7. Holzmann, H., Heinz, XH. Tick-borne encephalitis outbreak owing to consumption of fresh cheese from unpasteurized goat milk. Virusepidemiologische Information, 2008, Medizinische Universität Wien, 17, 8, 2-4.
8. Jezyna, C., Weglinska, T., Nawrocka, E. et al. Milk-borne outbreak of TBE in Otsztyn Province, Przegl Epidemiol 1976, 30, 4, 479-489.
9. Juceviciene, A., Vapalahti, O., Laiskonis, A., Ceplikiene., J, Leinikki, P. Prevalence of tick-borne-encephalitis virus antibodies in Lithuania. J Clin Virol 2002, Jul, 25, 1, 23-27.
10. Kerbo, N., Donchenko, I., Kutsar, K., Vasilenko, V. Tick-borne encephalitis outbreak in Estonia linked to raw goat milk. Euro Surveill 2005, 10, 6,
11. Kohl, I, Kozuch, O, Eleckova, E, Labuda, M, Zaludko, J. Family outbreak of alimentary tick-borne encephalitis in Slovakia associated with a natural focus of infection. Eur J Epidemiol 1996, Aug, 12, 4, 373-375.
12. Krejcí, J. The epidemic of viral meningoencephalitis in Vyskov. Lek Listy, 1949, 73 – 75, 112 – 116, 132 – 134.
13. Krejcí, J. Isolation of the virus of human meningo-encephalitis from a tick. Lek Listy 1950, Jul, 15, 5, 14, 6-9.
14. Labuda, M., Eleckova, E., Lickova, M., Sabo, A. Tick-borne encephalitis virus foci in Slovakia. Int J Med Microbiol 2002, Jun, 291, Suppl. 33, 43-47.
15. Materna, J., Daniel, M., Metelka, L., Harcarik, J. The vertical distribution, density and the development of the tick Ixodes ricinus in mountain areas influenced by climate changes. (The Krkonoše Mts., Czech Republic). Int J Med Microbiol 2008, 298, S1, 25 – 27.
16. Materna, J., Daniel, M., Danielova, V. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in Central Europe: results of the three years monitoring in the Krkonoše Mts. (Czech Republic). Centr Eur J Publ Health, 2005, 13, 24-28.
17. Matuszczyk, I, Tarnowska, H, Zabicka, J, Gut, W. The outbreak of an epidemic of tick-borne encephalitis in Kielec province induced by milk ingestion. Przegl Epidemiol 1997, 51, 4, 381-388.
18. Orolinova, M. The epidemic of TBE -Sheep cheese - in the district Vsetin. Proceedings of Czech Microbiol.-epidemiol. Conference, 2000, Breclav.
19. Pazdiora, P., Moravkova, J., Brij, Z., et al. Alimentary transmission in Familiar Incidence of Tick-borne Encephalitis. Prakticky lekar 1994, 74, 5, 209-210.
20. Pazdiora, P., Benesova, J., Bohmova, Z et al. The prevalence of tick-borne encephalitis in the region of West Bohemia (Czech Republic) between 1960-2005. Wien Med Wochenschr 2008, Feb.158, 3-4, 91-97.
21. Rampas, J., Gallia, F. Isolation of encephalitis virus from tics Ixodes ricinus. Cas Lek Ces 1949, 88, 1179 – 1180.
22. Raska K. et al. 1954, Epidemiology of Roznava encephalitis. 93-110, in Blaskovic, D.ed. The epidemic of encephalitis in Roznava natural focus of infection, Bratislava Slovak Academy of Sciences, 314.
23. Vereta, LA, Skorobrekha, VZ, Nikolaeva, SP, et al. The transmission of the tick-borne encephalitis virus via cow‘s milk. Med Parazitol (Mosk). 1991, May-Jun, 3, 54-56.
24. Zeman, P., Januska, J., Orolinova, M., Stuen, S., Struhar, V., Jebavy, L. High seroprevalence of granulocytic ehrlichiosis distinguishes sheep that were the source of an alimentary epidemic of tick-borne encephalitis, Wien Klin Wochenschr 2004, Sep 30, 116, 17-18, 614-616.
25. Zaludko, J, Vrbova, O., Hachlicova, R. et al. Familiar epidemics of tick-borne encephalitis in central Povazie, Bratisl Lek Listy, 1994, Nov, 95, 11, 523-526.
26. Outbreak of tick –borne encephalitis, presumably milk-borne, Weekly Epidemiological Record 1994, 19, 140-141.
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2009 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- SDĚLENÍ Z ČLS JEP Společné prohlášení České lékařské společnosti Jana Evangelisty Purkyně a Asociace inovativního farmaceutického průmyslu O spolupráci mezi lékařskou odbornou veřejností a inovativním farmaceutickým průmyslem
- Životní jubileum doc. MUDr. Otto Lochmanna, CSc.
- K 85. narozeninám prof. MUDr. Vladislava Potužníka, DrSc.
- Recenze
- Nové možnosti očkování proti chřipce
- TEST Z IMUNOLOGIE
- Zprávy z internetu
- Laboratórna diagnostika toxoplazmózy
- Genotypová heterogenita viru hepatitidy C (HCV) u dárců krve v ČR
- Vliv náhlých změn teploty a tlaku vzduchu na úmrtnost v ČR
- Situace a trendy v zastoupení genotypů viru hepatitidy C v populaci injekčních uživatelů drog
- Borrelia burgdorferi s.l. v klíšťatech na ostravských haldách
- Alimentární přenos klíšťové encefalitidy v České republice (1997–2008)
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Laboratórna diagnostika toxoplazmózy
- TEST Z IMUNOLOGIE
- Vliv náhlých změn teploty a tlaku vzduchu na úmrtnost v ČR
- Genotypová heterogenita viru hepatitidy C (HCV) u dárců krve v ČR
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



